Mosquitocidal Chips Containing the Insect Growth Regulator Pyriproxyfen for Control of Aedes aegypti (Diptera: Culicidae)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Water Volume
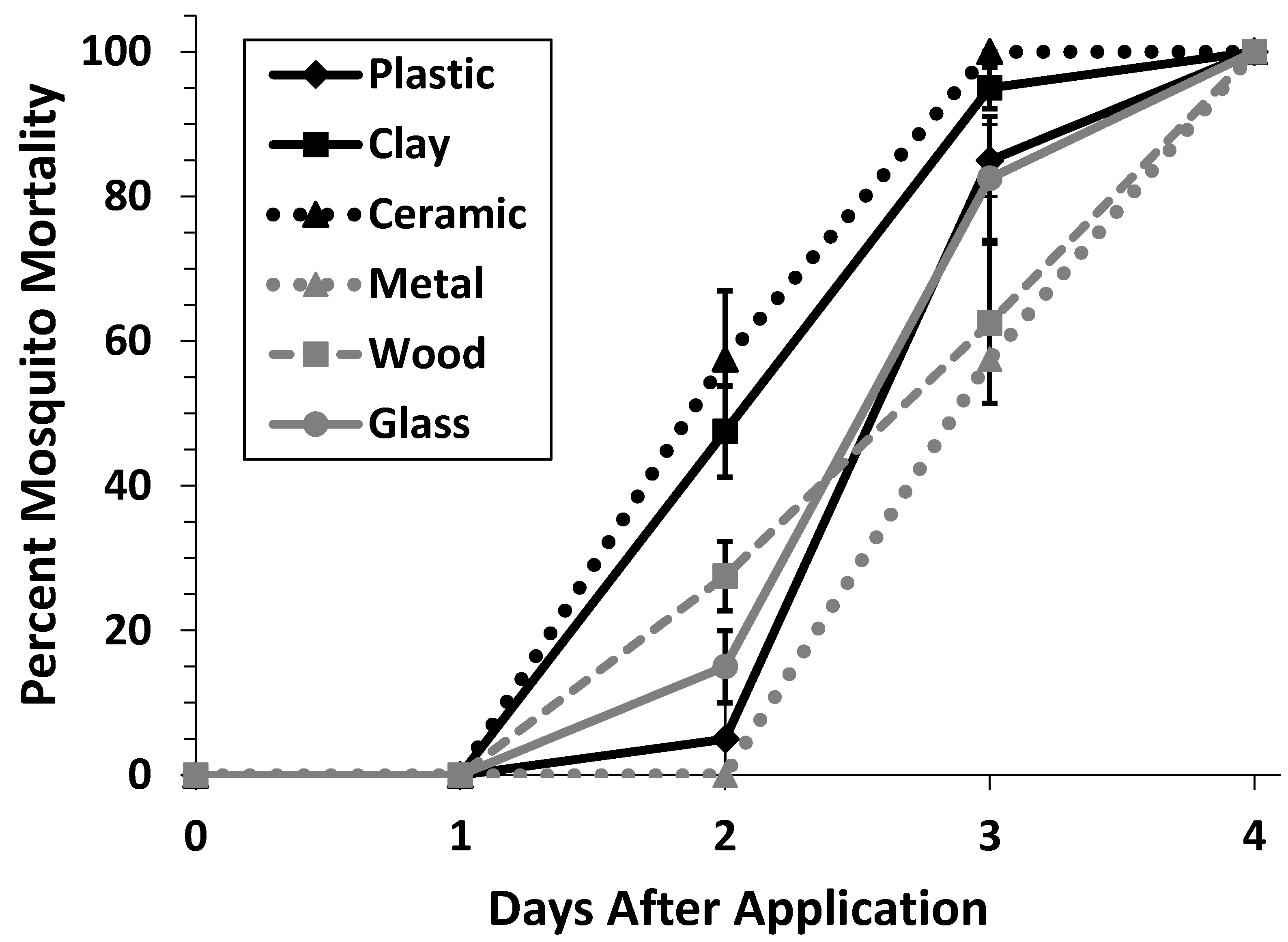
3.2. Container Material
3.3. Effects of Organic Matter
3.4. Population and Oviposition Effects of Chips
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belinato, T.A.; Martins, A.J.; Lima, J.B.P.; de Lima-Camara, T.N.; Peixoto, A.A.; Valle, D. Effect of the chitin synthesis inhibitor triflumuron on the development, viability and reproduction of Aedes aegypti. Mem. Inst. Oswaldo Cruz 2009, 104, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Dhadialla, T.S. Advances in Insect Physiology: Insect Growth Disruptors, Vol. 43; Academic Press: Boston, MA, USA, 2012; p. 249. [Google Scholar]
- Graf, J.-F. The role of insect growth regulators in arthropod control. J. Parasitol. Today 1993, 9, 471–474. [Google Scholar] [CrossRef]
- Sullivan, J. Environmental Fate of Pyriproxyfen. Available online: https://www.cdpr.ca.gov/docs/emon/pubs/fatememo/pyrprxfn.pdf (accessed on 17 May 2019).
