Effects of Epiphytes and Depth on Seagrass Spectral Profiles: Case Study of Gulf St. Vincent, South Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data Collection
2.3. Measuring Spectral Reflectance
2.4. Statistical Analyses
3. Results and Discussion
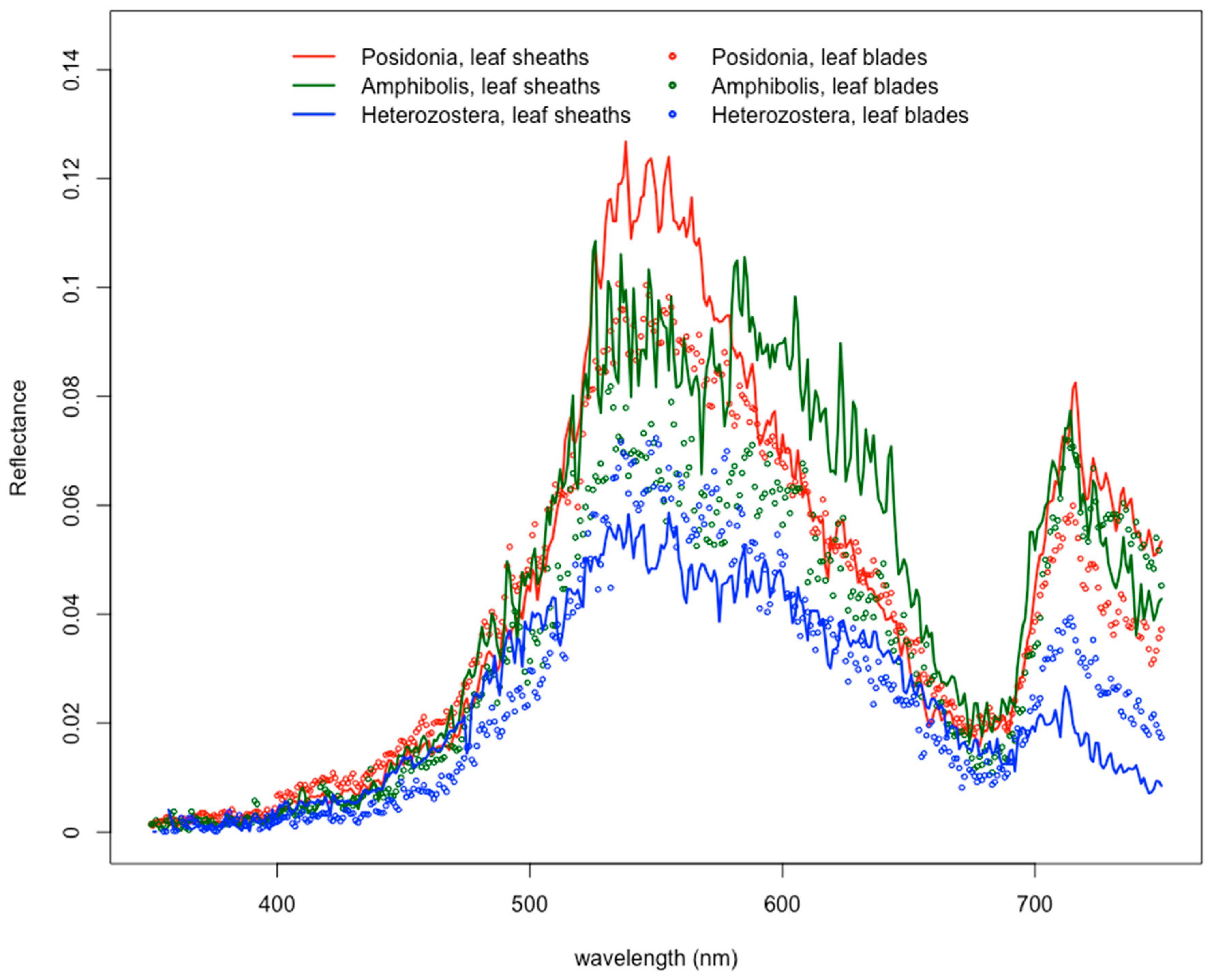
3.1. Epiphytes and the Reflectance Signal
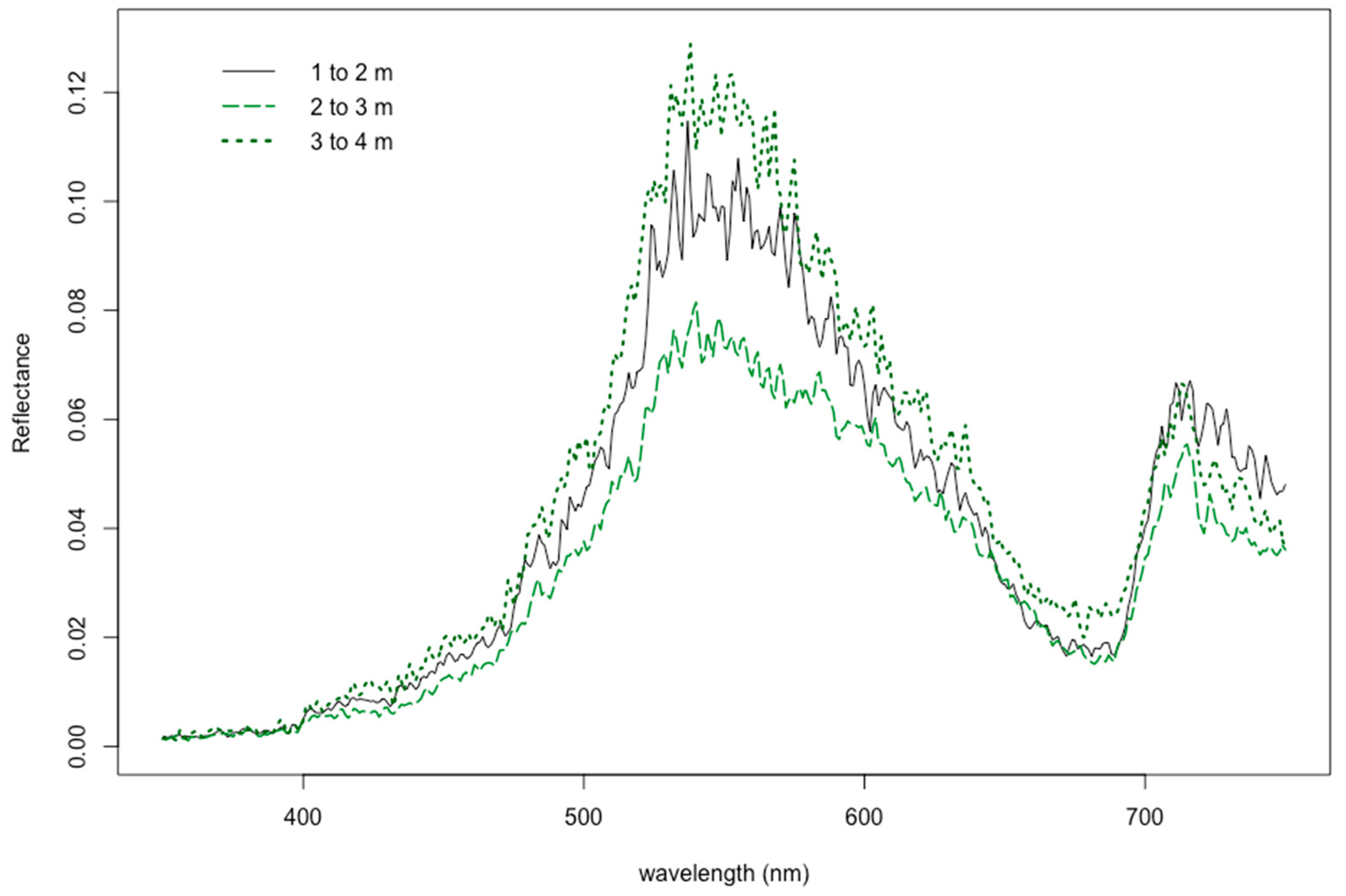
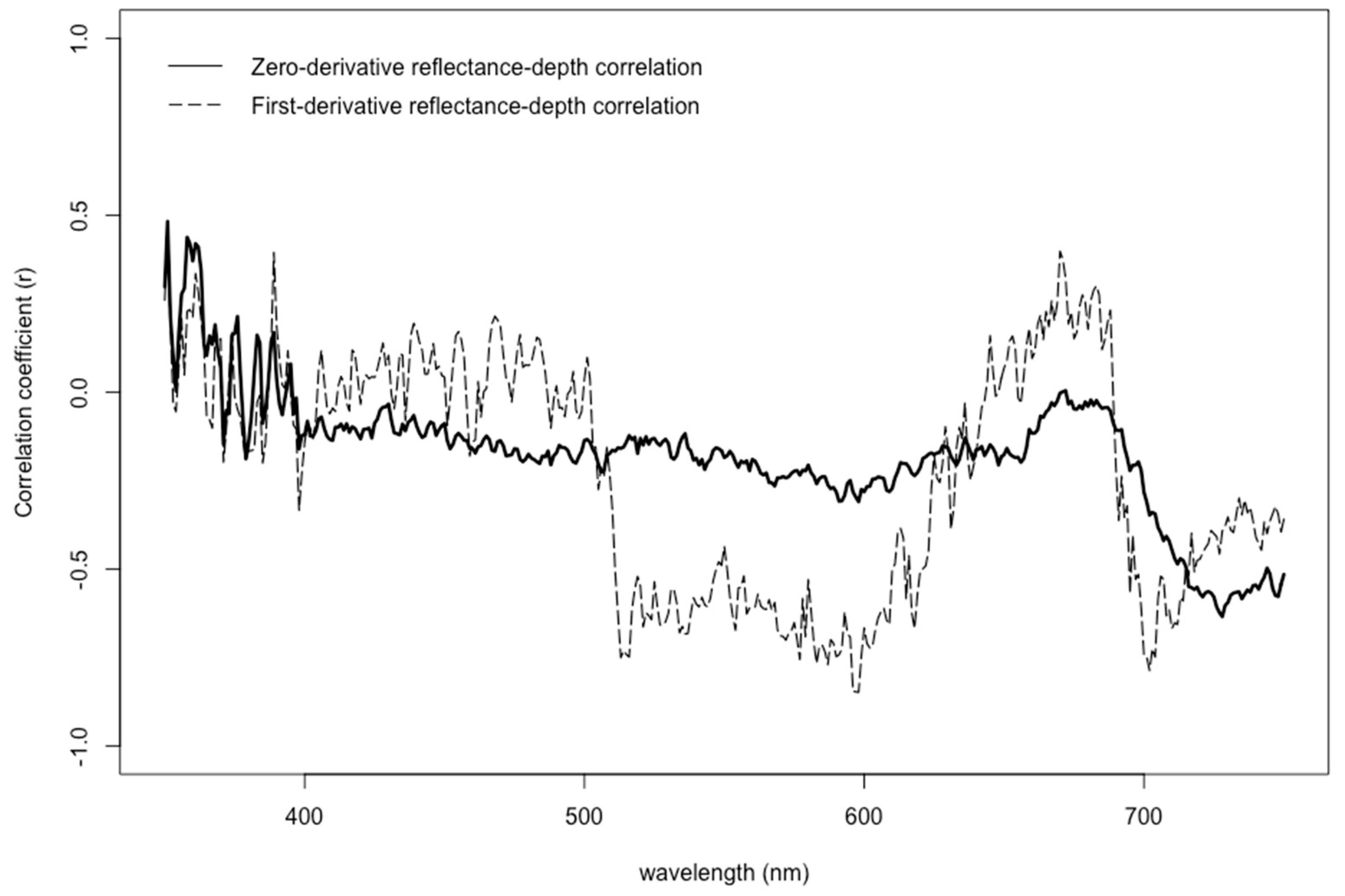
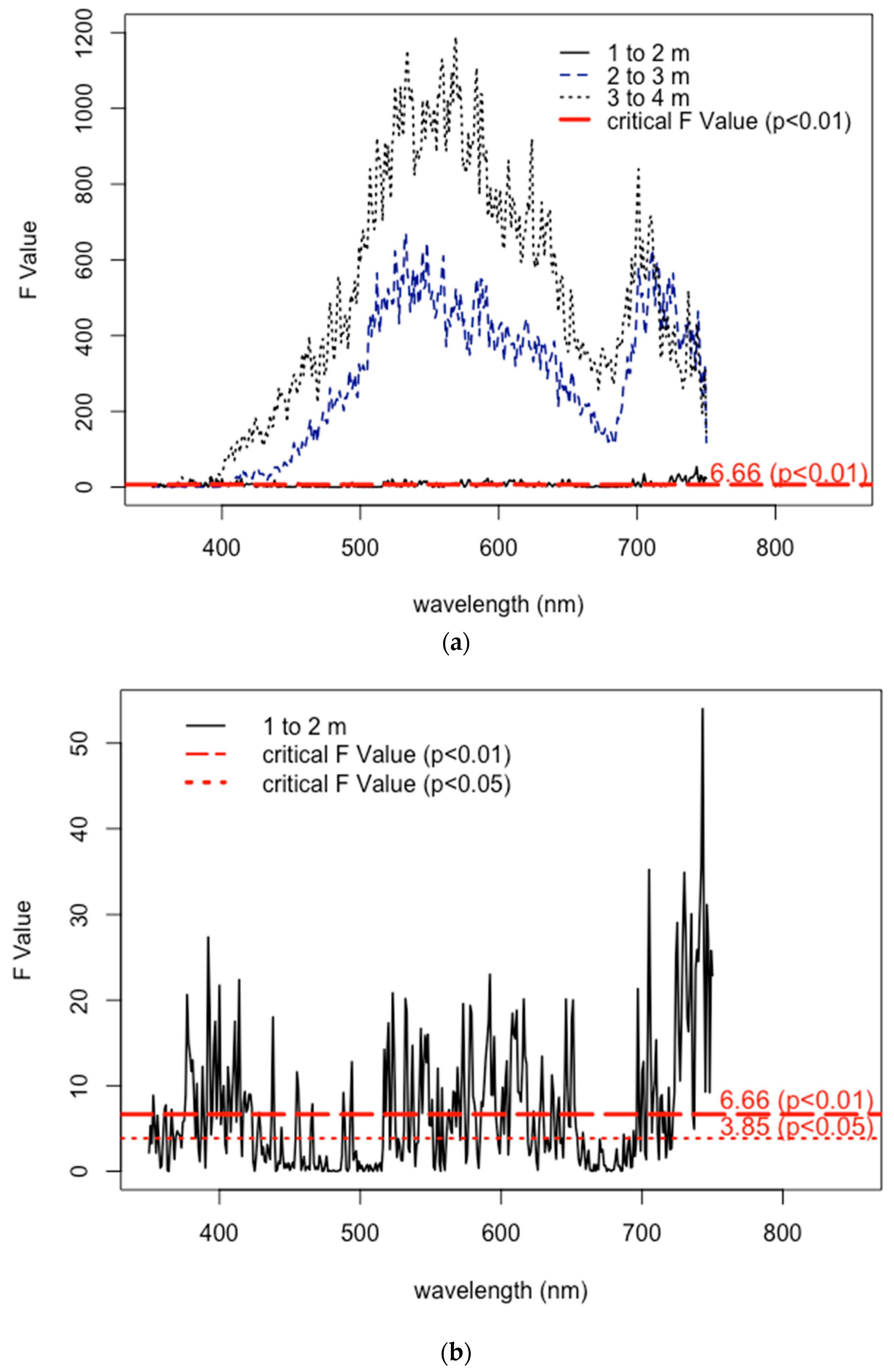
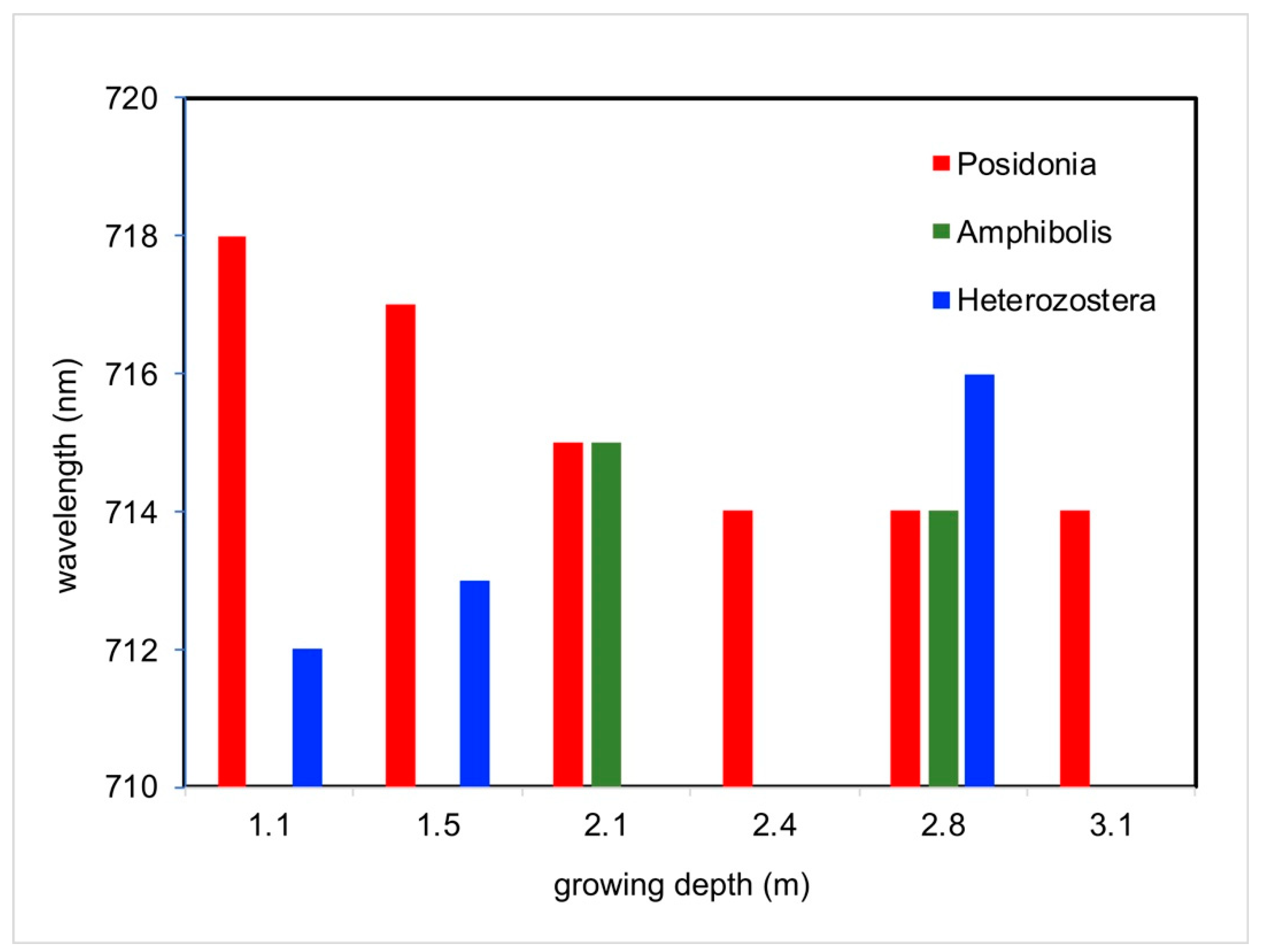
3.2. Reflectance Response due to Natural Growing Depth
3.3. Epiphyte “Preference” by Seagrass Genus
3.4. Influence of Natural Growing Depth on Seagrass Spectral Profiles by Genus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2008; p. 312. [Google Scholar]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J.; Brando, V. Mapping seagrass species, cover and biomass in shallow waters: An assessment of satellite multi-spectral and airborne hyper-spectral imaging systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Williams, S.L.; Heck, K.L., Jr. Seagrass community ecology. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2011; pp. 317–337. [Google Scholar]
