Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya
Abstract
:1. Introduction
2. Materials and Methods
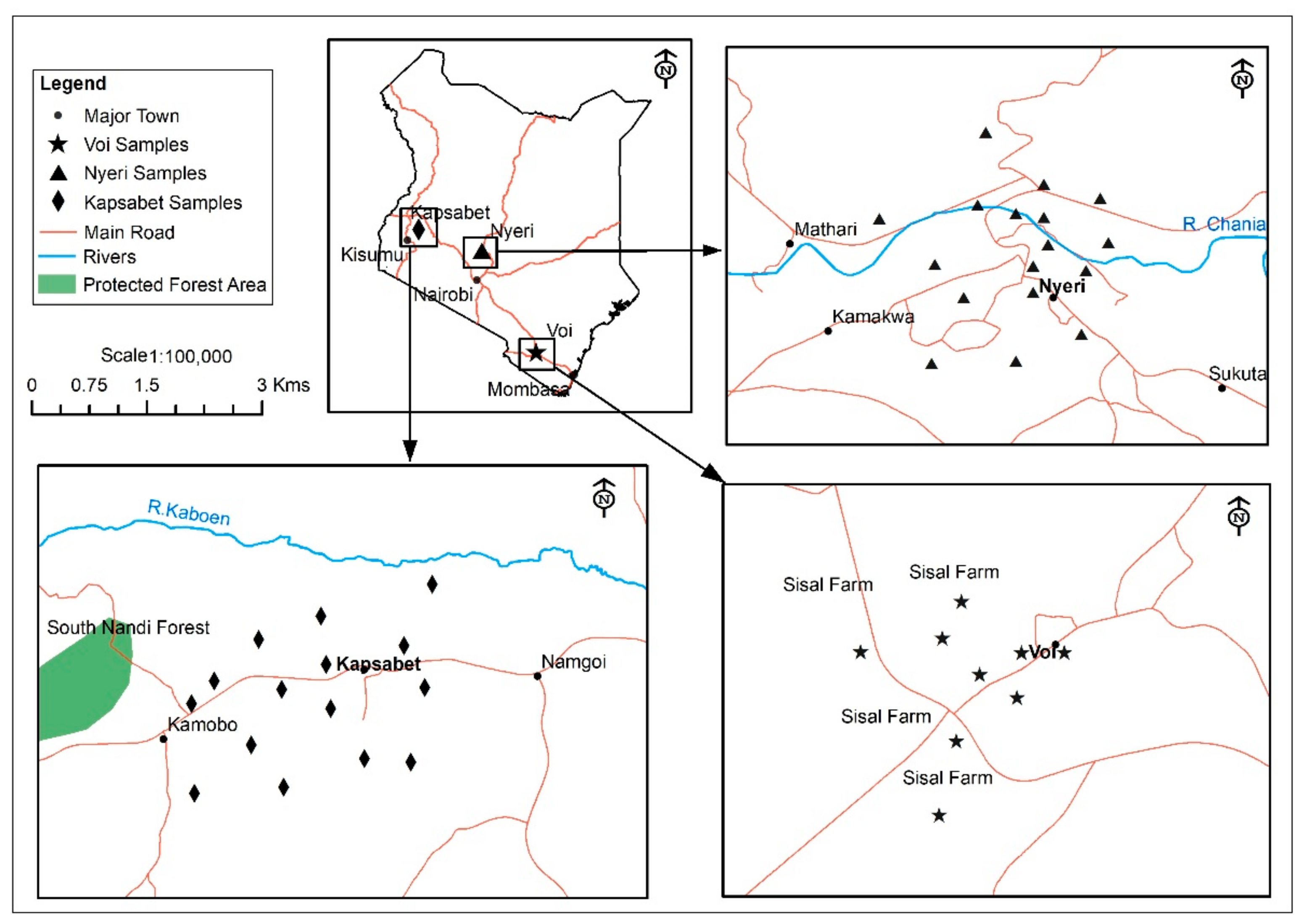
2.1. Sample Sites
2.2. Sampling and Pretreatment
2.3. Sample Extraction and Analysis
2.4. Instrumental Analysis
2.5. Quality Control
2.6. Carcinogenic Risk Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of DDTs and HCHs in Soils of Kenya
3.2. Sources of DDTs and HCHs in Soils
3.3. The Relationship between DDTs and HCHs Levels and TOC
3.4. Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gereslassie, T.; Workineh, A.; Atieno, O.J.; Wang, J. Determination of Occurrences, Distribution, Health Impacts of Organochlorine Pesticides in Soils of Central China. Int. J. Environ. Res. Public Health 2019, 16, 146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Shi, S.; Zhou, L.; Zhang, T.; Huang, Y. Organochlorine pesticides contamination in surface soils from two pesticide factories in Southeast China. Chemosphere 2009, 77, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Park, J.B.; Woo, M.S.; Lee, S.Y.; Kim, H.Y.; Yoo, Y.H. Persistent Organic Pollutant-Mediated Insulin Resistance. Int. J. Environ. Res. Public Health 2019, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Makris, S.L.; Rowe, J.N. Implementation of the Food Quality Protection Act (FQPA) as it relates to enhanced sensitivity of children. Teratology 1998, 57, 246. [Google Scholar]
- Ssebugere, P.; Wasswa, J.; Mbabazi, J.; Nyanzi, S.A.; Kiremire, B.T.; Marco, J.A. Organochlorine pesticides in soils from south-western Uganda. Chemosphere 2010, 78, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Xi, Z.; Cheng, H.; Xu, X. Organochlorine pesticides (DDTs and HCHs) in soils from the outskirts of Beijing, China. Chemosphere 2005, 60, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Kuhad, R.C.; Singh, A.; Tripathi, K.K.; Ghosh, P.K. Microbial degradation of the pesticide lindane (γ-Hexachlorocyclohexane). Adv. Appl. Microbiol. 2000, 47, 269–298. [Google Scholar] [PubMed]
- Bala, K.; Geueke, B.; Miska, M.E.; Rentsch, D.; Poiger, T.; Dadhwal, M.; Lal, R.; Holliger, C.; Kohler, H.P.E. Enzymatic conversion of ε-hexachlorocyclohexane and a hexachlorocyclohexane isomer, two neglected components of technical hexachlorocyclohexane. Environ. Sci. Technol. 2012, 46, 4051–4058. [Google Scholar] [CrossRef]
