Evaluation of Fresh Water Actinomycete Bioflocculant and Its Biotechnological Applications in Wastewaters Treatment and Removal of Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Cultivation and Isolation of Bioflocculant-Producing Actinomycetes
2.3. Screening for Bioflocculant-Producing Actinomycetes
2.4. Identification of Organism
2.5. Purification of Bioflocculant
2.6. Jar Test Determination of Bioflocculant Dosage
2.7. Effect of pH and Cations on Bioflocculant Activity
2.8. Characterization of Purified Bioflocculants
2.8.1. Chemical Composition Analyses
2.8.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.8.3. Thermogravimetric Analysis (TGA) and Thermal Stability
2.8.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Analysis
2.9. Application of Purified Bioflocculant
2.9.1. Treatment of Different Wastewaters by Bioflocculant Produced by Terrabacter sp.
2.9.2. Assay for Removal of Heavy Metals by Terrabacter sp.
2.9.3. Statistical Analysis
3. Results
3.1. Isolation of Bioflocculant Producing Bacteria and Component Analysis
3.2. Optimization of Flocculating Efficiency
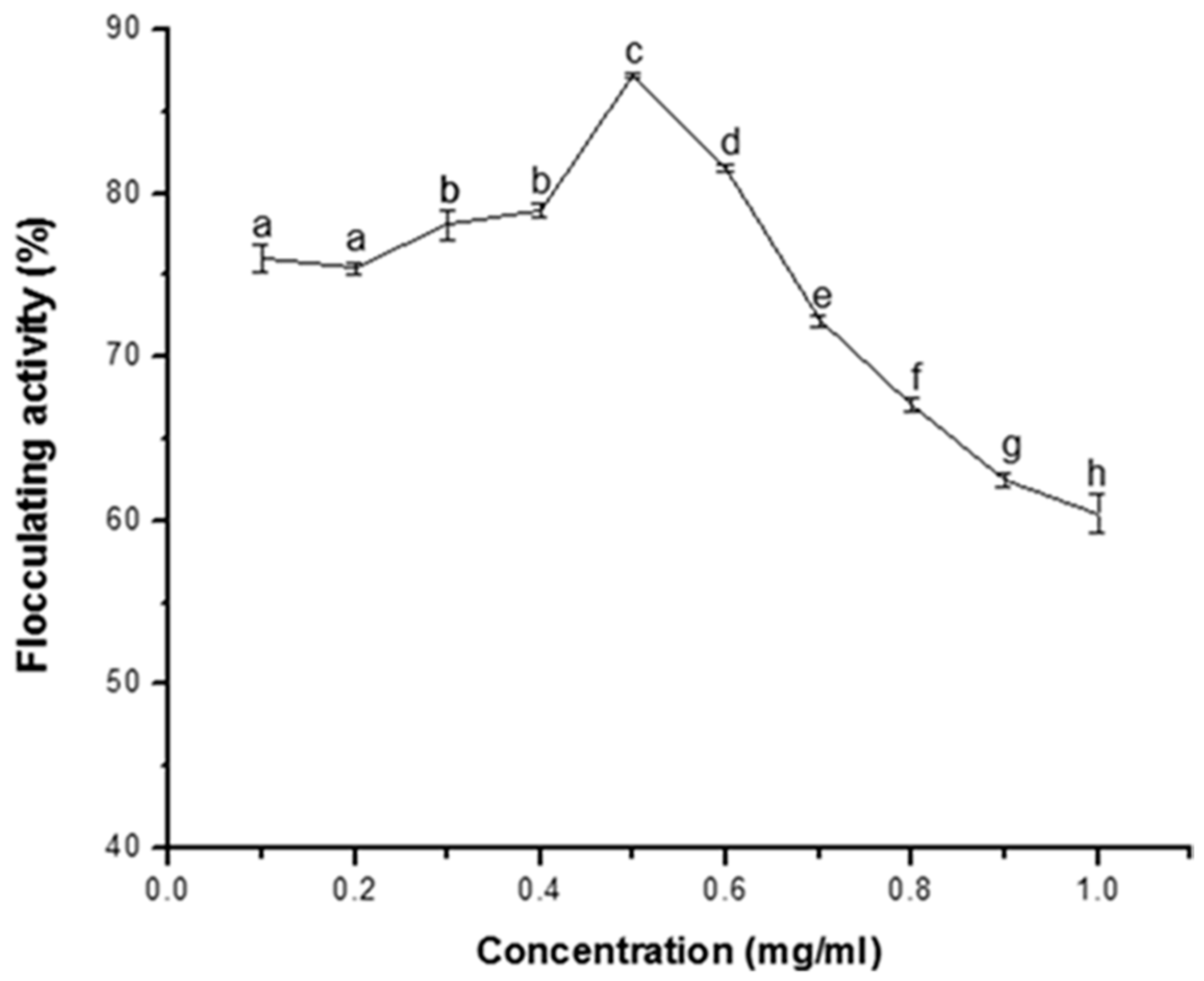
3.2.1. Effect of Bioflocculant Dosage
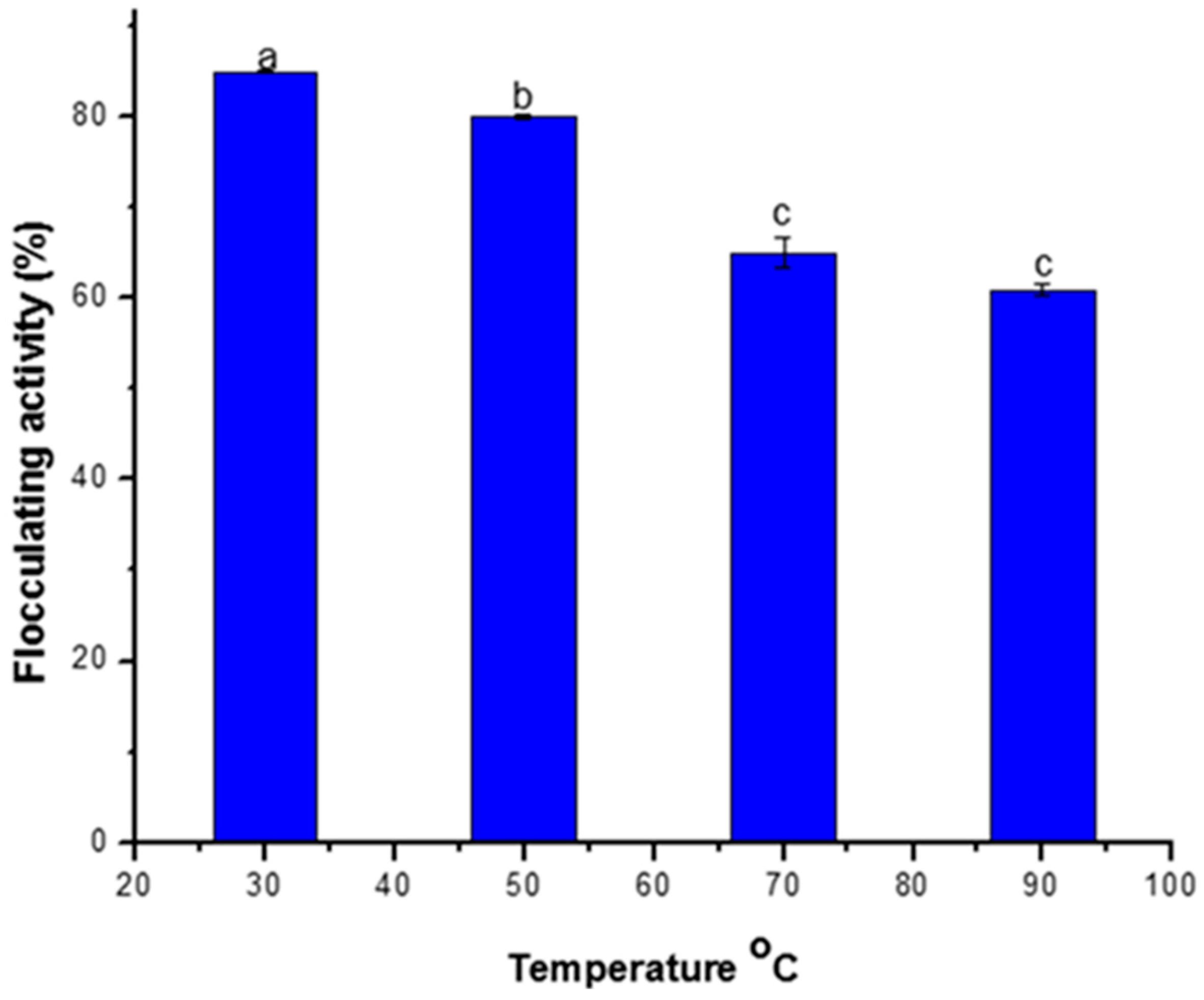
3.2.2. Effect of Temperature
3.2.3. Effect of Metal Ions
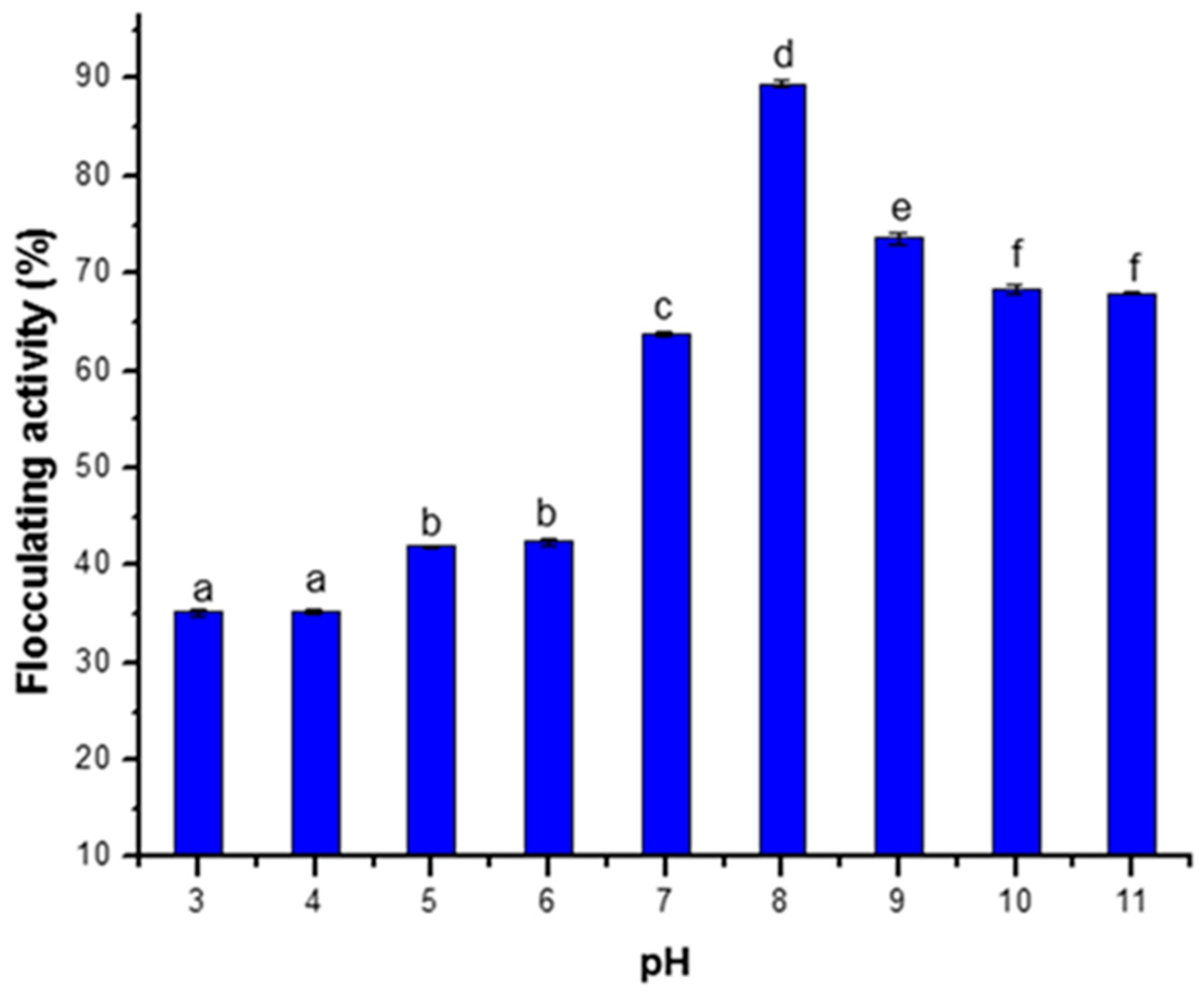
3.2.4. Effect of pH
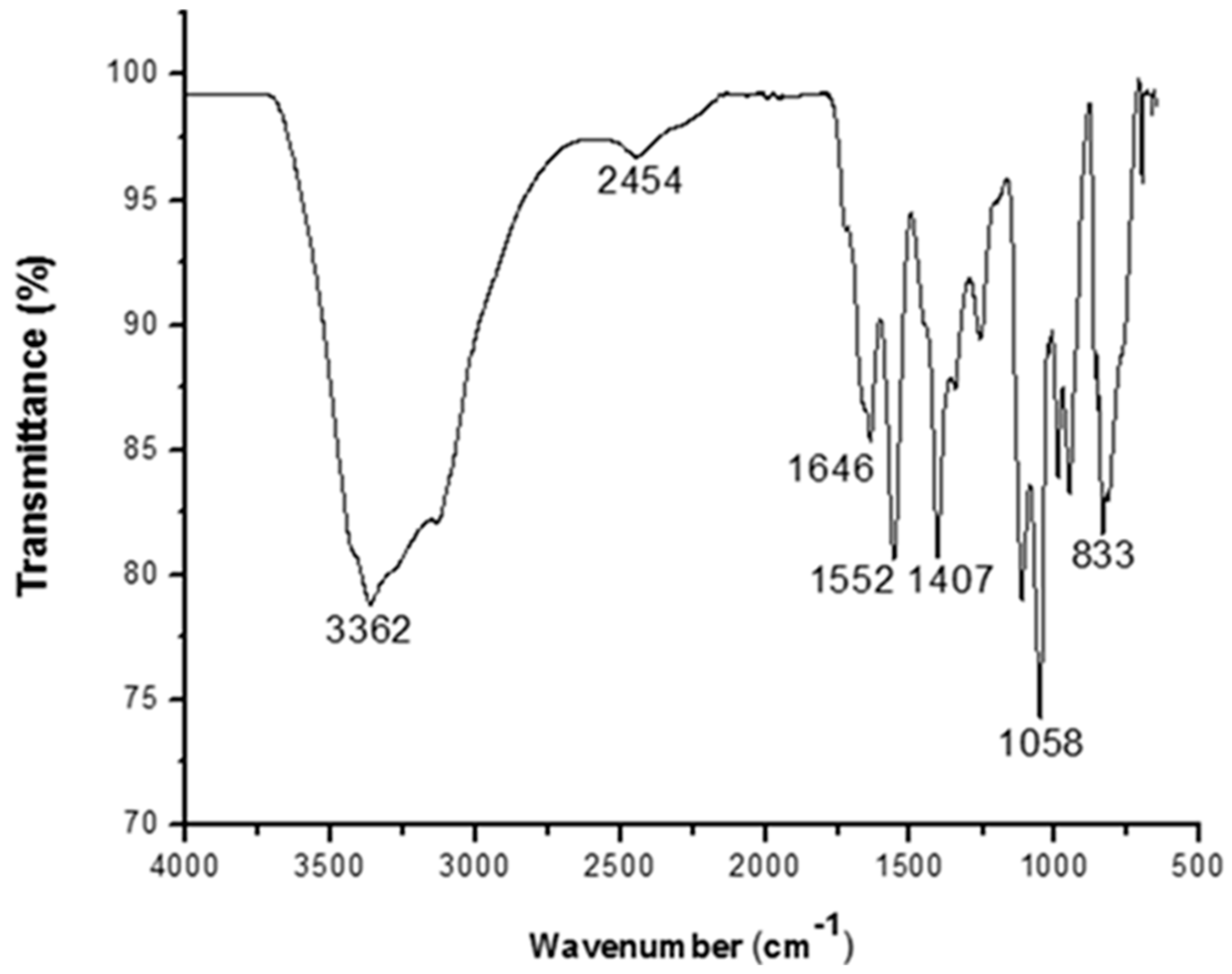
3.3. Functional Group Analysis
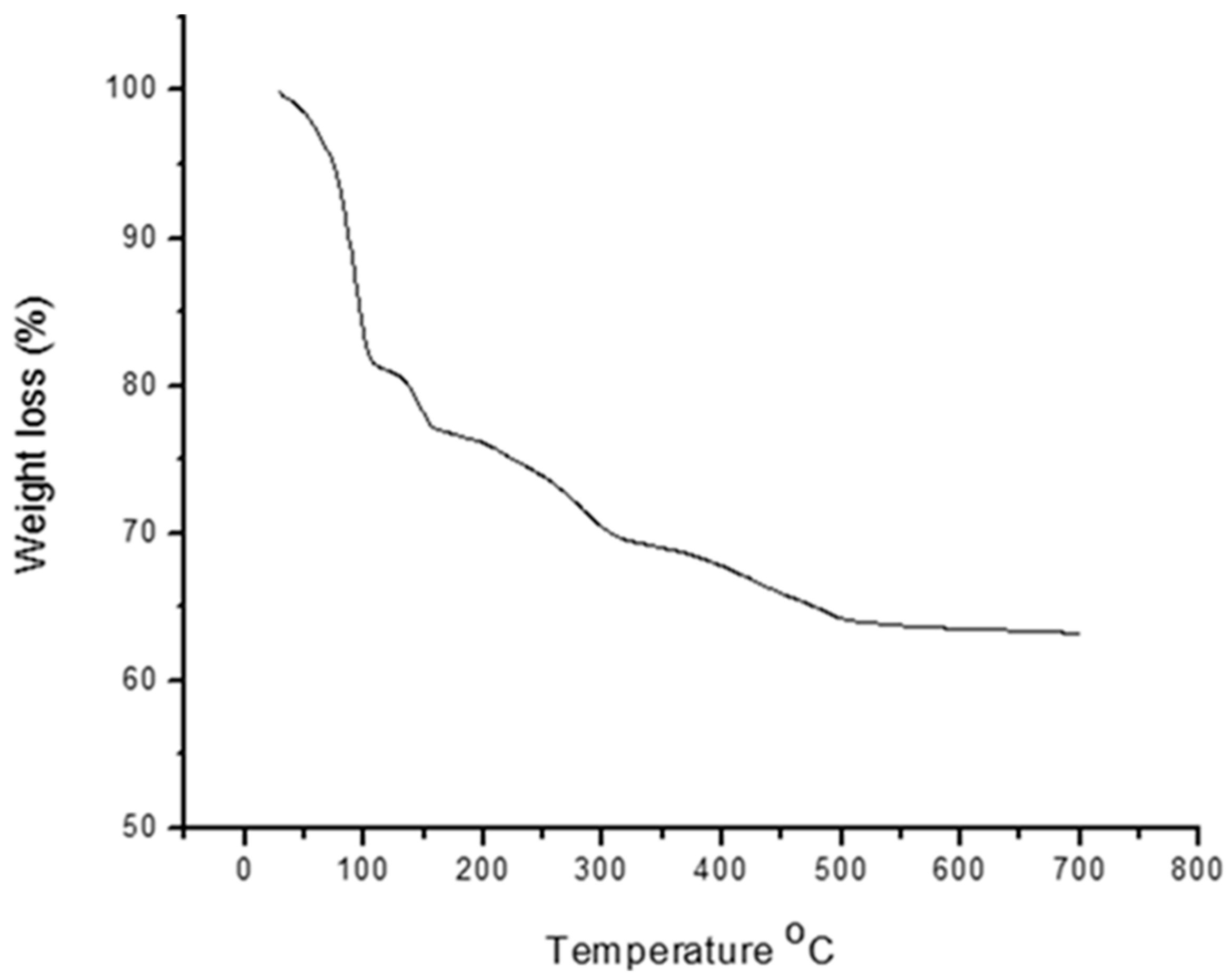
3.4. Thermogravimetric Analysis and Energy Dispersive X-ray and SEM Analyses
