Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
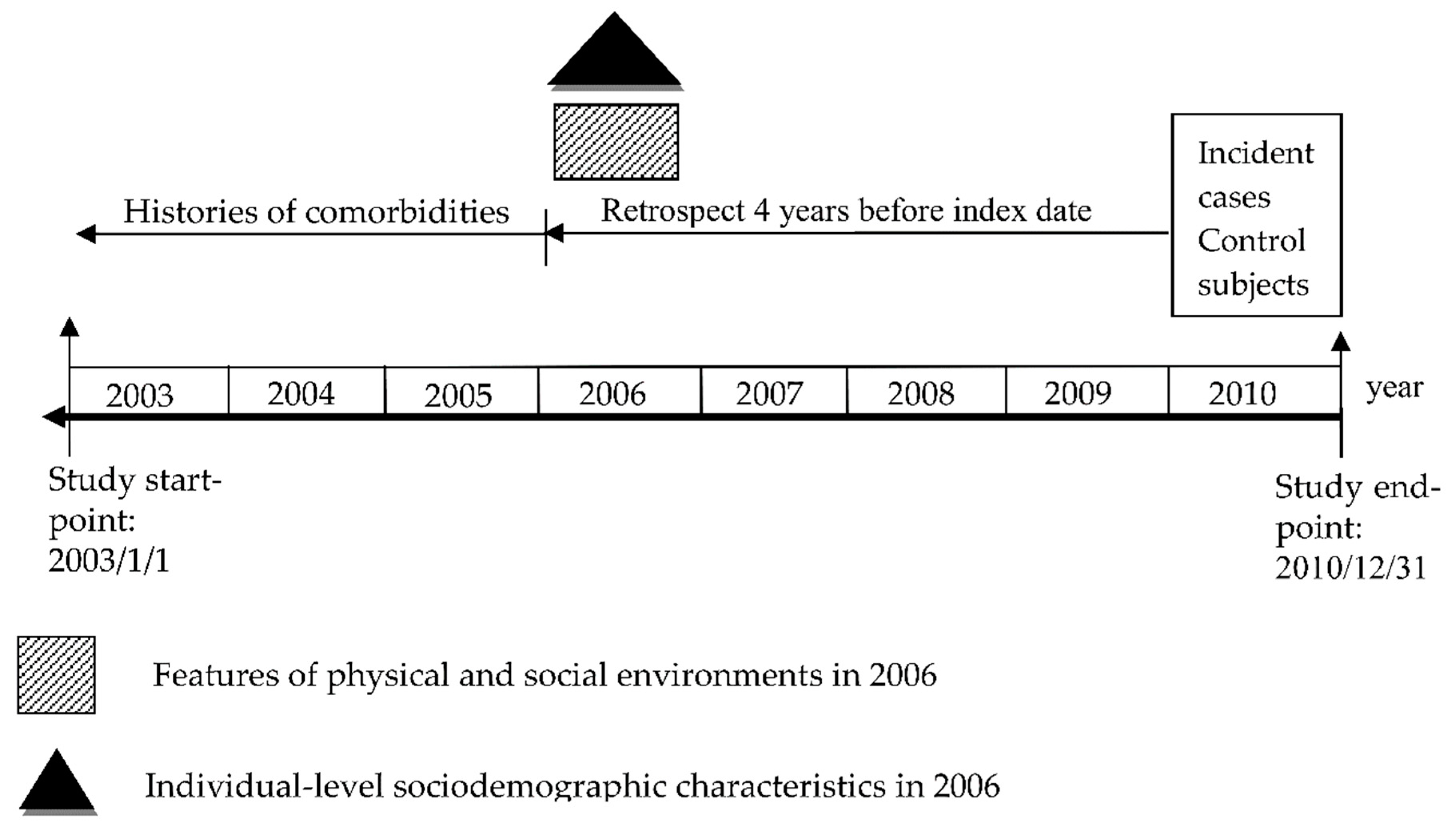
2.1. Study Design and Data Sources
2.2. Identification for Cases and Controls
2.3. Measurement for Physical and Social Environments
2.4. Levels of Urbanization
2.5. Potential Confounders
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings
4.2. Physical and Social Environments and AD in Later Life
4.3. Effects of the Urbanization Levels
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- Solomon, A.; Mangialasche, F.; Richard, E.; Andrieu, S.; Bennett, D.A.; Breteler, M.; Fratiglioni, L.; Hooshmand, B.; Khachaturian, A.S.; Schneider, L.S.; et al. Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 2014, 275, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, M.; Setti, A. Environment as ‘brain training’: A review of geographical and physical environmental influences on cognitive ageing. Ageing Res. Rev. 2015, 23, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Prina, A.M.; Brayne, C. The association between community environment and cognitive function: a systematic review. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Letellier, N.; Gutierrez, L.A.; Carriere, I.; Gabelle, A.; Dartigues, J.F.; Dufouil, C.; Helmer, C.; Cadot, E.; Berr, C. Sex-specific association between neighborhood characteristics and dementia: The Three-City cohort. Alzheimers Dement 2018, 14, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V.; Mujahid, M.S.; Hirsch, J.A.; Moore, K.; Moore, L.V. The Impact of Neighborhoods on CV Risk. Glob. Heart 2016, 11, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Braver, N.R.; Lakerveld, J.; Rutters, F.; Schoonmade, L.J.; Brug, J.; Beulens, J.W.J. Built environmental characteristics and diabetes: a systematic review and meta-analysis. BMC Med. 2018, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Julien, D.; Richard, L.; Gauvin, L.; Kestens, Y. Neighborhood characteristics and depressive mood among older adults: An integrative review. Int. Psychogeriatr. 2012, 24, 1207–1225. [Google Scholar] [CrossRef]
- Clarke, P.J.; Weuve, J.; Barnes, L.; Evans, D.A.; de Leon, C.F.M. Cognitive decline and the neighborhood environment. Ann. Epidemiol. 2015, 25, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.T.; Prina, A.M.; Jones, A.