- Fourqurean, J.; Manuel, S.; Coates, K.; Kenworthy, W.; Smith, S. Effects of excluding sea turtle herbivores from a seagrass bed: Overgrazing may have led to loss of seagrass meadows in Bermuda. Mar. Ecol. Prog. Ser. 2010, 419, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Cappello, T.; Giannetto, A.; Parrino, V.; Maisano, M.; Oliva, S.; De Marco, G.; Guerriero, G.; Mauceri, A.; Fasulo, S. Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 26, 32–39. [Google Scholar] [CrossRef]
- Esteban, N.; Unsworth, R.; Gourlay, J.; Hays, G. The discovery of deep-water seagrass meadows in a pristine Indian Ocean wilderness revealed by tracking green turtles. Mar. Pollut. Bull. 2018, 134, 99–105. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Manuel, S.A.; Coates, K.A.; Massey, S.C.; Kenworthy, W.J. Decadal Monitoring in Bermuda Shows a Widespread Loss of Seagrasses Attributable to Overgrazing by the Green Sea Turtle Chelonia mydas. Estuaries Coasts 2019, 1–17. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef]
- Han, L. Spectral reflectance of Thalassia testudinum with varying depths. In Proceedings of the International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; pp. 2123–2125. [Google Scholar]
- Dierssen, H.M.; Zimmerman, R.C.; Leathers, R.A.; Downes, T.V.; Davis, C.O. Ocean color remote sensing of seagrass and bathymetry in the Bahamas Banks by high-resolution airborne imagery. Limnol. Oceanogr. 2003, 48, 444–455. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Fyfe, S.K. Spatial and temporal variation in spectral reflectance: Are seagrass species spectrally distinct? Limnol. Oceanogr. 2003, 48, 464–479. [Google Scholar] [CrossRef]
- Wabnitz, C.C.; Andréfouët, S.; Torres-Pulliza, D.; Müller-Karger, F.E.; Kramer, P.A. Regional-scale seagrass habitat mapping in the Wider Caribbean region using Landsat sensors: Applications to conservation and ecology. Remote Sens. Environ. 2008, 112, 3455–3467. [Google Scholar] [CrossRef]
