Cardiac Vagal Control, Regulatory Processes and Depressive Symptoms: Re-Investigating the Moderating Role of Sleep Quality
Abstract
:1. Introduction
1.1. Cardiac Vagal Control and Regulatory Processes
1.2. Lower CVC as a Risk Factor for Depressive Symptoms
1.3. Sleep Quality as a Protective Factor Against the Development of Depressive Symptoms in the Presence of Other Risk Factors
1.4. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. CVC
2.3.2. Self-Regulation
2.3.3. Emotion Regulation
2.3.4. Sleep Quality
2.3.5. Depressive Symptoms
2.4. Statistical Analyses
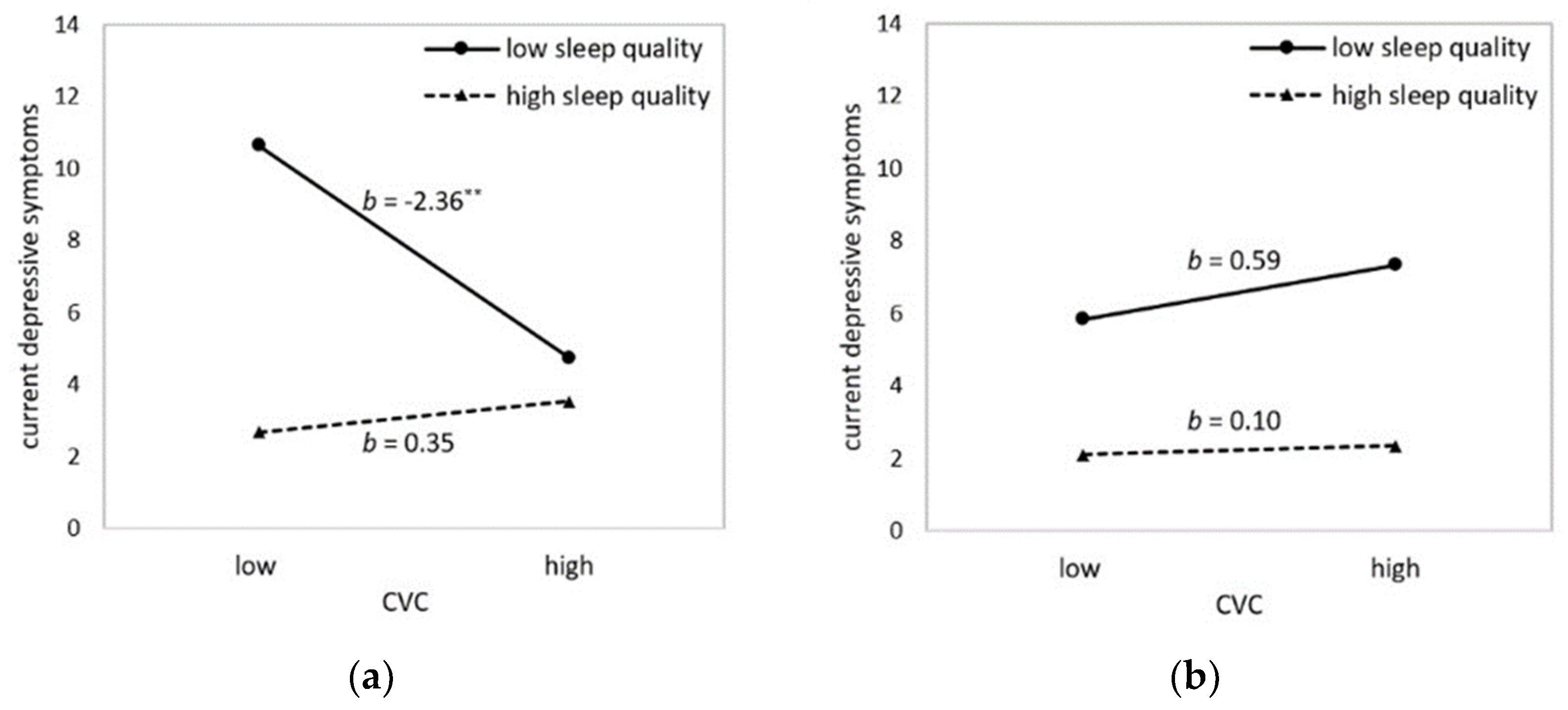
3. Results
3.1. Sleep Quality as Moderator in the Relationship between CVC and Depressive Symptoms
3.2. Sleep Quality as Moderator in the Relationship between Impaired Regulatory Processes and Depressive Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berntson, G.G.; Bigger, J.T.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Grossman, P.; Taylor, E.W. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Balzarotti, S.; Biassoni, F.; Colombo, B.; Ciceri, M.R. Cardiac vagal control as a marker of emotion regulation in healthy adults: A review. Biol. Psychol. 2017, 130, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D.; Adams, J.; Krohn, J.; Wenzel, M.; Mann, C.G.; Gomille, L.K.; Jacobi-Scherbening, V.; Kubiak, T. Heart rate variability and self-control-A meta-analysis. Biol. Psychol. 2016, 115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, J. Cardiac vagal control in depression: A critical analysis. Biol. Psychol. 2007, 74, 200–211. [Google Scholar] [CrossRef]
- Agelink, M. Autonomic neurocardiac function in patients with major depression and effects of antidepressive treatment with nefazodone. J. Affect. Disord. 2001, 62, 187–198. [Google Scholar] [CrossRef]
- Licht, C.M.M.; De Geus, E.J.C.; Zitman, F.G.; Hoogendijk, W.J.G.; Van Dyck, R.; Penninx, B.W.J.H. Association between major depressive disorder and heart rate variability in the Netherlands study of depression and anxiety (NESDA). Arch. Gen. Psychiatry 2008, 65, 1358–1367. [Google Scholar] [CrossRef]
- Gentzler, A.L.; Rottenberg, J.; Kovacs, M.; George, C.J.; Morey, J.N. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Dev. Psychobiol. 2012, 54, 556–567. [Google Scholar] [CrossRef]
- Hopp, H.; Shallcross, A.J.; Ford, B.Q.; Troy, A.S.; Wilhelm, F.H.; Mauss, I.B. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biol. Psychol. 2013, 93, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, L.; Blood, J.D.; Wu, J.; Chaplin, T.M.; Hommer, R.E.; Rutherford, H.J.V.; Potenza, M.N.; Mayes, L.C.; Crowley, M.J. High frequency heart-rate variability predicts adolescent depressive symptoms, particularly anhedonia, across one year. J. Affect. Disord. 2016, 196, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaptangco, M.; Crowell, S.E.; Baucom, B.R.; Bride, D.L.; Hansen, E.J. Examining the relation between respiratory sinus arrhythmia and depressive symptoms in emerging adults: A longitudinal study. Biol. Psychol. 2015, 110, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jandackova, V.K.; Britton, A.; Malik, M.; Steptoe, A. Heart rate variability and depressive symptoms: A cross-lagged analysis over a 10-year period in the Whitehall II study. Psychol. Med. 2016, 46, 2121–2131. [Google Scholar] [CrossRef]
