Removal of Pollutants in Mine Wastewater by a Non-Cytotoxic Polymeric Bioflocculant from Alcaligenes faecalis HCB2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Production Medium
2.2. Source of Bacterium
2.3. Determination of Bioflocculant Production
2.4. Optimisation of Culture Conditions
2.5. Extraction and Purification of the Bioflocculant
2.6. Effect of Bioflocculant Concentration and PH on Flocculating Activity
2.7. Physical and Chemical Characteristics
2.8. Cytotoxicity Assay of Bioflocculant
2.9. Proposed Flocculation Mechanism
2.10. Removal Efficiency of Bioflocculant on Wastewater
2.11. Software and Statistical Analysis
3. Results and Discussion
3.1. Effect of Inoculum Size
3.2. Effect of Carbon and Nitrogen Sources
3.3. Effect of Metal Cations
3.4. Effect of Shaking Speed
3.5. Effect of Cultivation Temperature
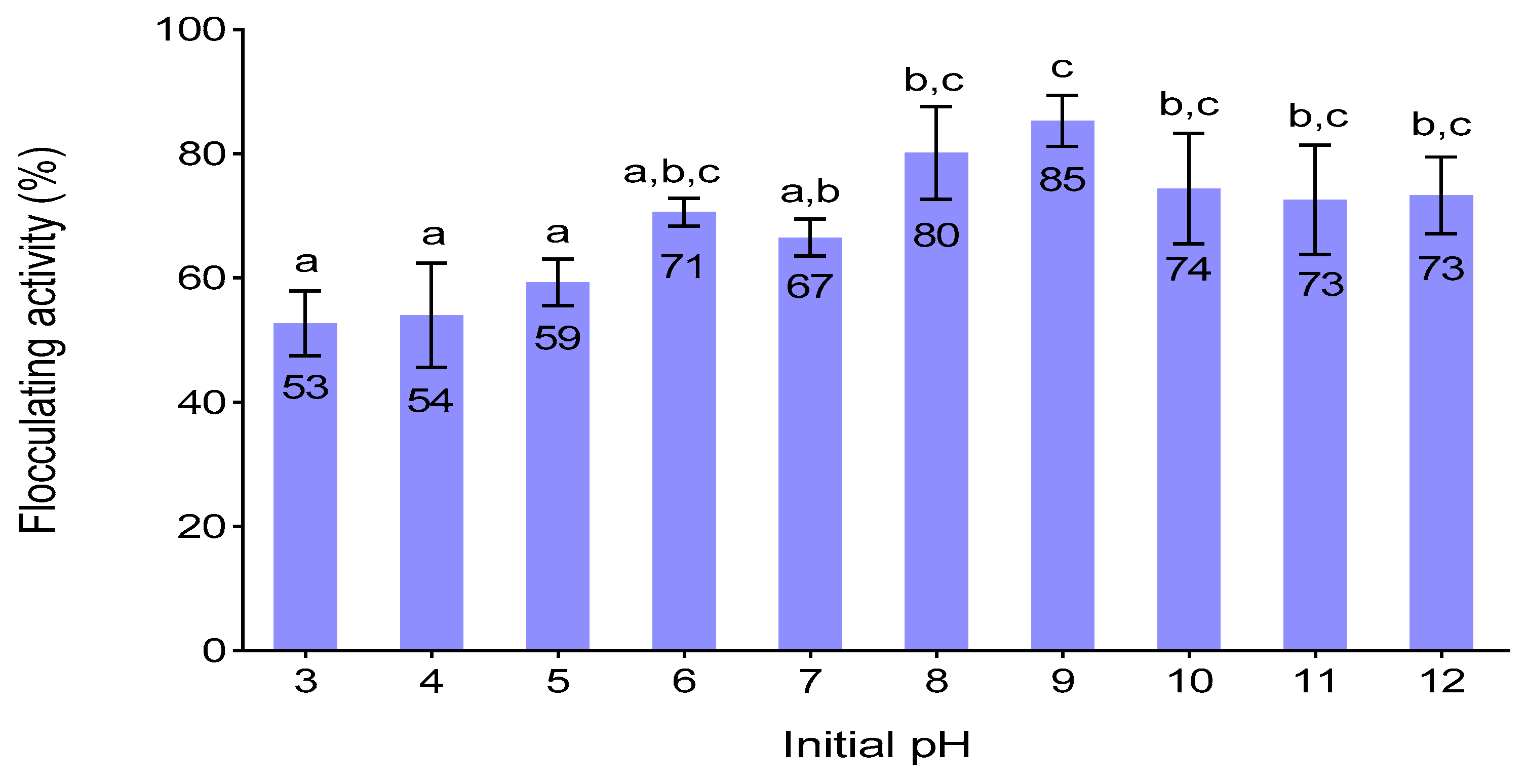
3.6. Effect of Initial PH on Flocculating Activity
3.7. Effect of Time on Flocculating Activity, Cell Number and PH
3.8. Bioflocculant Yield
3.9. Bioflocculant Dosage Size
3.10. Effect of PH on Flocculating Activity of the Purified Bioflocculant
3.11. Surface Morphological Structure
3.12. Bioflocculant Composition
3.13. Functional Groups of the Bioflocculant
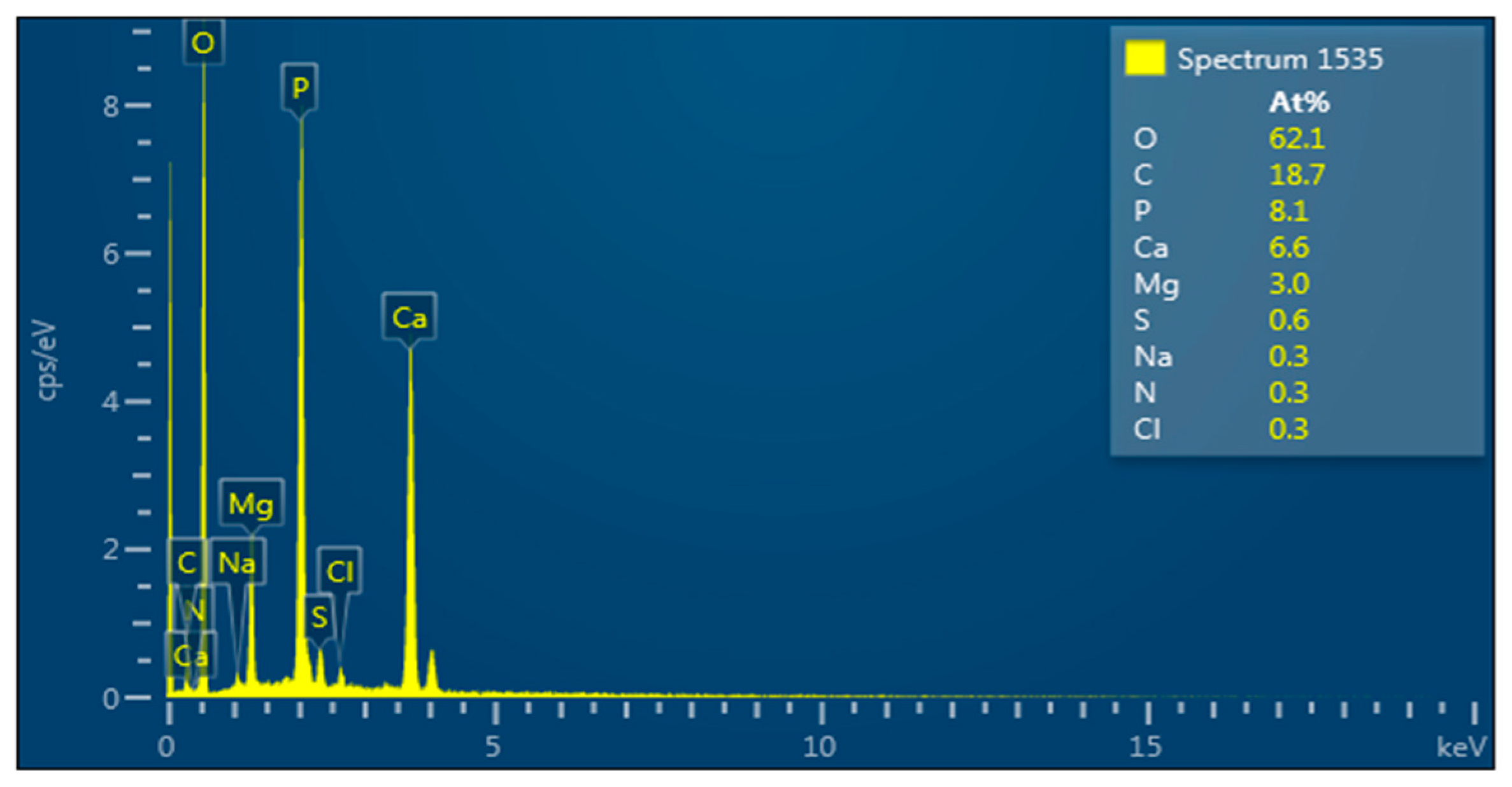
3.14. Elemental Composition of the Bioflocculant
3.15. Thermogravimetric Analysis of the Bioflocculant
3.16. Cytotoxic Effect of the Bioflocculant on HEK 293 Cell-Line
3.17. Proposed Flocculating Mechanisms of the Bioflocculant
3.18. Proposed Flocculating Mechanism of the Bioflocculant
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corcoran, E.; Nellemann, C.; Baker, E.K.; Bos, R.; Osborn, D.; Savelli, H. Sick Water? The Central Role of Wastewater Management in Sustainable Development: A Rapid Response Assessment. Available online: https://www.semanticscholar.org/paper/Sick-water.-The-central-role-of-wastewater-in-A-Corcoran-Nellemann/77b6ab256cd80d1557e5fda9a1804be77d21a971 (accessed on 28 September 2019).
- Yang, Z.; Liu, S.; Zhang, W.; Wen, Q.; Guo, Y. Enhancement of coal waste slurry flocculation by CTAB combined with bioflocculant produced by Azotobacter chroococcum. Sep. Purif. Technol. 2019, 211, 587–593. [Google Scholar] [CrossRef]
- Santschi, P.H. Marine colloids, agents of the self-cleansing capacity of aquatic systems: Historical perspective and new discoveries. Mar. Chem. 2018, 207, 124–135. [Google Scholar] [CrossRef]