- Dolch, T.; Reise, K. Long-term displacement of intertidal seagrass and mussel beds by expanding large sandy bedforms in the northern Wadden Sea. J. Sea Res. 2010, 63, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Borfecchia, F.; De Cecco, L.; Martini, S.; Ceriola, G.; Bollanos, S.; Vlachopoulos, G.; Valiante, L.M.; Belmonte, A.; Micheli, C. Posidonia oceanica genetic and biometry mapping through high-resolution satellite spectral vegetation indices and sea-truth calibration. Int. J. Remote Sens. 2013, 34, 4680–4701. [Google Scholar] [CrossRef]
- Cunha, A.H.; Assis, J.F.; Serrão, E.A. Reprint of “Seagrasses in Portugal: A most endangered marine habitat. Aquat. Bot. 2014, 115, 3–13. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, D.T.; Dekker, A.G. Remote Sensing Study of Marine and Coastal Features and Interpretation of Changes in Relation to Natural and Anthropogenic Processes: Final Technical Report; ACWS Technical Report No. 6. Prepared for the Adelaide Coastal Waters Study Steering Committee; South Australian Environment Protection Authority (SA EPA): Adelaide, Australia, 2006.
- Nayar, S.; Collings, G.; Pfennig, P.; Royal, M. Managing nitrogen inputs into seagrass meadows near a coastal city: Flow-on from research to environmental improvement plans. Mar. Pollut. Bull. 2012, 64, 932–940. [Google Scholar] [CrossRef]
- Tanner, J.E.; Irving, A.D.; Fernandes, M.; Fotheringham, D.; McArdle, A.; Murray-Jones, S.; Murray-Jones, S. Seagrass rehabilitation off metropolitan Adelaide: A case study of loss, action, failure and success. Ecol. Manag. Restor. 2014, 15, 168–179. [Google Scholar] [CrossRef]
- Hart, D. Seagrass Extent Change 2007-13-Adelaide Coastal Waters; DEWNR Technical Note 2013/07; Government of South Australia, Department of Environment Water and Natural Resources: Adelaide, Australia, 2013; p. 19.
- Tanner, J.E.; Theil, M.; Fotheringham, D.G. Seagrass Condition Monitoring: Encounter Bay and Port Adelaide; Final Report Prepared for the Adelaide and Mount Lofty Ranges Natural Resources Management Board. SARDI Publication No. F2012/000139-2. SARDI Research Report Series No. 799; Theil, M., Ed.; South Australian Research and Development Institution: West Beach, Australia, 2014.