- Patron, E.; Messerotti Benvenuti, S.; Favretto, G.; Valfrè, C.; Bonfà, C.; Gasparotto, R.; Palomba, D. Biofeedback Assisted Control of Respiratory Sinus Arrhythmia as a Biobehavioral Intervention for Depressive Symptoms in Patients After Cardiac Surgery: A Preliminary Study. Appl. Psychophysiol. Biofeedback 2013, 38, 1–9. [Google Scholar] [CrossRef]
- Caldwell, Y.T.; Steffen, P.R. Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int. J. Psychophysiol. 2018, 131, 96–101. [Google Scholar] [CrossRef]
- Keen, L.; Turner, A.D.; Mwendwa, D.; Callender, C.; Campbell, A. Depressive symptomatology and respiratory sinus arrhythmia in a non-clinical sample of middle-aged African Americans. Biol. Psychol. 2015, 108, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Lehofer, M.; Moser, M.; Hoehn-Saric, R.; McLeod, D.; Liebmann, P.; Drnovsek, B.; Egner, S.; Hildebrandt, G.; Zapotoczky, H.G. Major depression and cardiac autonomic control. Biol. Psychiatry 1997, 42, 914–919. [Google Scholar] [CrossRef]
- Rottenberg, J.; Wilhelm, F.H.; Gross, J.J.; Gotlib, I.H. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. J. Affect. Disord. 2002, 71, 265–272. [Google Scholar] [CrossRef]
- Baglioni, C.; Battagliese, G.; Feige, B.; Spiegelhalder, K.; Nissen, C.; Voderholzer, U.; Lombardo, C.; Riemann, D. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J. Affect. Disord. 2011, 135, 10–19. [Google Scholar] [CrossRef]
- Hertenstein, E.; Feige, B.; Gmeiner, T.; Kienzler, C.; Spiegelhalder, K.; Johann, A.; Jansson-Fröjmark, M.; Palagini, L.; Rücker, G.; Riemann, D.; et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Barber, L.K.; Munz, D.C. Consistent-sufficient sleep predicts improvements in self-regulatory performance and psychological strain. Stress Heal. 2011, 27, 314–324. [Google Scholar] [CrossRef]
- Werner, G.G.; Ford, B.Q.; Mauss, I.B.; Schabus, M.; Blechert, J.; Wilhelm, F.H. Cardiac Vagal Control and Depressive Symptoms: The Moderating Role of Sleep Quality. Behav. Sleep Med. 2016, 15, 451–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silk, J.S.; Vanderbilt-Adriance, E.; Shaw, D.S.; Forbes, E.E.; Whalen, D.J.; Ryan, N.D.; Dahl, R.E. Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Dev. Psychopathol. 2007, 19, 841–865. [Google Scholar] [CrossRef] [Green Version]
- El-Sheikh, M.; Erath, S.A.; Keller, P.S. Children’s sleep and adjustment: The moderating role of vagal regulation. J. Sleep Res. 2007, 16, 396–405. [Google Scholar] [CrossRef]
- Berking, M.; Wirtz, C.M.; Svaldi, J.; Hofmann, S.G. Emotion regulation predicts symptoms of depression over five years. Behav. Res. Ther. 2014, 57, 13–20. [Google Scholar] [CrossRef]