- Bampole, D.L.; Bafubiandi, M. Removal Performance of Silica and Solid Colloidal Particles from Chalcopyrite Bioleaching Solution: Effect of Coagulant (Magnafloc Set# 1597) for Predicting an Effective Solvent Extraction. Eng. J. 2018, 22, 123–139. [Google Scholar]
- Abdullah, M.; Roslan, A.; Kamarulzaman, M.; Erat, M. Colloids removal from water resources using natural coagulant: Acacia auriculiformis. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Evaluation of flocculating performance of a thermostable bioflocculant produced by marine Bacillus sp. Environ. Technol. 2016, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, J. Sorption characteristics and mechanisms of Pb (II) from aqueous solution by using bioflocculant MBFR10543. Appl. Microbiol. Biotechnol. 2014, 98, 6431–6441. [Google Scholar] [CrossRef] [PubMed]
- Abu Tawila, Z.; Ismail, S.; Dadrasnia, A.; Usman, M. Production and characterization of a bioflocculant produced by Bacillus salmalaya 139SI-7 and its applications in wastewater treatment. Molecules 2018, 23, 2689. [Google Scholar] [CrossRef]
- Ntozonke, N.; Okaiyeto, K.; Okoli, A.; Olaniran, A.; Nwodo, U.; Okoh, A. A Marine Bacterium, Bacillus sp. Isolated from the Sediment Samples of Algoa Bay in South Africa Produces a Polysaccharide-Bioflocculant. Int. J. Environ. Res. Public Health 2017, 14, 1149. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Mnif, W.; M. Siddeeg, S. Microbial Flocculants as an Alternative to Synthetic Polymers for Wastewater Treatment: A Review. Symmetry 2018, 10, 556. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Shojaosadati, S. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Czemierska, M.; Szcześ, A.; Hołysz, L.; Wiater, A.; Jarosz-Wilkołazka, A. Characterisation of exopolymer R-202 isolated from Rhodococcus rhodochrous and its flocculating properties. Eur. Polym. J. 2017, 88, 21–33. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Bo, L.; Xia, S.Q.; Wang, X.J.; Yang, A.M. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Guo, H.; Hong, C.; Zhang, C.; Zheng, B.; Jiang, D.; Qin, W. Bioflocculants’ production from a cellulase-free xylanase-producing Pseudomonas boreopolis G22 by degrading biomass and its application in cost-effective harvest of microalgae. Bioresour. Technol. 2018, 255, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dih, C.C.; Jamaluddin, N.A.; Zulkeflee, Z. Removal of Heavy Metals in Lake Water Using Bioflocculant Produced by Bacillus subtilis. Pertanika J. Trop. Agric. Sci. 2019, 42, 1. [Google Scholar]
