Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds
Abstract
:1. Introduction
1.1. Background
1.2. Motivation, Aim and Objectives
2. Materials and Methods
2.1. Set-Up and Experimental Operation
2.2. Quality Analysis of Water, Soil and Plants
2.3. Analysis of Data
3. Results and Discussion
3.1. Overview Regarding Irrigation Water Quality
3.1.1. Oxygen Demand Parameters
3.1.2. Irrigation Water Nutrients and Trace Elements
3.1.3. Particles, pH and Salinity
3.1.4. Microbial Indicators within the Irrigation Water
3.2. Analysis of Soil Quality
3.2.1. pH and Redox Potential Comparison
3.2.2. Soil Salinity Comparison
3.2.3. Soil Microbes
3.2.4. Soil Aluminum
3.2.5. Soil Zinc
3.2.6. Soil Boron
3.2.7. Other Elements
3.3. Chilli (First Generation) Microbial and Mineral Contents
3.3.1. Overview
3.3.2. Aluminum
3.3.3. Calcium
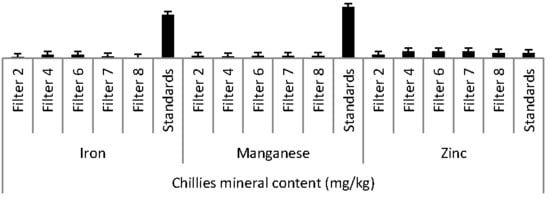
3.3.4. Iron
3.3.5. Potassium
3.3.6. Magnesium
3.3.7. Manganese
3.3.8. Zinc
3.3.9. Boron
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. User’s Manual for Irrigation with Treated Wastewater; Food and Agriculture Organization (FAO) of the United Nations, FAO Regional Office of the Near East: Cairo, Egypt, 2003. [Google Scholar]
- Pinto, U.; Maheshwari, B.L.; Grewal, H.S. Effects of greywater irrigation on plant growth, water use and soil properties. Resour. Conserv. Recycl. 2010, 54, 429–435. [Google Scholar] [CrossRef]
- Zavadil, J. The effect of municipal wastewater irrigation on the yield and quality of vegetables and crops. Soil Water Res. 2009, 4, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Sani, A.; Scholz, M.; Bouillon, L. Seasonal assessment of experimental vertical-flow constructed wetlands treating domestic wastewater. Bioresour. Technol. 2013, 147, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M. Wetland System—Storm Water Management Control; Springer: Berlin, Germany, 2010. [Google Scholar]
- Almuktar, S.A.A.A.N.; Scholz, M. Mineral and biological contamination of soil and Capsicum annuum irrigated with recycled domestic wastewater. Agric. Water Manag. 2016, 167, 95–109. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Scholz, M. Experimental assessment of recycled diesel spill-contaminated domestic wastewater treated by reed beds for irrigation of Sweet Peppers. Int. J. Environ. Res. Public Health 2016, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Recycling of domestic wastewater treated by vertical-flow wetlands for irrigation of two consecutive Capsicum annuum generations. Ecol. Eng. 2017, 107, 82–98. [Google Scholar] [CrossRef]
- Cirelli, G.L.; Consoli, S.; Licciardello, F.; Aiello, R.; Giuffrida, F.; Leonardi, C. Treated municipal wastewater reuse in vegetable production. Agric. Water Manag. 2012, 104, 163–170. [Google Scholar] [CrossRef]
- Aiello, R.; Cirelli, G.L.; Consoli, S. Effects of reclaimed wastewater irrigation on soil and tomato fruits: A case study in Sicily (Italy). Agric. Water Manag. 2007, 93, 65–72. [Google Scholar] [CrossRef]
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Almuktar, S.A.A.A.N.; Scholz, M.; Al-Isawi, R.H.K.; Sani, A. Recycling of domestic wastewater treated by vertical-flow wetlands for irrigating Chillies and Sweet Peppers. Agric. Water Manag. 2015, 149, 1–22. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Scholz, M.; Al-Isawi, R.H.K.; Sani, A. Recycling of domestic wastewater treated by vertical-flow wetlands for watering of vegetables. Water Pract. Technol. 2015, 10, 445–464. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Scholz, M. Microbial contamination of Capsicum annuum irrigated with recycled domestic wastewater treated by vertical-flow wetlands. Ecol. Eng. 2015, 82, 404–414. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture; FAO Agriculture Series Number 27; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 1994. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA), American Water Works Association, and Water and Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- EPA. Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; Method 200.7, Revision 4.4 (EMMC Version); US Environmental Protection Agency (EPA): Cincinnati, OH, USA, 1994.