- Clarke, K.; Hennessy, A.; Lewis, M. Adelaide Metropolitan Coastline Benthic Habitat Mapping from Hyperspectral Imagery; Product A: Bare-Substrate vs Non-Bare Substrate. Prepared for SA Water, SA Department for Environment and Water, and SA Environmental Protection Authority, School of Biological Sciences, The University of Adelaide, Adelaide, South Australia; The University of Adelaide: Adelaide, Australia, 2018. [Google Scholar]
- Dunk, I.; Lewis, M. Seagrass and shallow water feature discrimination using HyMap imagery. In Proceedings of the 10th Australasian Remote Sensing Photogrammetry Conference, Adelaide, Australia, 21–25 August 2000. [Google Scholar]
- Brando, V.; Dekker, A. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Costa, M. Mapping eelgrass (Zostera marina) in the Gulf Islands National Park Reserve of Canada using high spatial resolution satellite and airborne imagery. Remote Sens. Environ. 2013, 133, 152–167. [Google Scholar] [CrossRef]
- Kakuta, S.; Takeuchi, W.; Prathep, A. Seaweed and seagrass mapping in Thailand measured by using Landsat 8 optical and texture properties. In Proceedings of the International Symposium on Remote Sensing (ISRS), Tainan, Taiwan, 22–24 April 2015. [Google Scholar]
- Medjahed, S.A.; Ouali, M. Band selection based on optimization approach for hyperspectral image classification. Egypt. J. Remote Sens. Space Sci. 2018, 21, 413–418. [Google Scholar] [CrossRef]
- Vahtmäe, E.; Kutser, T.; Martin, G.; Kotta, J. Feasibility of hyperspectral remote sensing for mapping benthic macroalgal cover in turbid coastal waters—A Baltic Sea case study. Remote Sens. Environ. 2006, 101, 342–351. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, C.-C.; Chung, H.-W.; Lee, L.-J.; Yang, W.-C. Development and evaluation of a genetic algorithm-based ocean color inversion model for simultaneously retrieving optical properties and bottom types in coral reef regions. Appl. Opt. 2014, 53, 605–617. [Google Scholar] [CrossRef]
- Veys, C.; Hibbert, J.; Davis, P.; Grieve, B. An ultra-low-cost active multispectral crop diagnostics device. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar]
- Dekker, A.G.; Brando, V.E.; Anstee, J.M. Retrospective seagrass change detection in a shallow coastal tidal Australian lake. Remote Sens. Environ. 2005, 97, 415–433. [Google Scholar] [CrossRef]
- Dekker, A.; Brando, V.; Anstee, J.; Fyfe, S.; Malthus, T.; Karpouzli, E. Remote Sensing of Seagrass Ecosystems: Use of Spaceborne and Airborne Sensors. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2006; pp. 347–359. [Google Scholar]
- Mumby, P.J.; Skirving, W.; Strong, A.E.; Hardy, J.T.; LeDrew, E.F.; Hochberg, E.J.; Stumpf, R.P.; David, L.T. Remote sensing of coral reefs and their physical environment. Mar. Pollut. Bull. 2004, 48, 219–228. [Google Scholar] [CrossRef]
- Karpouzli, E.; Malthus, T.J.; Place, C.J. Hyperspectral discrimination of coral reef benthic communities in the western Caribbean. Coral Reefs 2004, 23, 141–151. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Costa, M.; Sharma, T. Remote Sensing of Shallow Coastal Benthic Substrates: In situ Spectra and Mapping of Eelgrass (Zostera marina) in the Gulf Islands National Park Reserve of Canada. Remote Sens. 2011, 3, 975. [Google Scholar] [CrossRef]
- Fulton, S. An analysis of the spectral reflectance separability of Amphibolis griffithii and Posidonia sinuosa at different water depths. In School of the Environment; The Flinders University of South Australia: Adelaide, Australia, 2014; p. 97. [Google Scholar]
- Tsai, F.; Philpot, W. Derivative Analysis of Hyperspectral Data. Remote Sens. Environ. 1998, 66, 41–51. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Spectral discrimination of coral reef benthic communities. Coral Reefs 2000, 19, 164–171. [Google Scholar] [CrossRef]
- Bargain, A.; Robin, M.; Le Men, E.; Huete, A.; Barillé, L. Spectral response of the seagrass Zostera noltii with different sediment backgrounds. Aquat. Bot. 2012, 98, 45–56. [Google Scholar] [CrossRef]
- Bargain, A.; Robin, M.; Méléder, V.; Rosa, P.; le Menn, E.; Harin, N.; Barillé, L. Seasonal spectral variation of Zostera noltii and its influence on pigment-based Vegetation Indices. J. Exp. Mar. Biol. Ecol. 2013, 446, 86–94. [Google Scholar] [CrossRef]
- Taylor, P.; Wilson, M. Palaeoecology and evolution of marine hard substrate communities. Earth Sci. Rev. 2003, 62, 1–103. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Lavery, P.S.; van Keulen, M. Epiphytes of Seagrasses. In Seagrasses: Biology, Ecology and Conservation; Larkum, A.W.D., Orth, R.J., Duarte, C.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 441–461. [Google Scholar]
- Larkum, A.W.; Orth, R.J.; Duarte, C.M. Seagrasses; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Cornell Cooperative Extension Marine Program. Fauna and Flora: Epiphytes. 2017. Available online: http://www.seagrassli.org/ecology/fauna_flora/epiphytes.html (accessed on 26 May 2019).
- Trautman, D.; Borowitzka, M. Distribution of the epiphytic organisms on Posidonia australis and P. sinuosa, two seagrasses with differing leaf morphology. Mar. Ecol. Prog. Ser. 1999, 179, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Drake, L.A.; Dobbs, F.C.; Zimmerman, R.C. Effects of epiphyte load on optical properties and photosynthetic potential of the seagrasses Thalassia testudinum Banks ex König and Zostera marina L. Limnol. Oceanogr. 2003, 48, 456–463. [Google Scholar] [CrossRef]
- Westphalen, G.; Collings, G.; Wear, R.; Fernandes, M.; Bryars, S.; Cheshire, A. A Review of Seagrass Loss on the Adelaide Metropolitan Coastline; ACWS Technical Report No. 2. Prepared for the Adelaide Coastal Waters Study Steering Committee (Publication No. RD04/0073); South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2005.