- Czemierska, M.; Szcześ, A.; Pawlik, A.; Wiater, A.; Jarosz-Wilkołazka, A. Production and characterisation of exopolymer from Rhodococcus opacus. Biochem. Eng. J. 2016, 112, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Jiang, X.; Zhang, K.; Zheng, T.; Wang, H. First evidence of bioflocculant from Shinella albus with flocculation activity on harvesting of Chlorella vulgaris biomass. Bioresour. Technol. 2016, 218, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Naresh Dholakiya, R.; Anil Kumar, M.; Mody, K.H. Recycling of starch processing industrial wastewater as a sole nutrient source for the bioflocculant production. Environ. Prog. Sustain. Energy 2017, 36, 1458–1465. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Zhou, J. Characterization of a bioflocculant MBF-5 by Klebsiella pneumoniae and its application in Acanthamoeba cysts removal. Bioresour. Technol. 2013, 137, 226–232. [Google Scholar] [CrossRef]
- Cosa, S.; Ugbenyen, A.M.; Mabinya, L.V.; Rumbold, K.; Okoh, A.I. Characterization and flocculation efficiency of a bioflocculant produced by a marine Halobacillus. Environ. Technol. 2013, 34, 2671–2679. [Google Scholar] [CrossRef]
- Choi, C.W.; Yoo, S.A.; Oh, I.H.; Park, S.H. Characterization of an extracellular flocculating substance produced by a planktonic cyanobacterium, Anabaena sp. Biotechnol. Lett. 1998, 20, 643–646. [Google Scholar] [CrossRef]
- Yu, L.; Tang, Q.W.; Zhang, Y.J.; Chen, R.P.; Liu, X.; Qiao, W.C.; Li, W.W.; Ruan, H.H.; Song, X. A novel Fe (III) dependent bioflocculant from Klebsiella oxytoca GS-4-08: Culture conditions optimization and flocculation mechanism. Sci. Rep. 2016, 6, 34980. [Google Scholar] [CrossRef]
- Natarajan, K. Production and characterization of bioflocculants for mineral processing applications. Int. J. Miner. Process. 2015, 137, 15–25. [Google Scholar]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis: A Practical Approach, 2rd ed.; IRL Press Ltd.: Oxford, UK, 1994. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rasulov, B.A.; Li, L.; Liu, Y.H.; Mohamad, O.A.; Xiao, M.; Ma, J.B.; Li, W.J. Production, characterization and structural modification of exopolysaccharide-based bioflocculant by Rhizobium radiobacter SZ4S7S14 and media optimization. 3 Biotech 2017, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Nwodo, U.; Mabinya, L.; Okoh, A. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Int. J. Environ. Res. Public Health 2013, 10, 5097–5110. