Seasonal Variations in Water-Quality, Antibiotic Residues, Resistant Bacteria and Antibiotic Resistance Genes of Escherichia coli Isolates from Water and Sediments of the Kshipra River in Central India
Abstract
:1. Introduction
2. Material and Methods
2.1. Setting
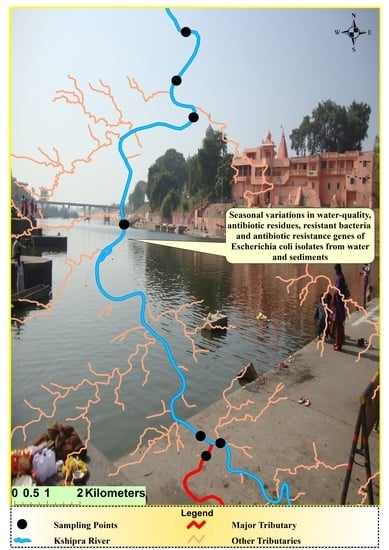
2.2. Sample Collection
2.3. Sample Analysis
2.3.1. Water Quality Parameters Measured in the Field
2.3.2. Water and Sediment Quality Parameters Examined in the Laboratory
2.3.3. Antibiotic Residue Analysis
2.3.4. Microbiological Methods
2.3.5. Molecular Methods
DNA Extraction
PCR Detection of Genes
2.4. Data Management and Statistical Analysis
3. Results
3.1. Water Quality
3.2. Antibiotic Residues
3.3. Antibiotic Resistance
3.4. Antibiotic Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gotha, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Lienert, J.; Bürki, T.; Escher, B.I. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2007, 56, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Kolpin, D.W.; Halling-Sørensen, B.; Tolls, J. Are veterinary medicines causing environmental risks? Environ. Sci. Technol. 2003, 37, 286A–294A. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.G.; Truman, C.C.; Kim, S.C.; Ascough, J.C.; Carlson, K. Antibiotic transport via runoff and soil loss. J. Environ. Qual. 2006, 35, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ. Sci Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Meyer, M.T.; Liu, X.; Zhao, Q.; Chen, H.; Chen, J.; Qiu, Z.; Yang, L.; Cao, J.; Shu, W. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut. Barking Essex 2010, 158, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Tuc Dinh, Q.; Clément, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Ying, G.-G.; Zhao, J.-L.; Yang, J.-F.; Wang, L.; Yang, B.; Liu, S. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ. Pollut. Barking Essex 2011, 159, 1877–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hari, A.C.; Paruchuri, R.A.; Sabatini, D.A.; Kibbey, T.C.G. Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ. Sci. Technol. 2005, 39, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Beausse, J. Selected Drugs in solid matrices: A review of environmental determination, occurrence and properties of principal substances. TrAC Trends Anal. Chem. 2004, 23, 753–761. [Google Scholar] [CrossRef]
- Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2007, 41, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, M.R.; Chandran, S.; Shah, H.; Diwan, V.; Tamhankar, A.J.; Stålsby Lundborg, C. Antibiotic resistance in an Indian rural community: A ”One-Health” observational study on commensal coliform from humans, animals, and water. Int. J. Environ. Res. Public Health 2017, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.K.; Pinyon, J.L.; Anantham, S.; Hall, R.M. Commensal Escherichia coli of healthy humans: A reservoir for antibiotic-resistance determinants. J. Med. Microbiol. 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Edge, T.A.; Hill, S. Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can. J. Microbiol. 2005, 51, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Micalizzi, G.B.; Graham, G.M.; Bates, J.B.; Costanzo, S.D. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol. 2007, 73, 5667–5670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Zhang, T.; Fang, H.H.P. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, E.A.; Seyfried, E.E.; McMahon, K.D. Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res. 2007, 41, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef] [PubMed]
- WHO. Communicable Disease Alert and Response for Mass Gatherings. 2008. Available online: http://www.who.int/csr/Mass_gatherings2.pdf?ua=1 (accessed on 10 March 2018).
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.R.; Shanmugam, G.; Velu, G.; Rengarajan, B.; Larsson, D.G.J. GC-MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 2011, 186, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Gotha, R.; Thatikonda, S. Mathematical model for the transport of fluoroquinolone and its resistant bacteria in aquatic environment. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef]
- Farook Ahmed, T.; Sushil, M.; Krishna, M. Impact of Dye Industrial effluent on physicochemical characteristics of Kshipra river, Ujjain city, India. Int. Res. J. Environ. Sci. 2012. Available online: http://www.isca.in/IJENS/Archive/v1/i2/8.ISCA-IRJEvsS-2012–041.pdf (accessed on 10 March 2018).