- Haugen, J.E.; Wania, F.; Ritter, N.; Schlabach, M. Hexachlorocyclohexanes in air in southern Norway. Temporal variation, source allocation, and temperature dependence. Environ. Sci Technol 1998, 32, 217–224. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Rodrigues, P.N.R.; Basto, M.C.P.; Vasconcelos, M.T.S.D. Organochlorine pesticides levels in Portuguese coastal areas. Chemosphere 2009, 75, 595–600. [Google Scholar] [CrossRef]
- Zhang, H.B.; Luo, Y.M.; Zhao, Q.G.; Wong, M.H.; Zhang, G.L. Residues of organochlorine pesticides in Hong Kong soils. Chemosphere 2006, 63, 633–641. [Google Scholar] [CrossRef]
- Nakata, H.; Kawazoe, M.; Arizono, K.; Abe, S.; Kitano, T.; Shimada, H.; Li, W.; Ding, X. Organochlorine Pesticides and Polychlorinated Biphenyl Residues in Foodstuffs and Human Tissues from China: Status of Contamination, Historical Trend, and Human Dietary Exposure. Arch. Environ. Contam. Toxicol. 2002, 43, 0473–0480. [Google Scholar] [CrossRef] [PubMed]
- Saadati, N.; Abdullah, M.P.; Zakaria, Z.; Rezayi, M.; Hosseinizare, N. Distribution and fate of HCH isomers and DDT metabolites in a tropical environment case study Cameron Highlands Malaysia. Chem. Cent. J. 2012, 6, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Christiane, R. WHO recommends DDT to control malaria. BMJ 2006, 333, 622. [Google Scholar]
- Ray, D. Nervous system and behavioral toxicology. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Harrison School of Pharmacy, Auburn University: Auburn, AL, USA, 2010; Volume 2, p. 8624. [Google Scholar]
- Carvalho, F.P.; Montenegro-Guillén, S.; Villeneuve, J.P.; Cattini, C.; Tolosa, I.; Bartocci, J.; Lacayo-Romero, M.; Cruz-Granja, A. Toxaphene residues from cotton fields in soils and in the coastal environment of Nicaragua. Chemosphere 2003, 53, 627–636. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Ximenes, V.F.; de Andrade, R.M.; Talamoni, J.L.; Libânio, M.; de Almeida, M.V.; Apon, B.M.; Cavalari, A.A. Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the Northeastern part of São Paulo State, Brazil. Chemosphere 2006, 65, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Naz, F.; Jan, M.I.; Khan, A.; Khan, I. Analysis of nutritional components of some wild edible plants components of some wild edible plants. J. Chem. Soc. Pak. 2007, 29, 500–508. [Google Scholar]
- Mansour, S.A. Persistent organic pollutants (POPs) in Africa: Egyptian scenario. Hum. Exp. Toxicol. 2009, 28, 531. [Google Scholar] [CrossRef]
- Wandiga, S.O. Use and distribution of organochlorine pesticides. The future in Africa. Pure Appl. Chem. 2001, 73, 1147–1156. [Google Scholar] [CrossRef]