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study Collaboration. The Built Environment and Cognitive Disorders: Results From the Cognitive Function and Ageing Study II. Am. J. Prev. Med. 2017, 53, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.T.; Prina, A.M.; Jones, A.P.; Barnes, L.E.; Matthews, F.E.; Brayne, C.; Medical Research Council Cognitive Function and Ageing Study. Community environment, cognitive impairment and dementia in later life: results from the Cognitive Function and Ageing Study. Age Ageing 2015, 44, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Keijzer, C.; Tonne, C.; Basagana, X.; Valentin, A.; Singh-Manoux, A.; Alonso, J.; Anto, J.M.; Nieuwenhuijsen, M.J.; Sunyer, J.; Dadvand, P. Residential Surrounding Greenness and Cognitive Decline: A 10-Year Follow-up of the Whitehall II Cohort. Environ. Health Perspect. 2018, 126, 077003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liu, S.; Sun, F.; Dong, X. Neighborhood social cohesion and cognitive function in U.S. Chinese older adults-findings from the PINE study. Aging Ment. Health 2019, 23, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Yeo, W.X.; Yang, G.R.; Hannan, N.; Lim, K.; Chua, C.; Tan, M.Y.; Fong, N.; Yeap, A.; Chen, L.; et al. Individual and Area Level Socioeconomic Status and Its Association with Cognitive Function and Cognitive Impairment (Low MMSE) among Community-Dwelling Elderly in Singapore. Dement. Geriatr. Cogn. Dis. Extra 2012, 2, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Llewellyn, D.J.; Langa, K.M.; Wallace, R.B.; Huppert, F.A.; Melzer, D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J. Am. Geriatr. Soc. 2008, 56, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.A.; Ghosh-Dastidar, B.; Margolis, K.L.; Slaughter, M.E.; Jewell, A.; Bird, C.E.; Eibner, C.; Denburg, N.L.; Ockene, J.; Messina, C.R.; et al. Neighborhood socioeconomic status and cognitive function in women. Am. J. Public Health 2011, 101, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, J.T.; Tan, M.S.; Tan, L. Cognitive reserve and Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Sun, Y.; Lee, P.C.; Li, C.Y.; Hu, S.C. Risk of dementia after Parkinson’s disease in Taiwan: a population-based retrospective cohort study using National Health Insurance claims. BMJ Open 2019, 9, e025274. [Google Scholar] [CrossRef] [PubMed]
- National Health Insurance Administration. Universal Health Coverage in Taiwan. 2017. Available online: https://www.nhi.gov.tw/English/Content_List.aspx?n=8FC0974BBFEFA56D&topn=ED4A30E51A609E49 (accessed on 8 May 2018).