- Gupta, R.C.; Gupta, A.K.; Shrivastava, R.K. Assessment of water quality status of holy river Kshipra using water quality index. J. Indian Water Resour. Soc. 2012, 32, 1–7. Available online: http://www.iwrs.org.in/journal/jan2012/4jan.pdf (accessed on 22 January 2018).
- David, S.; Roy, N. Public health perspectives from the biggest human mass gathering on earth: Kumbh mela, India. Int. J. Infect. Dis. 2016, 47, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.S.; Bhatia, R.K.; Pawar, R.S.; Bhatia, R.K. Assessment of Water Quality of River Kshipra during Simhastha Mahakumbh Mela 2016 in Ujjain, Madhya Pradesh. Int. J. Innov. Res. Sci. Technol. 2016. Available online: http://www.ijirst.org/articles/IJIRSTV3I4132.pdf (accessed on 12 January 2018).
- Diwan, V.; Purohit, M.; Chandran, S.; Parashar, V.; Shah, H.; Mahadik, V.K.; Lundborg, C.S.; Tamhankar, A.J. A three-year follow-up study of antibiotic and metal residues, antibiotic resistance and resistance genes, focusing on Kshipra-a river associated with holy religious mass-bathing in India: Protocol paper. Int. J. Environ. Res. Public Health 2017, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Stålsby Lundborg, C.; Tamhankar, A.J. Seasonal and temporal variation in release of antibiotics in hospital wastewater: Estimation using continuous and grab sampling. PLoS ONE 2013, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3704537/ (accessed on 31 January 2018). [CrossRef] [PubMed]
- Diwan, V.; Tamhankar, A.J.; Khandal, R.K.; Sen, S.; Aggarwal, M.; Marothi, Y. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health 2010, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Renew, J.; Smeby, K.; Pinkston, K.; Sedlak, D. Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. J. Contemp. Water Res. Educ. 2011, 120. Available online: http://opensiuc.lib.siu.edu/jcwre/vol120/iss1/4 (accessed on 21 January 2018).
- Nataro, J.; Bopp, C.; Fields, P.; Kaper, J.; Strockbine, N. Escherichia, Shigella, and Salmonella. In Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2007; Available online: http://www.asmscience.org/content/book/10.1128/9781555816728.chap35 (accessed on 13 January 2018).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Clin. Lab. Stand. Inst. 2014. Available online: http://www.facm.ucl.ac.be/intranet/CLSI/CLSI-2015-M100-S25-original.pdf (accessed on 27 January 2018).
- Chandran, S.P.; Diwan, V.; Tamhankar, A.J.; Joseph, B.V.; Rosales-Klintz, S.; Mundayoor, S.; Lundborg, C.S.; Macaden, R. Detection of carbapenem resistance genes and cephalosporin, and quinolone resistance genes along with oqxAB gene in Escherichia coli in hospital wastewater: A matter of concern. J. Appl. Microbiol. 2014, 117, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Titilawo, Y.; Obi, L.; Okoh, A. Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: Implications for public health. Sci. Total Environ. 2015, 523, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J.; Grabow, W.O.; Snozzi, M. Indicators of Microbial Water Quality; IWA Publishing: London, UK, 2001. [Google Scholar]
- Ishii, S.; Sadowsky, M.J. Escherichia coli in the environment: Implications for water quality and human health. Microbes Environ. 2008, 23, 101–108. [Google Scholar] [CrossRef] [PubMed]
- EPA. Revised Total Coliform Rule (RTCR): A Quick Reference Guide. 2013; Tillgänglig vid. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100K9MP.txt (accessed on 15 January 2018).
- Bhasin, S.; Shukla, A.N.; Shrivastava, S. Impact of mass bathing on water quality of river Kshipra at Triveni, Ujjain, M.P. India. Int. J. Adv. Life Sci. 2015. Available online: http://www.unitedlifejournals.com/ms_files/ijals/5._Impact_of_mass_bathing_on_waster_quality.pdf (accessed on 30 January 2018).
- Ingerslev, F.; Halling-Sorensen, B. Biodegradability properties of sulfonamides in activated sludge. Environ. Toxicol. Chem. 2000, 19, 2467–2473. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Blackwell, P.; Cavallo, R.; Kay, P.; Tolls, J. The sorption and transport of a sulphonamide antibiotic in soil systems. Toxicol. Lett. 2002, 131, 19–28. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Beek, B. (Ed.) Biodegradation and Persistence; Springer-Verlag: Berlin/Heidelberg, Germany, 2001; (Reactions and Processes); Available online: //www.springer.com/la/book/9783662146903 (accessed on 27 January 2018).