- Bryars, S.; Collings, G.; Nayar, S.; Westphalen, G.; Miller, D.; O’Loughlin, E.; Fernandes, M.; Mount, G.; Tanner, J.; Wear, R.; et al. Assessment of the Effects of Inputs to the Adelaide Coastal Waters on the Meadow Forming Seagrasses, Amphibolis and Posidonia; Task EP 1 Final Technical Report. ACWS Technical Report No. 15. Prepared for the Adelaide Coastal Waters Study Steering Committee (Publication No. RD01/0208-19); South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2006.
- Ralph, P.; Durako, M.; Enríquez, S.; Collier, C.; Doblin, M.; Ralph, P.; Doblin, M. Impact of light limitation on seagrasses. J. Exp. Mar. Boil. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Jernakoff, P.; Nielsen, J. Plant–animal associations in two species of seagrasses in Western Australia. Aquat. Bot. 1998, 60, 359–376. [Google Scholar] [CrossRef]
- Ierodiaconou, D.A.; Laurenson, L.J.B. Estimates of Heterozostera tasmanica, Zostera muelleri and Ruppia megacarpa distribution and biomass in the Hopkins Estuary, western Victoria, by GIS. Aust. J. Bot. 2002, 50, 215–228. [Google Scholar] [CrossRef]
- Greve, T.M.; Binzer, T. Which factors regulate seagrass growth and distribution? In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; EU Monitoring and Managing of European Seagrasses (M&MS) EVK3-CT-2000-00044: Brussels, Belgium, 2004; pp. 19–23. Available online: http://www.seagrasses.org (accessed on 26 July 2019).
- Lavery, P.; McMahon, K.; Mulligan, M.; Tennyson, A. Interactive effects of timing, intensity and duration of experimental shading on Amphibolis griffithii. Mar. Ecol. Prog. Ser. 2009, 394, 21–33. [Google Scholar] [CrossRef] [Green Version]
- McMahon, K.; Lavery, P.S. Canopy-scale modifications of the seagrass Amphibolis griffithii in response to and recovery from light reduction. J. Exp. Mar. Boil. Ecol. 2014, 455, 38–44. [Google Scholar] [CrossRef]
- Han, L.; Rundquist, D.C. The spectral responses of Ceratophyllum demersum at varying depths in an experimental tank. Int. J. Remote Sens. 2003, 24, 859–864. [Google Scholar] [CrossRef]
- West, R. Depth-related structural and morphological variations in an Australian Posidonia seagrass bed. Aquat. Bot. 1990, 36, 153–166. [Google Scholar] [CrossRef]
- Bryars, S.; Rowling, K. Benthic habitats of eastern Gulf St Vincent: Major changes in benthic cover and composition following European settlement of Adelaide. Trans. R. Soc. S. Aust. 2009, 133, 318–338. [Google Scholar]
- Bryars, S.; Miller, D.; Collings, G.; Fernandes, M.; Mount, G.; Wear, R. Field Surveys 2003–2005: Assessment of the Quality of Adelaide’s Coastal Waters, Sediments and Seagrasses; ACWS Technical Report No. 14. Prepared for the Adelaide Coastal Waters Study Steering Committee. Publication No. RD01/0208-15; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2006.
- Bryars, S.; Rowling, K. Chapter 1. Benthic habitats of eastern Gulf St Vincent: Major changes in seagrass distribution and composition since European settlement of Adelaide. In Restoration of Coastal Seagrass Ecosystems: Amphibolis antarctica in Gulf St Vincent, South Australia; Bryars, S., Ed.; SARDI Aquatic Sciences Publication No. F2008/000078, SARDI Research Report Series No. 277; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2008; pp. 5–27. [Google Scholar]
- Bone, Y.; Deer, L.; Edwards, S.; Campbell, E. Adelaide Coastal Waters Study Coastal Sediment Budget; ACWS Technical Report No. 16 Prepared for the Adelaide Coastal Waters Study Steering Committee; Adelaide University, Department of Geology: Adelaide, Australia, 2006. [Google Scholar]
- Mueller, J.L.; Davis, C.; Arnone, R.; Frouin, R.; Carder, K.; Lee, Z.; Steward, R.; Hooker, S.; Mobley, C.D.; McLean, S. Above-water radiance and remote sensing reflectance measurements and analysis protocols. In Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 2; Fargion, G.S., Mueller, J.L., Eds.; Goddard Space Flight Space Center: Greenbelt, MD, USA, 2000; Volume 2, pp. 98–107. [Google Scholar]
- Mueller, J.L.; Morel, A.; Frouin, R.; Davis, C.; Arnone, R.; Carder, K.; Lee, Z.; Steward, R.; Hooker, S.; Mobley, C. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4, Volume III: Radiometric Measurements and Data Analysis Protocols; Goddard Space Flight Space Center: Greenbelt, MD, USA, 2003; pp. 1–84. [Google Scholar]
- Joyce, K.E.; Phinn, S.R. Bi-directional reflectance of corals. Int. J. Remote Sens. 2002, 23, 389–394. [Google Scholar] [CrossRef]
- Ocean Optics, Ocean View Installation and Operation Manual; Document Number 000-20000-310-02-201503; Ocean Optics: Winter Park, FL, USA, 2013.
- Ocean Optics. Cosine Correctors. 2014. Available online: https://oceanoptics.com/product-category/cosine-correctors (accessed on 19 July 2019).
- Ocean Optics, JAZ Installation and Operation Manual; Document Number 013-RD000-000-02-201502; Ocean Optics: Winter Park, FL, USA, 2010.