- Hu, S.C. Construction of International Leading Monitoring and Decision-Making System for Active Ageing—Project of Active Ageing Database Integration, Value-Added Analysis and Project Management (2015–2018)-2017 Subsequent expansion project. 2017. Available online: https://www.grb.gov.tw/search/planDetail?id=12078480&docId=502322 (accessed on 1 May 2018).
- Cheng, C.; Zandi, P.; Stuart, E.; Lin, C.H.; Su, P.Y.; Alexander, G.C.; Lan, T.H. Association Between Lithium Use and Risk of Alzheimer’s Disease. J. Clin. Psychiatry 2017, 78, e139–e145. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.W.; Chien, W.C.; Chung, C.H.; Chao, P.C.; Chang, H.A.; Kao, Y.C.; Chou, Y.C.; Tzeng, N.S. Electroconvulsive Therapy and Risk of Dementia-A Nationwide Cohort Study in Taiwan. Front Psychiatry 2018, 9, 397. [Google Scholar] [CrossRef]
- Hung, Y.N.; Kadziola, Z.; Brnabic, A.J.; Yeh, J.F.; Fuh, J.L.; Hwang, J.P.; Montgomery, W. The epidemiology and burden of Alzheimer’s disease in Taiwan utilizing data from the National Health Insurance Research Database. Clinicoecon. Outcomes Res. 2016, 8, 387–395. [Google Scholar] [PubMed]
- Ministry of the Interior. Monthly Bulletin of Interior Statistics. 2006. Available online: https://www.moi.gov.tw/files/site_stuff/321/1/month/month.html (accessed on 29 July 2019).
- Shimada, K.; Yamazaki, S.; Nakano, K.; Ngoma, A.M.; Takahashi, R.; Yasumura, S. Prevalence of Social Isolation in Community-Dwelling Elderly by Differences in Household Composition and Related Factors: From a Social Network Perspective in Urban Japan. J. Aging Health 2014, 26, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Kooshiar, H.; Yahaya, N.; Hamid, T.A.; Abu Samah, A.; Sedaghat Jou, V. Living arrangement and life satisfaction in older Malaysians: the mediating role of social support function. PLoS ONE 2012, 7, e43125. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Kung, S.F.; Hu, S.C. The Relationship between Urbanization, the Built Environment, and Physical Activity among Older Adults in Taiwan. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Ene, M.; Leighton, E.A.; Blue, G.L.; Bell, B.A. Multilevel Models for Categorical Data Using SAS® PROC GLIMMIX: The Basics. 2015. Available online: https://support.sas.com/resources/papers/proceedings15/3430-2015.pdf (accessed on 2 August 2018).