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, H.; Yang, Z.; Zhang, Y.; Yu, B.; Liu, Z. Highly mesoporous carbons derived from biomass feedstocks templated with eutectic salt ZnCl 2/KCl. J. Mater. Chem. A 2014, 2, 19324–19329. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Al-joubory, H.H.R.; Uemura, Y.; Abubakar, B.I. Flocculation behavior and mechanism of bioflocculant produced by Aspergillus flavus. J. Environ. Manag. 2015, 150, 466–471. [Google Scholar] [CrossRef]
- Mohammed, J.N.; Dagang, W.R.Z.W. Development of a new culture medium for bioflocculant production using chicken viscera. MethodsX 2019, 6, 1467–1472. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Wu, Y.; Hu, C. Comparison of coagulation performance and floc properties of a novel zirconium-glycine complex coagulant with traditional coagulants. Environ. Sci. Pollut. Res. 2014, 21, 6632–6639. [Google Scholar] [CrossRef]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant production from Streptomyces platensis and its potential for river and waste water treatment. Braz. J. Microbiol. 2018, 49, 731–741. [Google Scholar] [CrossRef]
- Nwodo, U.; Agunbiade, M.; Green, E.; Nwamadi, M.; Rumbold, K.; Okoh, A. Characterization of an Exopolymeric Flocculant Produced by a Brachybacterium sp. Materials 2013, 6, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, A.E.A.; Abd-El-Haleem, D.; Youssef, A.S.; Zaki, S.; Abu-Elreesh, G.; El-Assar, S.A. Isolation, characterization, optimization, immobilization and batch fermentation of bioflocculant produced by Bacillus aryabhattai strain PSK1. J. Genet. Eng. Biotechnol. 2017, 15, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kurane, R.; Nohata, Y. Microbial flocculation of waste liquids and oil emulsion by a bioflocculant from Alcaligenes latus. Agric. Biol. Chem. 1991, 55, 1127–1129. [Google Scholar]
- Manivasagan, P.; Kang, K.H.; Kim, D.G.; Kim, S.K. Production of polysaccharide-based bioflocculant for the synthesis of silver nanoparticles by Streptomyces sp. Int. J. Biol. Macromol. 2015, 77, 159–167. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ye, H.F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtilis DYU1 isolate. Process Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Optimization and Application of Bioflocculant Passivated Copper Nanoparticles in the Wastewater Treatment. Int. J. Environ. Res. Public Health 2019, 16, 2185. [Google Scholar] [CrossRef]