- Gitahi, S.M.; Harper, D.M.; Muchiri, S.M.; Tole, M.P.; Ng’Ang’A, R.N. Organochlorine and organophosphorus pesticide concentrations in water, sediment, and selected organisms in Lake Naivasha (Kenya). Hydrobiologia 2002, 488, 123–128. [Google Scholar] [CrossRef]
- Sun, H.; Qi, Y.; Zhang, D.; Li, Q.X.; Wang, J. Concentrations, distribution, sources and risk assessment of organohalogenated contaminants in soils from Kenya, Eastern Africa. Environ. Pollut. 2016, 209, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Wang, X.T.; Jia, Y.; Wang, F.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. J. Hazard. Mater. 2009, 170, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Chio, C.P.; Chiang, Y.H.; Liao, C.M. Assessing hazardous risks of human exposure to temple airborne polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2009, 166, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Qi, S.; Yang, D.; Huang, H.; Zhang, J.; Chen, W.; Yohannes, H.K.; Sandy, E.H.; Yang, J.; Xing, X. Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: A case study from Ningde, southeast China. J. Geochem. Explor. 2015, 149, 43–51. [Google Scholar] [CrossRef]
- USEPA. Risk assessment guidance for (RAGS): Part F. Human health evaluation manual. In Supplemental Guidance for Inhalation Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2009. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/partf_200901_final.Pdf (accessed on 8 December 2018).
- U.S. EPA. Exposure Factors Handbook; Environmental Protection Agency: Washington, DC, USA, 1997.
- Osoro, E. Organochlorine Pesticides Residues in Water and Sediment from Rusinga Island, Lake Victoria, Kenya. J. Appl. Chem. 2016, 9, 56–63. [Google Scholar]
- Cui, L.; Wei, L.; Wang, J. Residues of organochlorine pesticides in surface water of a megacity in central China: Seasonal-spatial distribution and fate in Wuhan. Environ. Sci. Pollut. Res. 2016, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.M.; Seech, A.G.; Lee, H.; Trevors, J.T. Biodegradation of hexachlorocyclohexane (HCH) by microorganisms. Biodegradation 2005, 16, 363–392. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wenxin, L.; Yao, L.; Yu, Y.; Qian, Z.; Bengang, L. Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environ. Sci. Technol. 2008, 42, 8395. [Google Scholar] [CrossRef]
- Netherlands Ministry of Housing, Spatial Planning and Environment’s Circular on Target Values and Intervention Values for Soil Remediation. Available online: http://www2.minvrom.nl/Docs/internationaal/annexS_I2000.pdf (accessed on 27 July 2019).