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States; Southern Cooperative Series Bulletin Number 368; University of Georgia: Athens, GA, USA, 1992. [Google Scholar]
- Bar-Tal, A.; Aloni, B.; Karni, L.; Rosenberg, R. Nitrogen nutrition of greenhouse pepper. II. Effects of nitrogen concentration and NO3: NH4 ratio on growth, transpiration, and nutrient uptake. HortScience 2001, 36, 1252–1259. [Google Scholar]
- Pescod, M.B. Wastewater treatment and use in agriculture. In FAO Irrigationand Drainage Paper Number 47; Food and Agriculture Organisation (FAO): Rome, Italy, 1992. [Google Scholar]
- Scholz, M. Wetlands Systems to Control Urban Runoff; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- McCauly, A.; Jones, C.; Jacobsen, J. Plant Nutrient Functions and Deficiency and Toxicity Symptoms; Nutrient Management Module No. 9; Montana State University: Bozeman, MT, USA, 2011. [Google Scholar]
- FAO. Trace Elements in Soils and Agriculture; FAO Water Report 17; Food and Agriculture Organisation (FAO) of the United Nations: Rome, Italy, 1972. [Google Scholar]
- Wiseman, I.M.; Edwards, P.J. Constructed wetlands for minewater treatment: Performance and sustainability. Water Environ. J. 2004, 18, 127–132. [Google Scholar] [CrossRef]
- Lesley, B.; Daniel, H.; Paul, Y. Iron and manganese removal in wetland treatment systems: Rates, processes and implications for management. Sci. Total Environ. 2008, 394, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, Z.; Aboutalebi, A.; Zakerin, A. Comparison of different medium for production of sweet pepper transplant. Int. Res. J. Appl. Basic Sci. 2013, 4, 307–310. [Google Scholar]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Chou, C.C.; Pan, C.T. Heavy metal removal within pilot-scale constructed wetlands receiving river water contaminated by confined swine operations. Desalination 2009, 249, 368–373. [Google Scholar] [CrossRef]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environ. Pollut. 2004, 132, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Galletti, A.; Verlicchi, P.; Ranieri, E. Removal and accumulation of Cu, Ni and Zn in horizontal subsurface flow constructed wetlands: Contribution of vegetation and filling medium. Sci. Total Environ. 2010, 408, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Guittonny-Philippe, A.; Masotti, V.; Höhener, P.; Boudenne, J.L.; Viglione, J.; Laffont-Schwob, I. Constructed wetlands to reduce metal pollution from industrial catchments in aquatic Mediterranean ecosystems: A review to overcome obstacles and suggest potential solutions. Environ. Int. 2014, 64, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.J.; Wiel-Shafran, A.; Weisbrod, N.; Adar, E.; Gross, A. Greywater reuse for irrigation: Effect on soil properties. Sci. Total Environ. 2010, 408, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.; Knight, R. Treatment Wetlands; Lewis Publishers: Boca Raton, FL, USA, 1996. [Google Scholar]
- Green, M.; Friedler, E.; Ruskol, Y.; Safrai, I. Investigation of alternative method for nitrification in constructed wetlands. Water Sci. Technol. 1997, 35, 63–70. [Google Scholar] [CrossRef]
- Garcia, J.; Rousseau, D.P.; Morato, J.; Lesage, E.L.S.; Matamoros, V.; Bayona, J.M. Contaminant removal processes in subsurface-flow constructed wetlands: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 561–661. [Google Scholar] [CrossRef]
- Hua, G.F.; Li, L.; Zhao, Y.Q.; Zhu, W.; Shen, J.Q. An integrated model of substrate clogging in vertical flow constructed wetlands. J. Environ. Manag. 2013, 119, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Sundaravadivel, M.; Vigneswaran, S. Constructed wetlands for wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2001, 31, 351–409. [Google Scholar] [CrossRef]
- Manios, T.; Stentiford, E.I.; Millner, P. Removal of total suspended solids from wastewater in constructed horizontal flow subsurface wetlands. J. Environ. Sci. Health Part A 2003, 38, 1073–1085. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. Agronomy 1999, 38, 55–110. [Google Scholar]
- USEPA. Guidelines for Water Reuse; United States (US) Environmental Protection Agency (EPA): Washington, DC, USA, 2004.
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Essington, M.E. Soil and Water Chemistry: An Integrative Approach; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- EPA. Guidelines for Water Reuse: Manual; US Environmental Protection Agency (EPA) and US Agency for International Development: Cincinnati, OH, USA, 1992.