3.5. Application of Bioflocculant in Wasteswater Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.I. Implications for public health demands alternatives to inorganic and synthetic flocculants: Bioflocculants as important candidates. Microbiologyopen 2016, 5, 177–211. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Bali, G.; Sarangi, S.K. Effect of lead on metallothionein concentration in lead-resistant bacteria Bacillus cereus isolated from industrial effluent. Afr. J. Biotechnol. 2011, 10, 15966–15972. [Google Scholar] [CrossRef]
- Mu, J.; Zhou, H.; Chen, Y.; Yang, G.; Cui, X. Reavealing a novel natural bioflocculant resource from Ruditapes philippinarum: Effectivepolysaccharides and synergisticsflocculation. Carbohydr. Polym. 2018, 186, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ben Rebah, F.; Siddeeg, S.M. Cactus an eco-friendly material for wastewater treatment: A review. JMES 2017, 8, 1770–1782. [Google Scholar]
- Ajao, V.; Bruning, H.; Rijnaartsb, H.; Temmink, H. Natural flocculants from fresh and saline wastewater: Comparative properties and flocculation performances. Chem. Eng. J. 2018, 349, 622–632. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O.; Aremu, O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 2019, 231, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, M.O.; Van Heerden, E.; Pohl, C.H.; Ashafa, A.O.T. Flocculating performance of a bioflocculant produced by Arthrobacter humicola in sewage waste water treatment. BMC Biotechnol. 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Okoh, A. Bioflocculant production by a consortium of two bacterial species and its potential application in industrial wastewater and river water treatment. Pol. J. Environ. Stud. 2014, 23, 689–696. [Google Scholar]
- Salehizadeh, H.; Yana, N.; Farnoo, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef]
- Gong, W.; Wang, S.; Sun, F.; Liu, X.W.; Yue, Q.Y.; Gao, B.Y. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008, 99, 4668–4674. [Google Scholar] [CrossRef]