- Ware, G.W.; Whitacre, D.M. The Pesticide Book, 6th ed.; MeisterPro Information Resources: Willoughby, OH, USA, 2004; p. 496. [Google Scholar]
- Suman, D.S.; Wang, Y.; Dong, L.; Gaugler, R. Effects of larval habitat substrate on pyriproxyfen efficacy against Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Pyriproxyfen in Drinking-Water: Use for Vector Control in Drinking-Water Sources Sand Containers. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/pyriproxyfenvector.pdf (accessed on 17 May 2019).
- Harrington, L.C.; Edman, J.D. Indirect evidence against delayed “skip-oviposition” behavior by Aedes aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2001, 38, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Hales, S.; van Panhuis, W. A new strategy for dengue control. Lancet 2005, 365, 551–552. [Google Scholar] [CrossRef]
- Sihuincha, M.; Zamora-Perea, E.; Orellana-Rios, W.; Stancil, J.D.; Lopez-Sifuentes, V.; Vidal-Ore, C.; Devine, G.J. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J. Med. Entomol. 2005, 42, 620–630. [Google Scholar] [CrossRef]
- Ohba, S.; Ohashi, K.; Pujiyati, E.; Higa, Y.; Kawada, H.; Mito, N.; Takagi, M. The effect of pyriproxyfen as a “Population Growth Regulator” against Aedes albopictus under semi-field conditions. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Parens, R.; Nijhout, H.F.; Morales, A.; Costa, F.X.; Bar-Yam, Y. A possible link between pyriproxyfen and microcephaly. PLoS Curr. Outbreaks 2017. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P.; Amador, M.A.; Colon, N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J. Am. Mosq. Control Assoc. 1991, 7, 52–55. [Google Scholar] [PubMed]
- Espinoza-Gómez, F.; Hernández-Suárez, C.M.; Coll-Cárdenas, R. Educational campaign versus malathion spraying for the control of Aedes aegypti in Colima, Mexico. J. Epidemio. Commun. Health 2002, 56, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.G.; Mulla, M.S. Evaluation of two new insect growth regulators against mosquitoes in the laboratory. J. Am. Mosq. Control Assoc. 1986, 2, 57–60. [Google Scholar] [PubMed]
- Darriet, F.; Corbel, V. Laboratory evaluation of pyriproxyfen and spinosad, alone in combination, against Aedes aegypti larvae. J. Med. Entomol. 2006, 43, 1190–1194. [Google Scholar] [CrossRef]
- Kamal, H.A.; Khater, E.I.M. The biological effects of the insect growth regulators; pyriproxyfen and diflubenzuron on the mosquito Aedes aegypti. J. Egypt. Soc. Parasitol. 2010, 40, 565–574. [Google Scholar] [PubMed]
- Vythilingam, I.; Luz, B.M.; Hanni, R.; Beng, T.S.; Huat, T.C. Laboratory and field evaluation of the insect growth regulator pyriproxyfen (Sumilarv 0.5g) against dengue vectors. J. Am. Mosq. Control Assoc. 2005, 21, 296–300. [Google Scholar] [CrossRef]
- Schaefer, C.H.; Miura, T.; Dupras, E.F.; Mulligan, F.S., III; Wilder, W.H. Efficacy, nontarget effects, and chemical persistence of S-31183, a promising mosquito (Diptera: Culicidae) control agent. J. Econ. Entomol. 1988, 81, 1648–1655. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Treated Containers | Untreated Containers | ||
|---|---|---|---|---|
| No. of Eggs | % of Eggs | No. of Eggs | % of Eggs | |
| 100% TRT | 516 ± 71.9 | 100% | - | - |
| 75% TRT + 25% UT | 355 ± 75.8 | 74% | 122 ± 62.5 | 26% |
| 50% TRT + 50% UT | 420 ± 75.7 | 78% | 116 ± 49.6 | 22% |
| 25% TRT + 75% UT | 140 ± 40.5 | 24% | 439 ± 83.7 | 76% |
| 100% TRT | - | - | 625 ± 82.9 | 100% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, K.C.; Pereira, R.M.; Koehler, P.G. Mosquitocidal Chips Containing the Insect Growth Regulator Pyriproxyfen for Control of Aedes aegypti (Diptera: Culicidae). Int. J. Environ. Res. Public Health 2019, 16, 2152. https://doi.org/10.3390/ijerph16122152
Stevens KC, Pereira RM, Koehler PG. Mosquitocidal Chips Containing the Insect Growth Regulator Pyriproxyfen for Control of Aedes aegypti (Diptera: Culicidae). International Journal of Environmental Research and Public Health. 2019; 16(12):2152. https://doi.org/10.3390/ijerph16122152
Chicago/Turabian StyleStevens, Kristen C., Roberto M. Pereira, and Philip G. Koehler. 2019. "Mosquitocidal Chips Containing the Insect Growth Regulator Pyriproxyfen for Control of Aedes aegypti (Diptera: Culicidae)" International Journal of Environmental Research and Public Health 16, no. 12: 2152. https://doi.org/10.3390/ijerph16122152
APA StyleStevens, K. C., Pereira, R. M., & Koehler, P. G. (2019). Mosquitocidal Chips Containing the Insect Growth Regulator Pyriproxyfen for Control of Aedes aegypti (Diptera: Culicidae). International Journal of Environmental Research and Public Health, 16(12), 2152. https://doi.org/10.3390/ijerph16122152