- Barnett, D.W.; Barnett, A.; Nathan, A.; Van Cauwenberg, J.; Cerin, E. Built environmental correlates of older adults’ total physical activity and walking: a systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, M.; Genereux, M.; Bruneau, J.F.; Vanasse, A.; Chabot, E.; Beaulac, C.; Bedard, M.M. Importance of proximity to resources, social support, transportation and neighborhood security for mobility and social participation in older adults: results from a scoping study. BMC Public Health 2015, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 631–648. [Google Scholar] [CrossRef]
- Domenech-Abella, J.; Lara, E.; Rubio-Valera, M.; Olaya, B.; Moneta, M.V.; Rico-Uribe, L.A.; Ayuso-Mateos, J.L.; Mundo, J.; Haro, J.M. Loneliness and depression in the elderly: the role of social network. Soc. Psychiatry Psychiatr. Epidemiol. 2017, 52, 381–390. [Google Scholar] [CrossRef]
- Echeverria, S.; Diez-Roux, A.V.; Shea, S.; Borrell, L.N.; Jackson, S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: the Multi-Ethnic Study of Atherosclerosis. Health Place 2008, 14, 853–865. [Google Scholar] [CrossRef]
- Mujahid, M.S.; Diez Roux, A.V.; Morenoff, J.D.; Raghunathan, T.E.; Cooper, R.S.; Ni, H.; Shea, S. Neighborhood characteristics and hypertension. Epidemiology 2008, 19, 590–598. [Google Scholar] [CrossRef]
- Chaix, B.; Lindstrom, M.; Rosvall, M.; Merlo, J. Neighbourhood social interactions and risk of acute myocardial infarction. J. Epidemiol. Community Health 2008, 62, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamble, K.R.; Howard, J.H.; Howard, D.V. Not just scenery: viewing nature pictures improves executive attention in older adults. Exp. Aging Res. 2014, 40, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Hartig, T. Green space, psychological restoration, and health inequality. Lancet 2008, 372, 1614–1615. [Google Scholar] [CrossRef]
- Wu, Y.T.; Prina, A.M.; Jones, A.; Barnes, L.E.; Matthews, F.E.; Brayne, C.; Mrc, C. Micro-scale environment and mental health in later life: Results from the Cognitive Function and Ageing Study II (CFAS II). J. Affect. Disord. 2017, 218, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macintyre, S.; Ellaway, A.; Cummins, S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc. Sci. Med. 2002, 55, 125–139. [Google Scholar] [CrossRef]
- Commission for Rural Communities. Social isolation experienced by older people in rural communities. 2012. Available online: http://cdn.basw.co.uk/upload/basw_111815-1.pdf (accessed on 1 March 2019).
- Kuiper, J.S.; Zuidersma, M.; Oude Voshaar, R.C.; Zuidema, S.U.; van den Heuvel, E.R.; Stolk, R.P.; Smidt, N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2015, 22, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Chien, I.C.; Chou, Y.J.; Lin, C.H.; Bih, S.H.; Chou, P.; Chang, H.J. Prevalence and incidence of schizophrenia among national health insurance enrollees in Taiwan, 1996–2001. Psychiatry Clin. Neurosci. 2004, 58, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsychiatr. Dis. Treat. 2018, 14, 2047–2055. [Google Scholar] [CrossRef]
- Lin, Y.J.; Tian, W.H.; Chen, C.C. Urbanization and the utilization of outpatient services under National Health Insurance in Taiwan. Health Policy 2011, 103, 236–243. [Google Scholar] [CrossRef]