- López, E.; Ramos, I.; Sanromán, M.A. Extracellular polysaccharides production by Arthrobacter viscosus. J. Food Eng. 2003, 60, 463–467. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.K.; Tripathi, V.R.; Khare, S.K.; Garg, S.K. Comparative one-factor-at-a-time, response surface (statistical) and bench-scale bioreactor level optimization of thermoalkaline protease production from a psychrotrophic Pseudomonas putida SKG-1 isolate. Microb. Cell Factories 2011, 10, 114. [Google Scholar] [CrossRef]
- Fatokun, E.; Nwodo, U.; Okoh, A. Classical optimization of cellulase and xylanase production by a marine Streptomyces species. Appl. Sci. 2016, 6, 286. [Google Scholar] [CrossRef]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.; Babalola, O.O.; Aghdasi, F.; Okoh, A. Thermostable bacterial bioflocculant produced by Cobetia spp. isolated from Algoa Bay (South Africa). Int. J. Environ. Res. Public Health 2012, 9, 2108–2120. [Google Scholar] [CrossRef]
- Subudhi, S.; Bisht, V.; Batta, N.; Pathak, M.; Devi, A.; Lal, B. Purification and characterization of exopolysaccharide bioflocculant produced by heavy metal resistant Achromobacter xylosoxidans. Carbohydr. Polym. 2016, 137, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O.; Aremu, O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 2019, 231, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Production and characteristics of a heavy metals removing bioflocculant produced by Pseudomonas aeruginosa. Pol. J. Microbiol. 2012, 61, 281–289. [Google Scholar]
- Ugbenyen, A.; Cosa, S.; Mabinya, L.; Okoh, A. Bioflocculant production by Bacillus sp. Gilbert isolated from a marine environment in South Africa. Appl. Biochem. Microbiol. 2014, 50, 49–54. [Google Scholar]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Harichund, C. Isolation and characterization of heavy metal removing bacterial bioflocculants. Afr. J. Microbiol. Res. 2011, 5, 599–607. [Google Scholar]
- Li, Z.; Zhong, S.; Lei, H.Y.; Chen, R.W.; Yu, Q.; Li, H.L. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol. 2009, 100, 3650–3656. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, Q.; Huang, K.; Zeng, G.M.; Liao, D.X.; Liu, J.J.; Long, W.F. Screening and characterization of a bioflocculant produced by Aeromonas sp. Biomed. Environ. Sci. 2007, 20, 274–278. [Google Scholar]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011, 27, 2559–2565. [Google Scholar] [CrossRef]