- Hwang, C.; Chang, C.-H. Dimension Reduction and Spectral Deconvolution Analyses for Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia. In Proceedings of the 2019 IEEE Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability (2019 IEEE ECBIOS), Okinawa, Japan, 31 May–3 June 2019. [Google Scholar]
- Hwang, C.; Chang, C.-H.; Burch, M.; Fernandes, M.; Kildea, T. Spectral Deconvolution for Dimension Reduction and Differentiation of Seagrasses: Case Study of Gulf St. Vincent, South Australia. Sustainability 2019, 11, 3695. [Google Scholar] [CrossRef]
- Twardowski, M.S.; Lewis, M.R.; Barnard, A.H.; Ronald, J.; Zaneveld, V. In-Water Instrumentation and Platforms for Ocean Color Remote Sensing Applications. In Remote Sensing of Coastal Aquatic Environments; Miller, R.L., del Castillo, C.E., Mckee, B.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; p. 345. [Google Scholar]
- Dierssen, H.M.; Kudela, R.M.; Ryan, J.P.; Zimmerman, R.C. Red and black tides: Quantitative analysis of water-leaving radiance and perceived color for phytoplankton, colored dissolved organic matter, and suspended sediments. Limnol. Oceanogr. 2006, 51, 2646–2659. [Google Scholar] [CrossRef] [Green Version]
- Bryars, S. Chapter 2. Temporal and spatial variability in plant biomass, canopy structure, and epiphytes of Amphibolis Antarctica. In Restoration of Coastal Seagrass Ecosystems: Amphibolis Antarctica in Gulf St Vincent, South Australia; Bryars, S., Ed.; SARDI Aquatic Sciences Publication No. F2008/000078, SARDI Research Report Series No. 277; South Australian Research and Development Institute (Aquatic Sciences): Adelaide, Australia, 2008; pp. 28–53. [Google Scholar]
- Transon, J.; D’Andrimont, R.; Maugnard, A.; Defourny, P. Survey of Hyperspectral Earth Observation Applications from Space in the Sentinel-2 Context. Remote Sens. 2018, 10, 157. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Altman, N.; Krzywinski, M. Association, correlation and causation. Nat. Methods 2015, 12, 899. [Google Scholar] [CrossRef]
- Saakov, V.; Drapkin, V.Z.; Krivchenko, A.I.; Rozengart, E.V.; Bogachev, Y.; Knyazev, M.N. Derivative Spectrophotometry and Electron Spin Resonance (ESR) Spectroscopy for Ecological and Biological Questions; Springer: Wien, Austria, 2013; pp. 5–70. [Google Scholar]
- Dekker, A.G.; Phinn, S.R.; Anstee, J.; Moline, M.A.; Brando, V.E.; Casey, B.A.; Fearns, P.; Hedley, J.D.; Klonowski, W.M.; Lee, Z.P.; et al. Intercomparison of shallow water bathymetry, hydro-optics, and benthos mapping techniques in Australian and Caribbean coastal environments. Limnol. Oceanogr. Meth. 2011, 9, 30. [Google Scholar] [CrossRef]
- Roelfsema, C.M.; Lyons, M.; Kovacs, E.M.; Maxwell, P.; Saunders, M.I.; Samper-Villarreal, J.; Phinn, S.R. Multi-temporal mapping of seagrass cover, species and biomass: A semi-automated object based image analysis approach. Remote Sens. Environ. 2014, 150, 172–187. [Google Scholar] [CrossRef]
- Roelfsema, C.M.; Kovacs, E.M.; Saunders, M.I.; Phinn, S.R.; Lyons, M.; Maxwell, P. Challenges of remote sensing for quantifying changes in large complex seagrass environments. Estuar. Coast. Shelf Sci. 2013, 133, 161–171. [Google Scholar] [CrossRef]
- Kilminster, K.; McMahon, K.; Waycott, M.; Kendrick, G.A.; Scanes, P.; McKenzie, L.; O’Brien, K.R.; Lyons, M.; Ferguson, A.; Maxwell, P. Unravelling complexity in seagrass systems for management: Australia as a microcosm. Sci. Total Environ. 2015, 534, 97–109. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, C.; Chang, C.-H.; Burch, M.; Fernandes, M.; Kildea, T. Effects of Epiphytes and Depth on Seagrass Spectral Profiles: Case Study of Gulf St. Vincent, South Australia. Int. J. Environ. Res. Public Health 2019, 16, 2701. https://doi.org/10.3390/ijerph16152701
Hwang C, Chang C-H, Burch M, Fernandes M, Kildea T. Effects of Epiphytes and Depth on Seagrass Spectral Profiles: Case Study of Gulf St. Vincent, South Australia. International Journal of Environmental Research and Public Health. 2019; 16(15):2701. https://doi.org/10.3390/ijerph16152701
Chicago/Turabian StyleHwang, Charnsmorn, Chih-Hua Chang, Michael Burch, Milena Fernandes, and Tim Kildea. 2019. "Effects of Epiphytes and Depth on Seagrass Spectral Profiles: Case Study of Gulf St. Vincent, South Australia" International Journal of Environmental Research and Public Health 16, no. 15: 2701. https://doi.org/10.3390/ijerph16152701