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 37. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Report on the 32nd Session of the Codex Committee on Food Additives and Contaminants; Joint Food and Agriculture Organization (FAO) and World Health Organization (WHO) Food Standard Programme Codex Alimentarius Commission: Geneva, Switzerland, 2001. [Google Scholar]
- Li, Q.; Chen, Y.; Fu, H.; Cui, Z.; Shi, L.; Wang, L.; Liu, Z. Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazard. Mater. 2012, 227–228, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Proctor, J. Effects of aluminum on the growth and mineral composition of Betula pendula Roth. J. Exp. Bot. 2000, 51, 1057–1066. [Google Scholar] [CrossRef]
- Ciju, R.J. Chile Peppers; Amazon: Ridgmont, UK, 2013. [Google Scholar]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Zhu, K.; Prince, R.L. Calcium and bone. Clin. Biochem. 2012, 45, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Musso, C. Magnesium metabolism in health and disease. Int. Urol. Nephrol. 2009, 41, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hakala, M.; Rantamäki, S.; Puputti, E.M.; Tyystjärvi, T.; Tyystjärvi, E. Photoinhibition of manganese enzymes: insights into the mechanism of photosystem II photoinhibition. J. Exp. Bot. 2006, 57, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Setting Maximum Levels for Certain Contaminants in Food Stuffs; Commission Regulation No. 466/2001; European Commission: Brussels, Belgium, 2001; p. L77/1. [Google Scholar]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of zinc plants. In Zinc in Soils and Plants; Springer: New York, NY, USA, 1993; pp. 93–106. [Google Scholar]
- Lăcătuşu, R. Appraising levels of soil contamination and pollution with heavy metals. In Land Information Systems; Developments for Planning the Sustainable Use of Land Resources, EUR 17729; European Soil Bureau: Brussels, Belgium, 1998. [Google Scholar]
- Diana, G. Boron in the soil, from deficit to toxicity. Informatore Agrario 2006, 62, 54–58. [Google Scholar]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef] [PubMed]










| Filter | COD | BOD5 | DO | NH4-N | NO3-N |
| Filter 2 | 45.0 ± 18.14 (19) | 18.9 ± 14.98 (41) | 2.8 ± 1.56 (45) | 4.6 ± 4.57 (23) | 1.6 ± 2.06 (22) |
| Filter 4 | 43.3 ± 13.10 (18) | 15.6 ± 15.10 (42) | 3.1 ± 1.96 (44) | 2.9 ± 2.86 (22) | 0.4 ± 0.48 (21) |
| Filter 6 | 63.9 ± 36.81 (18) | 29.4 ± 26.99 (41) | 2.9 ± 1.53 (45) | 8.5 ± 7.30 (24) | 3.9 ± 3.57 (24) |
| Filter 7 | 42.2 ± 23.82 (16) | 14.2 ± 12.76 (48) | 3.1 ± 2.13 (46) | 3.5 ± 4.23 (17) | 4.0 ± 3.36 (15) |
| Filter 8 | 60.4 ± 34.41 (17) | 17.2 ± 13.46 (53) | 3.1 ± 2.39 (50) | 1.6 ± 2.33 (18) | 3.0 ± 2.29 (16) |
| Standards * | - | - | - | 5.0 | 30.0 |
| Filter | PO4-P | SS | NTU | pH | EC |
| Filter 2 | 4.1 ± 1.47 (24) | 12.1 ± 10.07 (53) | 9.2 ± 8.07 (53) | 6.6 ± 0.26 (52) | 462.6 ± 146.89 (44) |
| Filter 4 | 3.7 ± 1.24 (23) | 4.7 ± 6.25 (52) | 3.5 ± 4.19 (51) | 6.6 ± 0.28 (51) | 483.4 ± 155 (43) |
| Filter 6 | 5.0 ± 2.01 (23) | 11.0 ± 13.00 (53) | 8.2 ± 9.05 (51) | 6.8 ± 0.28 (52) | 832.4 ± 298.17 (44) |
| Filter 7 | 4.3 ± 2.53 (19) | 4.7 ± 8.16 (56) | 3.8 ± 3.71 (55) | 6.7 ± 0.32 (58) | 582.9 ± 442.86 (44) |
| Filter 8 | 4.0 ± 2.09 (20) | 3.1 ± 3.71 (56) | 3.1 ± 2.76 (55) | 6.6 ± 0.38 (61) | 584.1 ± 459.11 (47) |
| Standards * | 2.0 | 6.5-8.4 | 3000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds. Int. J. Environ. Res. Public Health 2018, 15, 1776. https://doi.org/10.3390/ijerph15081776
Almuktar SAAAN, Abed SN, Scholz M. Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds. International Journal of Environmental Research and Public Health. 2018; 15(8):1776. https://doi.org/10.3390/ijerph15081776
Chicago/Turabian StyleAlmuktar, Suhad A. A. A. N., Suhail N. Abed, and Miklas Scholz. 2018. "Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds" International Journal of Environmental Research and Public Health 15, no. 8: 1776. https://doi.org/10.3390/ijerph15081776
APA StyleAlmuktar, S. A. A. A. N., Abed, S. N., & Scholz, M. (2018). Contaminations of Soil and Two Capsicum annuum Generations Irrigated by Reused Urban Wastewater Treated by Different Reed Beds. International Journal of Environmental Research and Public Health, 15(8), 1776. https://doi.org/10.3390/ijerph15081776