- Ajao, V.; Millah, S.; Gagliano, M.C.; Bruning, H.; Rijnaart, H.; Temmink, H. Valorization of glycerol/ethanol-rich wastewater to bioflocculants: Recovery, properties, and performance. J. Harzad. Mater. 2019, 373, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kang, K.H.; Kim, D.G.; Kim, S.K. Production of polysaccharide-based bioflocculant for the synthesis of silver nanoparticles by Streptomyces sp. Int. J. Biol. Macromol. 2015, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jochen, S.; Volker, S.; Bernd, R. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 1–24. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V. Enzymatic transformations involved in the biosynthesis of microbial exo-polysaccharides based on the assembly of repeat units. Chem. Biochem. 2015, 16, 1141–1147. [Google Scholar] [CrossRef]
- Jensen, P.R.; Dwight, R.; Fenical, W. Distribution of actinomycetes in near shoretropical marine sediments. Appl. Environ. Microbiol. 1991, 57, 1102–1108. [Google Scholar] [PubMed]
- Xia, S.; Zhang, Z.; Wang, X.; Yang, A.; Chen, L.; Zhao, J.; Leonard, D.; Jaffrezic-Renault, N. Production and characterization of a bioflocculant by Proteus mirabilisTJ-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant production from Streptomyces platensis and its potential for river and waste water treatment. Braz. J. Microbiol. 2018, 49, 731–741. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.F.; Jiang, P.J.; Yang, S.L.; Liu, Z.L. Composition and characterization of microbiological flocculant SC06. Environ. Chem. 2002, 21, 360–364. [Google Scholar]