- Alexy, R.; Kümpel, T.; Kümmerer, K. Assessment of degradation of 18 antibiotics in the closed bottle test. Chemosphere 2004, 57, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Braselton, W.E.; Rumbeiha, W.K.; Johnson, M. Rapid and reliable identification of ionophore antibiotics in feeds by liquid chromatography-tandem mass spectrometry. J. Vet. Diagn Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn Inc. 2011, 23, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pedersen, J.A. Adsorption of sulfonamide antimicrobial agents to clay minerals. Environ. Sci. Technol. 2005, 39, 9509–9516. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Stamm, C. Time and pH-dependent sorption of the veterinary antimicrobial sulfathiazole to clay minerals and ferrihydrite. Chemosphere 2007, 68, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Daschner, F.D.; Kummerer, K. biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria. Arch. Environ. Contam. Toxicol. 1999, 37, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Höltge, S.; Kreuzig, R. Laboratory Testing of sulfamethoxazole and its metabolite acetyl-sulfamethoxazole in Soil. Clean Soil Air Water 2007, 35, 104–110. [Google Scholar] [CrossRef]
- Heise, J.; Höltge, S.; Schrader, S.; Kreuzig, R. Chemical and biological characterization of non-extractable sulfonamide residues in soil. Chemosphere 2006, 65, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, M.; Jiang, H.; Zhang, D.; Lin, L.; Ye, H.; Zhang, X. Occurrence, seasonal variation and removal efficiency of antibiotics and their metabolites in wastewater treatment plants, Jiulongjiang River Basin, South China. Environ. Sci. Process. Impacts. 2015, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. TrAC Trends Anal. Chem. 2001, 20, 419–434. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant. Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Picó, Y.; Andreu, V. Fluoroquinolones in soil—Risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Golet, E.M.; Strehler, A.; Alder, A.C.; Giger, W. Determination of fluoroquinolone antibacterial agents in sewage sludge and sludge-treated soil using accelerated solvent extraction followed by solid-phase extraction. Anal. Chem. 2002, 74, 5455–5462. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, X.; Cheng, D.; Liu, G.; Liang, B.; Cui, B.; Bai, J. Temporal-spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci. Total Environ. 2016, 569–570, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Cha, J.M.; Yang, S.; Carlson, K.H. Trace determination of beta-lactam antibiotics in surface water and urban wastewater using liquid chromatography combined with electrospray tandem mass spectrometry. J. Chromatogr. A 2006, 1115, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and antibiotic resistance genes in natural environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Ewers, C.; Wieler, L.H. Extended-spectrum beta-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Front. Microbiol. 2011, 2. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3244693/ (accessed on 30 January 2018). [CrossRef] [PubMed]
- Peirano, G.; van Greune, C.H.J.; Pitout, J.D.D. Characteristics of infections caused by extended-spectrum β-lactamase-producing Escherichia coli from community hospitals in South Africa. Diagn. Microbiol. Infect. Dis. 2011, 69, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Iwane, T.; Urase, T.; Yamamoto, K. Possible impact of treated wastewater discharge on incidence of antibiotic resistant bacteria in river water. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2001, 43, 91–99. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Zhang, J.; Liu, R.; Gu, X.; Zhang, Y.; Wang, Z. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river. Environ. Microbiol. 2009, 11, 1506–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, R.; Kim, S.-C.; Carlson, K.H.; Pruden, A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Séveno, N.A.; Kallifidas, D.; Smalla, K.; van Elsas, J.D.; Collard, J.M.; Karagouni, A.D.; Wellington, E.M. Occurrence of reservoirs of antibiotic resistance genes in the environment. Rev. Med. Microbiol. 2002, 13, 15–27. [Google Scholar] [CrossRef]
- Knapp, C.W.; Dolfing, J.; Ehlert, P.A.I.; Graham, D.W. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2010, 44, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef] [PubMed]
- Längin, A.; Alexy, R.; König, A.; Kümmerer, K. Deactivation and transformation products in biodegradability testing of beta-lactams amoxicillin and piperacillin. Chemosphere 2009, 75, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.K.; McKellar, J.F.; Phillips, G.O.; Reid, A.G. Photochemical oxidation of tetracycline in aqueous solution. J. Chem. Soc. Perkin Trans. 1979, 369–375. [Google Scholar] [CrossRef]
- Torniainen, K.; Tammilehto, S.; Ulvi, V. The effect of pH, buffer type and drug concentration on the photodegradation of ciprofloxacin. Int. J. Pharm. 1996, 132, 53–61. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011. Available online: https://www.hindawi.com/journals/ijmicro/2011/312956/cta/ (accessed on 30 January 2018).