- Yaya, S.; Uthman, O.A.; Amouzou, A.; Ekholuenetale, M.; Bishwajit, G. Inequalities in maternal health care utilization in Benin: a population based cross-sectional study. BMC Pregnancy Childbirth 2018, 18, 194. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Arvanitakis, Z.; Bang, W.; Bennett, D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007, 69, 2197–2204. [Google Scholar] [CrossRef] [PubMed]

| Variables a | Case | Control | pe | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 1.000 | ||||
| Men | 5071 | 40.9 | 5071 | 40.9 | |
| Female | 7330 | 59.1 | 7330 | 59.1 | |
| Age (years) | 1.000 | ||||
| 65–69 | 1135 | 9.2 | 1135 | 9.2 | |
| 70–74 | 2132 | 17.2 | 2132 | 17.2 | |
| 75–79 | 2692 | 21.7 | 2692 | 21.7 | |
| 80–84 | 3215 | 25.9 | 3215 | 25.9 | |
| ≥80 | 3227 | 26.0 | 3227 | 26.0 | |
| Mean ± SD b | 79.6 ± 7.2 | 79.3 ± 7.1 | |||
| Occupational status | <0.0001 | ||||
| White collar | 1479 | 11.9 | 1549 | 12.5 | |
| Blue collar | 4608 | 37.1 | 4984 | 40.2 | |
| Others | 6314 | 51.0 | 5868 | 47.3 | |
| Salary-based insurance premium (NTD) b | |||||
| dependents | 4592 | 37.4 | 4622 | 37.2 | <0.0001 |
| <Median (19,200) | 3093 | 25.3 | 2829 | 22.8 | |
| ≥Median | 4578 | 37.3 | 4950 | 39.9 | |
| Mean ± SD b,c | 8139.8 ± 11,021.6 | 8718.7 ± 11,517.4 | |||
| Number of comorbidities d | 0.0011 | ||||
| 0 | 4034 | 32.5 | 4181 | 33.7 | |
| 1–2 | 5985 | 48.3 | 6096 | 49.2 | |
| ≥3 | 2382 | 19.2 | 2124 | 17.1 | |
| Total | 12,401 | 100.0 | 12,401 | 100.0 | |
| Environmental Features | Percentile | ||||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD a | Q1a | Q2 a | Q3 a | IQR a | |
| Physical Environments | |||||||
| Parks, greeneries, and square area b | 0 | 25.98 | 0.54 ± 1.50 | 0.14 | 0.29 | 0.55 | 0.41 |
| Playgrounds and sport venues b | 0 | 17.76 | 0.97 ± 2.15 | 0.08 | 0.21 | 0.80 | 0.72 |
| Community centers c | 0 | 324.75 | 41.95 ± 43.49 | 10.11 | 31.17 | 59.42 | 49.31 |
| Social environments | |||||||
| Median annual family income d | 339 | 829 | 507.46 ± 68.16 | 465 | 494 | 539 | 74 |
| Illiterate people aged ≥65 e | 0.22 | 55.93 | 18.79 ± 11.03 | 10.08 | 16.74 | 26.73 | 16.65 |
| Elderly living alone f | 0 | 539.29 | 38.41 ± 51.27 | 10.85 | 18.89 | 46.46 | 35.61 |
| Other environments | |||||||
| Hospitals and clinics f | 0.72 | 33.82 | 6.43 ± 4.99 | 2.74 | 5.00 | 8.72 | 5.98 |
| Variables | No. of Township (Case/Control) | Case | Control | pf | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Total | 337/338 | 12,401 | 100.0 | 12,401 | 100.0 | |
| Physical environments | ||||||
| Parks, greeneries, and square area a | 0.6843 | |||||
| <Median | 172/172 | 6434 | 51.9 | 6402 | 51.6 | |
| ≥Median | 165/166 | 5967 | 48.1 | 5999 | 48.4 | |
| Playgrounds and sport venues a | 0.0628 | |||||
| <Median | 170/169 | 8351 | 67.3 | 8213 | 66.2 | |
| ≥Median | 167/169 | 4050 | 32.7 | 4188 | 33.8 | |
| Community centers b | 0.0499 | |||||
| <Median | 173/170 | 10,162 | 82.0 | 10,042 | 81.0 | |
| ≥Median | 164/168 | 2239 | 18.0 | 2359 | 19.0 | |
| Social environments | ||||||
| Median annual family income c | 0.0335 | |||||
| <Median | 163/166 | 2518 | 20.3 | 2654 | 21.4 | |
| ≥Median | 174/172 | 9883 | 79.7 | 9747 | 78.6 | |
| Illiterate people aged ≥65d | 0.7144 | |||||
| <Median | 165/168 | 8381 | 67.6 | 8354 | 67.4 | |
| ≥Median | 172/170 | 4020 | 32.4 | 4047 | 32.6 | |