- Piyo, N.; Cosa, S.; Mabinya, L.V.; Okoh, A.I. Assessment of bioflocculant production by Bacillus sp. Gilbert, a marine bacterium isolated from the bottom sediment of Algoa Bay. Mar. Drugs 2011, 9, 1232–1242. [Google Scholar]
- Lee, S.H.; Shin, W.S.; Shin, M.C.; Choi, S.J.; Park, L.S. Improvement of water treatment performance by using polyamine flocculants. Environ. Technol. 2001, 22, 653–659. [Google Scholar] [CrossRef]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011, 27, 2559–2565. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin Phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Patil, S.V.; Bathe, G.A.; Patil, A.V.; Patil, R.H.; Salunkea, B.K. Production of bioflocculant exopolysaccharide by Bacillus subtilis. Adv. Biotechnol. 2009, 58, 15–16. [Google Scholar]
- Abu Hassan, M.A.; Tan, P.L.; Zainon, N.Z. Coagulation and Flocculation Treatment of Wastewater in Textile Industry Using Chitosan. J. Chem. Nat. Resour. Eng. 2009, 4, 43–53. [Google Scholar]
- Zulkeflee, Z.; Ahmad, Z.A.; Zulkifli, H.S.; Mohd, K.Y. Cation dependence, pH tolerance, and dosage requirement of a bioflocculant produced by Bacillus spp. UPMB13: Flocculation performance optimization through kaolin assays. Sci. World J. 2012, 2012, 495659. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W. Characteristics and culture conditions of a bioflocculant produced by Penicillium sp. Biomed. Environ. Sci. 2010, 23, 213–218. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Characterization of a Bioflocculant (MBF-UFH) Produced by Bacillus sp. AEMREG7. Int. J. Mol. Sci. 2015, 16, 12986–13003. [Google Scholar] [CrossRef]