- Zhang, H.; Lu, Y.; Dawson, R.W.; Shi, Y.; Wang, T. Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere 2005, 60, 762–769. [Google Scholar] [CrossRef]
- Toan, V.D.; Thao, V.D.; Walder, J.; Schmutz, H.R.; Ha, C.T. Contamination by selected organochlorine pesticides (OCPs) in surface soils in Hanoi, Vietnam. Bull. Environ. Contam. Toxicol. 2007, 78, 195. [Google Scholar] [CrossRef]
- Falandysz, J.; Brudnowska, B.; Kawano, M.; Wakimoto, T. Polychlorinated Biphenyls and Organochlorine Pesticides in Soils from the Southern Part of Poland. Arch. Environ. Contam. Toxicol. 2001, 40, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Hua, D. Occurrence, distribution and possible sources of organochlorine pesticides in peri-urban vegetable soils of Changchun, Northeast China. Hum. Ecol. Risk Assess. 2017, 23, 2033–2045. [Google Scholar] [CrossRef]
- Lee, K.T.; Tanabe, S.; Koh, C.H. Distribution of organochlorine pesticides in sediments from Kyeonggi Bay and nearby areas, Korea. Environ. Pollut. 2001, 114, 207–213. [Google Scholar] [CrossRef]
- Yang, L.; Xia, X.; Liu, S.; Bu, Q. Distribution and sources of DDTs in urban soils with six types of land use in Beijing, China. J. Hazard. Mater. 2010, 174, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, B.; Hites, R.A. Concentration of organochlorine pesticides in wine corks. Chemosphere 2001, 44, 729–735. [Google Scholar] [CrossRef]
- Gonzalez, M.; Miglioranza, K.; Moreno, J.E.A.D.; Moreno, V.J. Occurrence and distribution of organochlorine pesticides (OCPs) in tomato (Lycopersicon esculentum) crops from organic production. Agric. Food Chem 2003, 51, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Donald, M.; Shiu, W.Y.; Ching, M.K. Illustrated Handbook of Physical-Chemical Properties of Environmental Fate for Organic Chemicals, 2nd ed.; Taylor & Francis Group: London, UK, 2006; pp. 922–2257. [Google Scholar]
- Makokha, V.A.; Ndung’u, A.W.; Mungai, T.M.; Yan, X.; Wang, J. Concentrations, Sources, and Risk Assessment of Organohalogen Compounds in Soils from Kiambu to Mombasa, Kenya. Bull. Environ. Contam. Toxicol. 2018, 101, 766–772. [Google Scholar] [CrossRef]
- Yahaya, A.; Okoh, O.O.; Okoh, A.I.; Adeniji, A.O. Occurrences of Organochlorine Pesticides along the Course of the Buffalo River in the Eastern Cape of South Africa and Its Health Implications. Int. J. Environ. Res. Public Health 2017, 14, 1372. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.V.D.; Manuweera, G.; Konradsen, F. Global trends in the production and use of DDT for control of malaria and other vector-borne diseases. Malar. J. 2017, 16, 401. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Zouboulis, A.; Samara, C. Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: Model predictions against experimental values. Chemosphere 2006, 65, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Szu-Chich, C.; Liao, C.M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar]
- Peng, C.; Chen, W.; Liao, X.; Wang, M.; Ouyang, Z.; Jiao, W.; Bai, Y. Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environ. Pollut. 2011, 159, 802–808. [Google Scholar] [CrossRef] [PubMed]

| OCPs | |||
|---|---|---|---|
| α-HCH | 6.30 | 4.49 | 6.30 |
| β-HCH | 1.80 | 1.98 | 1.86 |