- Sørensen, S.J.; Bailey, M.; Hansen, L.H.; Kroer, N.; Wuertz, S. Studying plasmid horizontal transfer in situ: A critical review. Nat. Rev. Microbiol. 2005, 3, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Klein, E.Y.; Laxminarayan, R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3141051/ (accessed on 30 January 2018). [CrossRef] [PubMed]
- Shakya, P.; Barrett, P.; Diwan, V.; Marothi, Y.; Shah, H.; Chhari, N. Antibiotic resistance among Escherichia coli isolates from stool samples of children aged 3 to 14 years from Ujjain, India. BMC Infect. Dis. 2013, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, H.; Mohapatra, S.S.; Mantri, C.K.; Colwell, R.R.; Singh, D.V. Vibrio cholerae non-O1, non-O139 strains isolated before 1992 from Varanasi, India are multiple drug resistant, contain intSXT, dfr18 and aadA5 genes. Environ. Microbiol. 2008, 10, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chakraborty, R. Incidence of class 1 integrons in multiple antibiotic-resistant Gram-negative copiotrophic bacteria from the River Torsa in India. Res. Microbiol. 2006, 157, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shi, J.; Chang, H.; Li, D.; Yang, M.; Kamagata, Y. Phenotyping and genotyping of antibiotic-resistant Escherichia coli isolated from a natural river basin. Environ. Sci. Technol. 2008, 42, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Dorland, W.A.N. Dorland’s Illustrated Medical Dictionary, 31st ed.; Saunders Press: Philadelphia, PA, USA, 2007. [Google Scholar]

| River Water Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Summer (N = 14) | Rain (N = 14) | Autumn (N = 14) | Winter (N = 14) | p-Value | ||||
| n | Mean (Range) µg/L | n | Mean (Range) µg/L | n | Mean (Range) µg/L | n | Mean (Range) µg/L | ||
| Ceftriaxone | 0 | BDL | 0 | BDL | 0 | BDL | 0 | BDL | - |
| Ciprofloxacin | 0 | BDL | 0 | BDL | 0 | BDL | 0 | BDL | - |
| Norfloxacin | 0 | BDL | 0 | BDL | 6 | 0.66 (0–0.98) | 0 | BDL | - |
| Ofloxacin | 0 | BDL | 0 | BDL | 7 | 0.99 (0.64–1.46) | 0 | BDL | - |
| Metronidazole | 0 | BDL | 0 | BDL | 0 | BDL | 0 | BDL | - |
| Sulfamethoxazole | 14 | 1.39 (0.24–2.21) | 4 | 0.04 (0–0.17) | 7 | 2.75 (1.05–4.66) | 7 | 2.18 (0.5–3.47) | <0.0001 * |
| Total Residual Antibiotics as Beta Lactam | n (%) | n (%) | n (%) | n (%) | |||||
| Present (>5 ppb) | 7 (50) | 0 (0) | 0 (0) | 0 (0) | - | ||||
| River Sediment Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Summer (N = 0) | Rain (N = 7) | Autumn (N = 7) | Winter (N = 14) | p-Value | ||||
| n | Mean (Range) µg/L | n | Mean (Range) µg/L | n | Mean (Range) µg/L | n | Mean (Range) µg/L | ||
| Ceftriaxone | - | - | BDL | BDL | BDL | BDL | BDL | BDL | - |
| Ciprofloxacin | - | - | BDL | BDL | BDL | BDL | BDL | BDL | - |
| Norfloxacin | - | - | BDL | BDL | BDL | BDL | BDL | BDL | - |
| Ofloxacin | - | - | BDL | BDL | BDL | BDL | 1 | 1.39 (0–9.74) | - |
| Metronidazole | - | - | BDL | BDL | BDL | BDL | BDL | BDL | - |