- Jing, L.; Yun, Y.; Li, X.; Song, L. Novel bioflocculant produced by salt-tolerant, alkaliphilic strain Oceanobacillus polygoni HG6 and its application in tannery wastewater treatment. Biosci. Biotechnol. Biochem. 2017, 81, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yang, Q.; Huang, K.; Zeng, G.; Liao, D.; Liu, J. Screening and characterization of a bioflocculant produced by Aeromonas sp. Biomed. Environ. Sci. 2007, 20, 274–278. [Google Scholar]
- Wu, J.Y.; Ye, H.F. Characterization and flocculanting properties of an extracelluar biopolymer produced from a Bacillus subtilis DYU1 isolate. Proc. Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Zhang, Z.; Ling, B.; Xia, S.; Wang, X.; Yang, A. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Yokoi, H.; Natsuda, O.; Hirose, J.; Hayashi, S.; Takasaki, Y. Characteristics of biopolymer flocculant produced by Bacillus sp. PY- 90. J. Ferment. Bioeng. 1995, 79, 378–380. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, X.Q.; Guo, S.L.; Alam, M.A.; Bai, F.W. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour. Technol. 2013, 135, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, Z.; Wang, T.; Chen, Y.; Zhang, J.; Yu, J.; Zhang, T.; Zhang, Y.; Li, Y. Production of novel microbial flocculants by Klebsiella sp. TG-1 using waste residue from the food industry and its use in defecating the trona suspension. Bioresour. Technol. 2013, 139, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ye, Z.; Fang, X.; Li, Y.; Cai, W. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Biosour. Technol. 2008, 99, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, N.Z.; Hamoda, M.F.; Al-Ghusain, I. Coagulant selection and sludge conditioning in a slaughterhouse wastewater treatment plant. Bioresour. Technol. 2004, 95, 115–119. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Talukdar, D.; Chatterjee, B.P.; Guha, A.K. Whey processing with chitosan and isolation of lactose. Process Biochem. 