| γ-HCH | 1.30 | 1.34 | 1.80 |
| δ-HCH | 1.80 | N/A | 1.80 |
| p,p′-DDE | 3.40 × 10−1 | 4.86 × 10−1 | N/A |
| p,p′-DDD | 2.40 × 10−1 | 3.43 × 10−1 | N/A |
| p,p′-DDT | 3.40 × 10−1 | 4.86 × 10−1 | 3.40 × 10−1 |
| o,p′-DDT | N/A | N/A | N/A |
| OCPs | Nyeri (n = 23) | Kapsabet (n = 20) | Voi (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean ± Std. Deviation | Sum of Statistics | Range | Mean ± Std. Deviation | Sum of Statistics | Range | Mean ± Std. Deviation | Sum of Statistics | Dutch Standard Limits [32] | |
| α-HCH | n.d.–0.62 | 0.20 ± 0.23 | 4.6 | 0.03–7.52 | 0.75 ± 1.7 | 14.9 | n.d.–0.75 | 0.30 ± 0.3 | 2.73 | 17,000 |
| γ-HCH | n.d.–1.32 | 0.39 ± 0.36 | 9.07 | n.d.–0.48 | 0.30 ± 0.16 | 5.95 | 0.06–1.03 | 0.46 ± 0.3 | 4.16 | 1200 |
| β-HCH | 0.24–2.12 | 0.83 ± 0.56 | 19.2 | n.d.–37.8 | 4.82 ± 10 | 96.3 | 0.02–4.16 | 1.99 ± 1.5 | 18.0 | 1600 |
| δ-HCH | n.d.–0.66 | 0.27 ± 0.23 | 6.19 | n.d.–2.25 | 0.25 ± 0.5 | 5.09 | n.d.–0.92 | 0.39 ± 0.3 | 3.51 | - |
| ∑HCHs | 0.24–4.72 | 1.69 ± 1.4 | 39.1 | 0.03–48.1 | 6.12 ± 13 | 122 | 0.06–6.86 | 3.14 ± 2.4 | 28.4 | - |
| α-HCH/γ-HCH | - | 0.51 | - | - | 2.51 | - | - | 0.66 | - | |
| p,p′-DDE | n.d.–2.16 | 0.44 ± 0.50 | 10.1 | n.d.–1.66 | 0.48 ± 0.44 | 9.65 | n.d.–3.3 | 0.91 ± 0.97 | 8.16 | 2300 |
| o,p′-DDT | n.d.–2.87 | 0.41 ± 0.95 | 9.49 | n.d.–3.02 | 0.37 ± 0.90 | 7.41 | n.d.–8.75 | 1.52 ± 3.2 | 13.6 | 1700 |
| p,p′-DDD | n.d.–2.92 | 0.30 ± 0.85 | 6.93 | n.d. | n.d. | n.d. | n.d.–3.46 | 0.75 ± 1.5 | 6.71 | 34,000 |
| p,p′-DDT | n.d.–11.6 | 0.51 ± 2.4 | 11.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1700 |
| ∑DDTs | n.d.–19.6 | 1.66 ± 4.7 | 38.1 | n.d.–4.68 | 0.85 ± 1.3 | 17.1 | n.d.–15.5 | 3.18 ± 5.6 | 28.5 | - |
| p,p′-DDE/p,p′-DDD | - | 0.82 | - | - | n.d. | - | - | 0.84 | - | |
| TOC | 0.21–2.65 | 0.90–0.8 | 20.7 | 0.03–1.71 | 0.54–0.51 | 10.7 | 0.25–1.25 | 0.56–0.38 | 5.0 | |
| ∑OCPs | 0.24–24.3 | 3.35 ± 6.1 | 0.03–52.7 | 6.97 ± 14 | 0.06–22.4 | 6.32 ± 8.02 | ||||
| OCPs | Nyeri | Kapsabet | Voi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC1 | PC2 | |
| α-HCH | 0.324 | 0.493 | −0.687 | 0.176 | 0.927 | −0.106 | −0.041 | 0.699 | 0.561 |
| β-HCH | 0.830 | −0.114 | 0.081 | −0.233 | −0.681 | −0.355 | 0.448 | 0.803 | 0.587 |
| γ-HCH | 0.648 | −0.266 | −0.229 | 0.439 | 0.967 | −0.065 | 0.021 | 0.854 | 0.306 |
| δ-HCH | 0.208 | 0.093 | 0.913 | 0.166 | −0.215 | −0.198 | −0.717 | 0.649 | 0.555 |
| p,p′-DDE | −0.204 | 0.039 | 0.106 | 0.918 | −0.373 | −0.287 | 0.701 | 0.579 | 0.755 |
| p,p′-DDD | −0.168 | 0.889 | −0.114 | −0.118 | −0.131 | −0.067 | 0.732 | 0.400 | 0.816 |
| p,p′-DDT | −0.051 | 0.910 | 0.056 | 0.099 | −0.026 | 0.993 | −0.029 | −0.054 | 0.82 |
| o,p′-DDT | 0.836 | 0.019 | 0.067 | −0.101 | −0.026 | 0.993 | −0.029 | −0.765 | 0.074 |
| Eigenvalues | 2.29 | 1.83 | 1.27 | 1.17 | 2.89 | 2.13 | 1.42 | 5.33 | 1.02 |
| % of variance | 28.6 | 22.9 | 15.9 | 14.7 | 36.2 | 26.6 | 17.6 | 66.5 | 12.7 |
| Cumulative % | 28.6 | 51.5 | 67.4 | 82.0 | 36.2 | 62.9 | 80.6 | 66.6 | 79.3 |
| OCPs | TOC | α-HCH | γ-HCH | β-HCH | δ-HCH | p,p′-DDE | o,p′-DDT | p,p′-DDD | p,p′-DDT |
|---|---|---|---|---|---|---|---|---|---|
| Nyeri | |||||||||
| TOC | 1 | 0.506 * | 0.377 | 0.240 | −0.153 | −0.077 | 0.270 | 0.094 | 0.066 |