| Sulfamethoxazole | - | - | BDL | BDL | BDL | BDL | 1 | 1.18 (0–8.23) | - |
| Total Residual Antibiotics as Beta Lactam | n (%) | n (%) | n (%) | n (%) | |||||
| Present (>5 ppb) | - | 4 (57.1) | 0 | 0 | - | ||||
| River Water Samples | |||||
|---|---|---|---|---|---|
| Antibiotic | Summer N = 70 * | Rain N = 80 ** | Autumn N = 70 *** | Winter N = 83 **** | p-Value |
| n (%) | n (%) | n (%) | n (%) | ||
| Ampicillin | 12 (17) | 27 (33) | 32 (45) | 33 (39) | 0.002 |
| Cefotaxime | 14 (20) | 15 (18) | 14 (20) | 24 (28) | 0.4 |
| Ceftazidime | 10 (14) | 12 (15) | 15 (21) | 13 (15) | 0.7 |
| Cefepime | 0 (0) | 6 (7) | 10 (14) | 9 (10) | 0.004 |
| Ceftriaxone | 9 (12) | - | - | - | - |
| Imipenem | 1 (1) | 1 (1) | 1 (1) | 4 (4) | 0.5 |
| Meropenem | 7 (10) | 0 (0) | 4 (5) | 22 (26) | <0.0001 |
| Ciprofloxacin | 4 (5) | 7 (8) | 10 (14) | 12 (14) | 0.2 |
| Nalidixic acid | 8 (11) | 11 (13) | 16 (22) | 21 (25) | 0.08 |
| Amikacin | 13 (15) | 0 (0) | 0 (0) | 13 (15) | <0.0001 |
| Gentamicin | 0 (0) | 2 (2) | 1 (1) | 8 (9) | 0.007 |
| Nitrofurantoin | 0 (0) | 3 (3) | 3 (4) | 5 (6) | 0.2 |
| Tetracycline | 3 (4) | 10 (12) | 12 (17) | 11 (13) | 0.09 |
| Tigecycline | 0 (0) | 0 (0) | 0 (0) | 7 (8) | 0.0002 |
| Sulfamethizole | 5 (7) | 11 (13) | 12 (17) | 7 (8) | 0.2 |
| Co-trimoxazole | 4 (5) | 11 (13) | 11 (15) | 9 (10) | 0.3 |
| Colistin | 0 (00) | - | - | - | - |
| ESBL | 7 (8) | 10 (11) | 10 (12) | 5 (6) | 0.4 |
| MDR | 2 (2) | 15 (17) | 19 (24) | 20 (23) | <0.0001 |
| Sediment Samples | ||||
|---|---|---|---|---|
| Antibiotics | Rain N = 31 * | Autumn N = 27 ** | Winter N = 39 *** | p-Value |
| n (%) | n (%) | n (%) | ||
| Ampicillin | 9 (29) | 9 (33) | 12 (30) | 0.96 |
| Cefotaxime | 9 (29) | 6 (22) | 18 (46) | 0.12 |
| Ceftazidime | 9 (29) | 6 (22) | 8 (20) | 0.76 |
| Cefepime | 6 (20) | 2 (7) | 6 (15) | 0.41 |
| Imipenem | 0 (0) | 0 (0) | 2 (5) | 0.33 |
| Meropenem | 0 (0) | 0 (0) | 11 (28) | <0.0001 |
| Ciprofloxacin | 6 (19) | 7 (25) | 6 (15) | 0.62 |
| Nalidixic acid | 7 (22) | 11 (40) | 11 (28) | 0.34 |
| Amikacin | 0 (0) | 1 (3) | 2 (5) | 0.62 |
| Gentamicin | 0 (0) | 0 (0) | 2 (5) | 0.33 |
| Nitrofurantoin | 2 (6) | 0 (0) | 0 (0) | 0.18 |
| Tetracycline | 3 (9) | 8 (29) | 7 (17) | 0.15 |
| Tigecycline | 0 (0) | 0 (0) | 2 (5) | 0.33 |
| Sulfamethizole | 5 (16) | 7 (25) | 7 (17) | 0.65 |
| Co-trimoxazole | 6 (19) | 8 (29) | 7 (17) | 0.55 |
| ESBL | 9 (26) | 4 (13) | 7 (17) | 0.49 |
| MDR | 9 (26) | 10 (34) | 10 (24) | 0.67 |
| River Water Samples | |||||
|---|---|---|---|---|---|
| Antibiotic Resistance Genes | Summer | Rain | Autumn | Winter | p-Value |
| n (%) | n (%) | n (%) | n (%) | ||
| CTX-M-1 a | 8 (23) | 10 (31) | 9 (50) | 8 (19) | 0.09 |
| CTX-M-2 a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| CTX-M-9 a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| qnrAb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| qnrBb | (5) | 0 (0) | 0 (0) | 0 (0) | 0.2 |
| qnrSb | 2 (10) | 6 (23) | 2 (8) | 7 (16) | 0.5 |
| Sul Ic | 2 (5) | 4 (12) | 3 (13) | 4 (12) | 0.6 |