- Maliehe, T.; Simonis, J.; Basson, A.; Reve, M.; Ngema, S.; Xaba, P. Production, characterisation and flocculation mechanism of bioflocculant TMT-1 from marine Bacillus pumilus JX860616. Afr. J. Biotechnol. 2016, 15, 2352–2367. [Google Scholar]
- Toeda, K.; Kurane, R. Microbial flocculant from Alcaligenes cupidus KT201. Agric. Biol. Chem. 1991, 55, 2793–2799. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and characterization of a novel bioflocculant from Bacillus licheniformis. Appl. Environ. Microbiol. 2010, 76, 2778–2782. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Devi, A.; Sarma, H.K.; Lal, B. Application of bioflocculating property of Pseudomonas aeruginosa strain IASST201 in treatment of oil-field formation water. J. Basic Microbiol. 2014, 54, 658–669. [Google Scholar] [CrossRef]
- Abd-El-Haleem, D.A.; Al-Thani, R.F.; Al-Mokemy, T.; Al-Marii, S.; Hassan, F. Isolation and characterization of extracellular bioflocculants produced by bacteria isolated from Qatari ecosystems. Pol. J. Microbiol. 2008, 57, 231–239. [Google Scholar]
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591. [Google Scholar] [CrossRef]
- Pathak, M.; Devi, A.; Bhattacharyya, K.; Sarma, H.; Subudhi, S.; Lal, B. Production of a non-cytotoxic bioflocculant by a bacterium utilizing a petroleum hydrocarbon source and its application in heavy metal removal. Rsc Adv. 2015, 5, 66037–66046. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, T.; Bridle, H.; Ghosh, M. Antimicrobial efficacy and safety of mucoadhesive exopolymer produced by Acinetobacter haemolyticus. Int. J. Biol. Macromol. 2017, 94, 187–193. [Google Scholar] [CrossRef]
- Mahmoud, N.; Hend, A.; Salma, M.; Rawhia, A.; Abd, E.; Zakia, A. Bioflocculants Produced by Isolated Bacteria from Egyptian Soil II-Cytopathological Effect of Extracellular and Intracellular Bacterial Extracts. Glob. J. Biotechnol. Biochem. 2015, 10, 47–61. [Google Scholar]
- Feng, J.; Yang, Z.; Zeng, G.; Huang, J.; Xu, H.; Zhang, Y.; Wei, S.; Wang, L. The adsorption behavior and mechanism investigation of Pb (II) removal by flocculation using microbial flocculant GA1. Bioresour. Technol. 2013, 148, 414–421. [Google Scholar] [CrossRef] [PubMed]








| Inoculum Size (%) | FA (%) ± SD | Carbon Source | FA (%) ± SD | Nitrogen Source | FA (%) ± SD | Cations | FA (%) ± SD |
|---|---|---|---|---|---|---|---|
| 1 | 70.8 ± 5.50 a | Molasses | 38.6 ± 23.20 c | Yeast extract | 22.4 ± 7.05 c | Control | 49.5 ± 3.35 a,c |