2003, 39, 381–385. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Shriner, R.L.; Hermann, C.K.F.; Morrill, T.C.; Curtin, D.Y.; Fuson, R.C. The Systematic Identification of Organic Compounds; John Wiley & Sons: New York, NY, USA, 1998; pp. 158–161. [Google Scholar]
- Ugbenyen, A.M.; Okoh, A.I. Characteristics of a bioflocculant produced by a consortium of Cobetia and Bacillus species and its application in the treatment of wastewaters. Water SA 2014, 40, 139–144. [Google Scholar] [CrossRef]
- Kumar, C.G.; Joo, H.S.; Kavali, R.; Choi, J.W.; Chang, C.S. Characterization of an extracellular biopolymer flocculant from a haloakalophilic Bacillus isolate. World J. Microbiol. Biotechnol. 2004, 20, 837–843. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S.A. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Agunbiade, M.O.; Pohl, C.H.; Ashafa, A.O.T. A Review of the Application of Bioflocculants in Wastewater Treatment. Polish J. Environ. Stud. 2016, 25, 1381–1389. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Production and characteristics of a heavy metals removing bioflocculant produced by Pseudomonas aeruginosa. Pol. J. Microbiol. 2012, 61, 281–289. [Google Scholar]
- Wang, Y.; Gao, B.Y.; Yue, Q.Y.; Wei, J.C.; Zhou, W.Z.; Gu, R. Color removal from textile industry wastewater using composite flocculants. Environ. Technol. 2013, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Morillo, J.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sanchez, M. Production of a metal-binding exopolysaccharide by Paenibacillus jamilae using two phase olive-mill waste as fermentation substrate. Curr. Microbiol. 2006, 53, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Rai, J.P.N. Adsorption of heavy metals by Paenibacillus validus strain MP5 isolated from industrial effluent-polluted soil. Bioremed. J. 2012, 16, 66–73. [Google Scholar] [CrossRef]