| Sul IIc | 4 (10) | 6 (18) | 5 (21) | 4 (12) | 0.6 |
| NDM d | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| VIM d | 0 (0) | 0 (0) | 0 (0) | 0 (0) | - |
| Phylogenic groups | |||||
| A e | 30 (63) | 38 (66) | 28 (60) | 34 (52) | 0.5 |
| B1 e | 14 (30) | 11 (19) | 15 (32) | 16 (25) | 0.4 |
| B2 e | 0 (0) | 2 (3) | 0 (0) | 6 (9) | 0.03 |
| D e | 4 (8) | 5 (9) | 4 (9) | 9 (14) | 0.8 |
| Sediment Samples | ||||
|---|---|---|---|---|
| Antibiotic Resistance Genes | Rain | Autumn | Winter | p-Value |
| n (%) | n (%) | n (%) | ||
| CTX-M-1 a | 5 (25) | 2 (40) | 7 (41) | 0.6 |
| CTX-M-2 a | 0 (0) | 0 (0) | 0 (0) | - |
| CTX-M-9 a | 0 (0) | 0 (0) | 0 (0) | - |
| qnr A b | 0 (0) | 0 (0) | 0 (0) | - |
| qnr B | 0 (0) | 0 (0) | 0 (0) | - |
| qnr S b | 0 (0) | 2 (15) | 4 (44) | 0.3 |
| sul Ic | 1 (25) | 3 (33) | 2 (33) | 0.9 |
| sul IIc | 1 (25) | 3 (33) | 4 (67) | 0.5 |
| NDM d | 0 (0) | 0 (0) | 0 (0) | - |
| VIM d | 0 (0) | 0 (0) | 0 (0) | - |
| Phylogenic groups | ||||
| A e | 16 (76) | 11 (56) | 15 (63) | 0.5 |
| B1 e | 4 (19) | 2 (11) | 5 (21) | 0.7 |
| B2 e | 0 (0) | 1 (5) | 0 (0) | 0.3 |
| D e | 1 (5) | 4 (21) | 4 (17) | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diwan, V.; Hanna, N.; Purohit, M.; Chandran, S.; Riggi, E.; Parashar, V.; Tamhankar, A.J.; Stålsby Lundborg, C. Seasonal Variations in Water-Quality, Antibiotic Residues, Resistant Bacteria and Antibiotic Resistance Genes of Escherichia coli Isolates from Water and Sediments of the Kshipra River in Central India. Int. J. Environ. Res. Public Health 2018, 15, 1281. https://doi.org/10.3390/ijerph15061281
Diwan V, Hanna N, Purohit M, Chandran S, Riggi E, Parashar V, Tamhankar AJ, Stålsby Lundborg C. Seasonal Variations in Water-Quality, Antibiotic Residues, Resistant Bacteria and Antibiotic Resistance Genes of Escherichia coli Isolates from Water and Sediments of the Kshipra River in Central India. International Journal of Environmental Research and Public Health. 2018; 15(6):1281. https://doi.org/10.3390/ijerph15061281
Chicago/Turabian StyleDiwan, Vishal, Nada Hanna, Manju Purohit, Salesh Chandran, Emilia Riggi, Vivek Parashar, Ashok J. Tamhankar, and Cecilia Stålsby Lundborg. 2018. "Seasonal Variations in Water-Quality, Antibiotic Residues, Resistant Bacteria and Antibiotic Resistance Genes of Escherichia coli Isolates from Water and Sediments of the Kshipra River in Central India" International Journal of Environmental Research and Public Health 15, no. 6: 1281. https://doi.org/10.3390/ijerph15061281
APA StyleDiwan, V., Hanna, N., Purohit, M., Chandran, S., Riggi, E., Parashar, V., Tamhankar, A. J., & Stålsby Lundborg, C. (2018). Seasonal Variations in Water-Quality, Antibiotic Residues, Resistant Bacteria and Antibiotic Resistance Genes of Escherichia coli Isolates from Water and Sediments of the Kshipra River in Central India. International Journal of Environmental Research and Public Health, 15(6), 1281. https://doi.org/10.3390/ijerph15061281