| 2 | 68.5 ± 3.46 a | Sucrose | 61.4 ± 0.78 b,c | Casein | 34.0 ± 4.09 c | Na+ | 62.3 ± 7.28 a,b |
| 3 | 68.3 ± 3.52 a | Glucose | 70.7 ± 3.55 a,b | Peptone | 80.4 ± 1.25 b | Li+ | 75.4 ± 2.31 b |
| 4 | 67.4 ± 6.78 a | Starch | 79.1 ± 2.81 a,b | (NH4)2SO4 | 89.2 ± 6.88 a,b | K+ | 78.1 ± 2.52 b |
| 5 | 67.7 ± 7.46 a | Lactose | 81.2 ± 1.80 a,b | Urea | 97.4 ± 0.84 a | Mn2+ | 63.2 ± 6.78 a,b |
| Fructose | 88.1 ± 1.85 a | Ba2+ | 63.9 ± 2.08 a,b | ||||
| Maltose | 90.6 ± 2.11 a | Ca2+ | 71.2 ± 5.42 b | ||||
| Fe3+ | 31.1 ± 3.15 d |
| Dosage (mg/mL) | FA (%) ± SD |
|---|---|
| 0.2 | 80.4 ± 1.04 a |
| 0.4 | 78.7 ± 1.29 a |
| 0.6 | 78.5 ± 2.72 a |
| 0.8 | 85.6 ± 1.35 b |
| 1 | 84.8 ± 1.04 b |
| Samples | Concentration (mg/mL) |
|---|---|
| Carbohydrates | 0.886 |
| Proteins | 0.095 |
| Samples | Zeta Potential (mV) |
|---|---|
| Bioflocculant | −17.1 ± 0.7 |
| Kaolin particles | −6.59 ± 3.0 |
| Kaolin particles with K+ | −7.01 ± 1.0 |
| Kaolin particles flocculated with bioflocculant in the presence of K+ | −4.41 ± 0.7 |
| Type of Flocculants | Water Quality before Treatment | Water Quality after Treatment | Removal Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BOD (mg/L) | COD (mg/L) | S (mg/L) | BOD (mg/L) | COD (mg/L) | S (mg/L) | BOD | COD | S | |
| Bioflocculant | 6.4 ± 0.0 | 1557 ± 0.0 | 4.1 ± 0.0 | 3.2 ± 0.2 | 436 ± 2.08 | 1.03 ± 0.13 | 59 b | 72 a | 75 a |
| Alum | 6.4 ± 0.0 | 1557 ± 0.0 | 4.1 ± 0.0 | 2.9 ± 0.2 | 828 ± 1 | 1.37 ± 0.12 | 50 a | 47 b | 66 b |
| FeCl3 | 6.4 ± 0.0 | 1557 ± 0.0 | 4.1 ± 0.0 | 2.6 ± 0.58 | 753 ± 2.65 | 1.08 ± 0.12 | 54 a,b | 52 c | 73 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of Pollutants in Mine Wastewater by a Non-Cytotoxic Polymeric Bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. https://doi.org/10.3390/ijerph16204001
Maliehe TS, Basson AK, Dlamini NG. Removal of Pollutants in Mine Wastewater by a Non-Cytotoxic Polymeric Bioflocculant from Alcaligenes faecalis HCB2. International Journal of Environmental Research and Public Health. 2019; 16(20):4001. https://doi.org/10.3390/ijerph16204001
Chicago/Turabian StyleMaliehe, Tsolanku Sidney, Albertus Kotze Basson, and Nkosinathi Goodman Dlamini. 2019. "Removal of Pollutants in Mine Wastewater by a Non-Cytotoxic Polymeric Bioflocculant from Alcaligenes faecalis HCB2" International Journal of Environmental Research and Public Health 16, no. 20: 4001. https://doi.org/10.3390/ijerph16204001