| α-HCH | 1 | 0.154 | 0.212 | −0.402 | 0.05 | 0.386 | 0.351 | 0.167 | |
| γ-HCH | 1 | 0.353 | 0.176 | −0.305 | −0.271 | −0.132 | 0.559 ** | ||
| β-HCH | 1 | 0.003 | 0.096 | −0.286 | −0.203 | 0.384 | |||
| δ-HCH | 1 | 0.159 | −0.066 | 0.088 | 0.151 | ||||
| p,p′-DDE | 1 | −0.063 | 0.116 | −0.191 | |||||
| o,p′-DDT | 1 | 0.696 ** | −0.095 | ||||||
| p,p′-DDD | 1 | −0.077 | |||||||
| p,p′-DDT | 1 | ||||||||
| Kapsabet | |||||||||
| TOC | 1 | 0.289 | −0.059 | 0.470 * | −0.161 | −0.236 | −0.076 | −0.131 | −0.131 |
| α-HCH | 1 | −0.585 ** | 0.842 ** | −0.065 | −0.29 | −0.12 | −0.101 | −0.101 | |
| γ-HCH | 1 | −0.622 ** | −0.285 | 0.534 * | 0.268 | −0.353 | −0.353 | ||
| β-HCH | 1 | −0.208 | −0.322 | −0.12 | −0.09 | −0.09 | |||
| δ-HCH | 1 | −0.18 | −0.147 | −0.12 | −0.12 | ||||
| p,p′-DDE | 1 | 0.576 ** | −0.257 | −0.257 | |||||
| o,p′-DDT | 1 | −0.067 | −0.067 | ||||||
| p,p′-DDD | 1 | 1.00 ** | |||||||
| p,p′-DDT | 1 | ||||||||
| Voi | |||||||||
| TOC | 1 | 0.447 | 0.406 | 0.126 | 0.289 | 0.514 | 0.471 | 0.971 ** | −0.24 |
| α-HCH | 1 | 0.871 ** | 0.874 ** | 0.646 | 0.756 * | 0.664 | 0.473 | −0.412 | |
| γ-HCH | 1 | 0.866 ** | 0.872 ** | 0.929 ** | 0.794 * | 0.412 | −0.543 | ||
| β-HCH | 1 | 0.603 | 0.705 * | 0.606 | 0.134 | −0.441 | |||
| δ-HCH | 1 | 0.801 ** | 0.696 * | 0.354 | −0.442 | ||||
| p,p′-DDE | 1 | 0.889 ** | 0.495 | −0.35 | |||||
| o,p′-DDT | 1 | 0.488 | −0.18 | ||||||
| p,p′-DDD | 1 | −0.189 | |||||||
| p,p′-DDT | 1 |
| Growth stage | Exposure Pathways | Maximum | Mean | Median |
|---|---|---|---|---|
| Childhood | Ingestion | 3.09 × 10−6 | 7.22 × 10−7 | 2.21 × 10−7 |
| Dermal contact | 7.55 × 10−7 | 1.48 × 10−7 | 2.74 × 10−8 | |
| Inhalation | 1.28 ×10−10 | 2.92 × 10−11 | 7.88 × 10−12 | |
| Cancer risk | 3.85 × 10−6 | 8.70 × 10−7 | 2.49 × 10−7 | |
| Adolescence | Ingestion | 1.61 × 10−6 | 3.76 × 10−7 | 1.15 × 10−7 |
| Dermal contact | 8.66 × 10−7 | 1.69 × 10−7 | 3.14 × 10−8 | |
| Inhalation | 2.17 ×10−10 | 4.95 × 10−11 | 1.33 × 10−11 | |
| Cancer risk | 2.48 × 10−6 | 5.45 × 10−7 | 1.47 × 10−7 | |
| Adult | Ingestion | 2.96 × 10−6 | 6.92 × 10−7 | 2.12 × 10−7 |
| Dermal contact | 1.03 × 10−6 | 2.02 × 10−7 | 3.75 × 10−8 | |
| Inhalation | 3.94 × 10−10 | 9.00 × 10−7 | 2.43 × 10−11 | |
| Cancer risk | 4.00 × 10−6 | 8.94 × 10−7 | 2.50 × 10−7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungai, T.M.; Wang, J. Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya. Int. J. Environ. Res. Public Health 2019, 16, 2937. https://doi.org/10.3390/ijerph16162937
Mungai TM, Wang J. Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya. International Journal of Environmental Research and Public Health. 2019; 16(16):2937. https://doi.org/10.3390/ijerph16162937
Chicago/Turabian StyleMungai, Teresiah M., and Jun Wang. 2019. "Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya" International Journal of Environmental Research and Public Health 16, no. 16: 2937. https://doi.org/10.3390/ijerph16162937
APA StyleMungai, T. M., & Wang, J. (2019). Occurrence and Toxicological Risk Evaluation of Organochlorine Pesticides from Suburban Soils of Kenya. International Journal of Environmental Research and Public Health, 16(16), 2937. https://doi.org/10.3390/ijerph16162937