| Elderly living alone e | 0.7760 | |||||
| <Median | 173/170 | 8373 | 67.5 | 8352 | 67.4 | |
| ≥Median | 164/168 | 4028 | 32.5 | 4049 | 32.6 | |
| Other environments | ||||||
| Hospitals and clinics e | 0.0080 | |||||
| <Median | 168/167 | 1971 | 15.9 | 2126 | 17.1 | |
| ≥Median | 169/171 | 10,430 | 84.1 | 10,275 | 82.9 | |
| Urbanization | 0.0005 | |||||
| Rural | 196/199 | 3076 | 24.8 | 3338 | 26.9 | |
| Suburban | 113/111 | 5937 | 47.9 | 5714 | 46.1 | |
| Urban | 28/28 | 3388 | 27.3 | 3349 | 27.0 | |
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Intercept (SD) a | 0.02 (0.03) | −0.45 (0.19) * | −0.21 (0.20) | −0.16 (0.21) | −0.20 (0.21) |
| Physical environments | |||||
| Parks, greeneries, and square area | 1.02 (0.99–1.05) | 1.02 (0.99–1.05) | 1.02 (0.99–1.05) | 1.01 (0.99–1.04) | |
| Playgrounds and sport venues | 0.97 (0.96–0.99) * | 0.97 (0.96–0.99) * | 0.97 (0.96–0.99) * | 0.97 (0.96–0.99) * | |
| Community centers | 0.92 (0.86–0.99) * | 0.91 (0.83–0.98) * | 0.92 (0.85–1.01) | 0.94 (0.86–1.03) | |
| Social environments | |||||
| Median annual family income | 1.05 (1.01–1.10) * | 1.02 (0.98–1.07) | 1.01 (0.96–1.06) | 1.01 (0.96–1.05) | |
| Illiterate aged ≥65 | 1.04 (0.97–1.12) | 1.04 (0.97–1.12) | 1.06 (0.99–1.14) | 1.08 (0.99–1.16) | |
| Elderly living alone | 1.03 (0.99–1.08) | 1.05 (1.01–1.10) * | 1.05 (1.01–1.11) * | 1.05 (1.01–1.11) * |
| Variables | Urban areas b | Suburban Areas b | Rural Areas b |
|---|---|---|---|
| Intercept (SD) a | −0.08 (0.65) | −0.65 (0.37) | 0.28 (0.45) |
| Physical environments | |||
| Parks, greeneries, and square area | 0.93 (0.76–1.14) | 0.99 (0.93–1.06) | 1.03 (0.99–1.07) |
| Playgrounds and sport venues | 1.24 (0.22–6.87) | 0.97 (0.95–1.00) | 0.97 (0.94–0.99) * |
| Community centers | 0.78 (0.42–1.44) | 0.96 (0.81–1.14) | 0.89 (0.79–0.99) * |
| Social environments | |||
| Median annual family income | 1.01 (0.91–1.12) | 1.05 (0.97–1.14) | 0.95 (0.84–1.06) |
| Illiterate people aged ≥65 | 1.12 (0.63–2.00) | 1.24 (1.04–1.48) * | 1.03 (0.95–1.13) |
| Elderly living alone | 1.02 (0.93–1.12) | 1.06 (0.96–1.16) | 1.11 (1.03–1.20) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-C.; Li, C.-Y.; Kung, S.-F.; Kuo, H.-W.; Huang, N.-C.; Sun, Y.; Hu, S.C. Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study. Int. J. Environ. Res. Public Health 2019, 16, 2828. https://doi.org/10.3390/ijerph16162828
Liu C-C, Li C-Y, Kung S-F, Kuo H-W, Huang N-C, Sun Y, Hu SC. Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study. International Journal of Environmental Research and Public Health. 2019; 16(16):2828. https://doi.org/10.3390/ijerph16162828
Chicago/Turabian StyleLiu, Chih-Ching, Chung-Yi Li, Shiann-Far Kung, Hsien-Wen Kuo, Nuan-Ching Huang, Yu Sun, and Susan C. Hu. 2019. "Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study" International Journal of Environmental Research and Public Health 16, no. 16: 2828. https://doi.org/10.3390/ijerph16162828
APA StyleLiu, C.-C., Li, C.-Y., Kung, S.-F., Kuo, H.-W., Huang, N.-C., Sun, Y., & Hu, S. C. (2019). Association of Environmental Features and the Risk of Alzheimer’s Dementia in Older Adults: A Nationwide Longitudinal Case-Control Study. International Journal of Environmental Research and Public Health, 16(16), 2828. https://doi.org/10.3390/ijerph16162828