| Bioflocculant Dosage (mg/mL) | (%) Flocculating Activity | ||||
|---|---|---|---|---|---|
| River | Dairy | Sewage | Meat Processed | Brewery | |
| 0.1 | 18.1 ± 1.5 | 61.0 ± 2.3 | - | - | - |
| 0.2 | 22.6 ± 1.9 | 61.7 ± 0.9 | - | - | - |
| 0.3 | 22.8 ± 0.2 | 63.0 ± 0.7 | - | - | - |
| 0.4 | 24.2 ± 2.9 | 69.8 ± 0.2 | - | - | - |
| 0.5 | 17.5 ± 0.3 | 85.1 ± 0.1 | - | - | - |
| 0.6 | 17.1 ± 1.6 | 79.4 ± 0.1 | - | - | - |
| 0.7 | 15.9 ± 0.8 | 74.0 ± 0.9 | - | - | - |
| 0.8 | 08.3 ± 2.4 | 73.5 ± 1.6 | - | - | - |
| 0.9 | 07.6 ± 1.6 | 71.7 ± 0.7 | - | - | - |
| 1.0 | 07.7 ± 1.3 | 70.8 ± 1.9 | - | - | - |
| Wastewater | pH | Turbidity (NTU) | COD (mg/L) | Nitrate (mg/L) | BOD (mg/L) | SS (mg/L) |
|---|---|---|---|---|---|---|
| Dairy | 7.21 ± 1.4 | 907 ± 0.9 | 1758 ± 2.1 | 5.24 ±1.3 | 623 ± 0.3 | 528 ± 2.4 |
| River | 7.10 ± 0.9 | 123 ± 1.6 | 170 ± 0.3 | 2.40± 0.6 | 34.0 ± 1.6 | 186 ± 1.1 |
| Meat | 8.90 ± 1.5 | 238 ± 1.9 | 356 ± 0.5 | 6.70 ± 0.4 | 305 ± 0.8 | 224 ± 0.1 |
| Sewage | 7.73 ± 0.2 | 128 ± 1.1 | 1360 ± 1.7 | 8.40 ± 0.3 | 49.2 ± 0.2 | 201 ± 1.4 |
| Brewery | 6.28 ± 1.6 | 442 ± 2.3 | 4033 ± 2.4 | 9.60 ± 1.8 | 1703 ± 2.1 | 461 ± 0.9 |
| Flocculant | Dosage (mg/mL) | BOD Removal (%) | COD Removal (%) | Turbidity Removal (%) | SS (mg/L) | F/A | Nitrate (mg/L) |
|---|---|---|---|---|---|---|---|
| SFD 11 | 0.5 | 63.3 ± 0.4 | 54.1 ± 0.5 | 89.7 ± 0.6 | 66.6 ±1.2 | 85.1 ± 0.1 | 75.6 ± 0.4 |
| PAC | 0.3 | 60.9 ± 0.7 | 43.3 ± 0.8 | 87.2 ± 1.1 | 71.2 ± 0.5 | 79.0 ± 0.6 | 68.1 ± 2.9 |
| PEI | 0.7 | 46.0 ± 1.4 | 36.0 ± 0.4 | 52.1 ± 0.5 | 43.6 ±1.4 | 56.0 ± 1.0 | 49.3 ± 1.2 |
| Alum | 1.0 | 33.4 ± 1.3 | 20.9 ± 0.6 | 43.0 ± 1.5 | 50.2 ± 0.9 | 38.1 ± 0.5 | 51.8 ± 0.8 |
| Metals | Treated Sample | Untreated Sample | (%) Removal |
|---|---|---|---|
| Aluminum | 0.029 | 0.115 | 77.7 |
| Manganese | 0.008 | 0.021 | 74.8 |
| Zinc | 0.042 | 0.099 | 61.9 |
| Iron | 0.029 | 0.130 | 57.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agunbiade, M.O.; Pohl, C.; Heerden, E.V.; Oyekola, O.; Ashafa, A. Evaluation of Fresh Water Actinomycete Bioflocculant and Its Biotechnological Applications in Wastewaters Treatment and Removal of Heavy Metals. Int. J. Environ. Res. Public Health 2019, 16, 3337. https://doi.org/10.3390/ijerph16183337
Agunbiade MO, Pohl C, Heerden EV, Oyekola O, Ashafa A. Evaluation of Fresh Water Actinomycete Bioflocculant and Its Biotechnological Applications in Wastewaters Treatment and Removal of Heavy Metals. International Journal of Environmental Research and Public Health. 2019; 16(18):3337. https://doi.org/10.3390/ijerph16183337
Chicago/Turabian StyleAgunbiade, Mayowa Oladele, Carolina Pohl, Esta Van Heerden, Oluwaseun Oyekola, and Anofi Ashafa. 2019. "Evaluation of Fresh Water Actinomycete Bioflocculant and Its Biotechnological Applications in Wastewaters Treatment and Removal of Heavy Metals" International Journal of Environmental Research and Public Health 16, no. 18: 3337. https://doi.org/10.3390/ijerph16183337
APA StyleAgunbiade, M. O., Pohl, C., Heerden, E. V., Oyekola, O., & Ashafa, A. (2019). Evaluation of Fresh Water Actinomycete Bioflocculant and Its Biotechnological Applications in Wastewaters Treatment and Removal of Heavy Metals. International Journal of Environmental Research and Public Health, 16(18), 3337. https://doi.org/10.3390/ijerph16183337