- Fehlinger, T.; Stumpenhorst, M.; Stenzel, N.; Rief, W. Emotion regulation is the essential skill for improving depressive symptoms. J. Affect. Disord. 2013, 144, 116–122. [Google Scholar] [CrossRef]
- Radkovsky, A.; McArdle, J.J.; Bockting, C.L.H.; Berking, M. Successful emotion regulation skills application predicts subsequent reduction of symptom severity during treatment of major depressive disorder. J. Consult. Clin. Psychol. 2014, 82, 248–262. [Google Scholar] [CrossRef]
- Williams, D.P.; Tracy, L.M.; Gerardo, G.M.; Rahman, T.; Spangler, D.P.; Koenig, J.; Thayer, J.F. Sex moderates the relationship between resting heart rate variability and self-reported difficulties in emotion regulation. Emotion 2018, 19, 992–1001. [Google Scholar] [CrossRef]
- Blechert, J.; Peyk, P.; Liedlgruber, M.; Wilhelm, F.H. ANSLAB: Integrated multichannel peripheral biosignal processing in psychophysiological science. Behav. Res. Methods 2016, 48, 1528–1545. [Google Scholar] [CrossRef]
- Wilhelm, F.H.; Grossman, P.; Roth, W.T. Analysis of cardiovascular regulation. Biomed. Sci. Instrum. 1999, 35, 135–140. [Google Scholar] [PubMed]
- Schwarzer, R. Selbstregulation (REG). In Skalen zur Erfassung von Lehrer- und Schülermerkmalen. Dokumentation der Psychometrischen Verfahren im Rahmen der Wissenschaftlichen Begleitung des Modellversuchs Selbstwirksame Schulen; Schwarzer, R., Jerusalem, M., Eds.; Freie Universität Berlin: Berlin, Germany, 1999; pp. 92–93. [Google Scholar]
- Ehring, T.; Fischer, S.; Schnülle, J.; Bösterling, A.; Tuschen-Caffier, B. Characteristics of emotion regulation in recovered depressed versus never depressed individuals. Pers. Individ. Dif. 2008, 44, 1574–1584. [Google Scholar] [CrossRef] [Green Version]
- Gratz, K.L.; Roemer, L. Multidimensional Assessment of Emotion Regulation and Dysregulation: Development, Factor Structure, and Initial Validation of the Difficulties in Emotion Regulation Scale. J. Psychopathol. Behav. Assess. 2004, 26, 41–54. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1988, 28, 193–213. [Google Scholar] [CrossRef]
- Riemann, D.; Backhaus, J. Behandlung von Schlafstörungen: Ein psychologisches Gruppenprogramm (Treatment of Sleep Disorders); Beltz Psychologie Verlags Union: Weilheim, Germany, 1996. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Kühner, C.; Bürger, C.; Keller, F.; Hautzinger, M. Reliabilität und Validität des revidierten Beck-Depressionsinventars (BDI-II). Nervenarzt 2007, 78, 651–656. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 2nd ed.; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Long, J.S.; Ervin, L.H. Using Heteroscedasticity Consistent Standard Errors in the Linear Regression Model. Am. Stat. 2000, 54, 217–224. [Google Scholar]
- Hayes, A.F.; Cai, L. Using heteroskedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behav. Res. Methods 2007, 39, 709–722. [Google Scholar] [CrossRef] [Green Version]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Berking, M.; Orth, U.; Wupperman, P.; Meier, L.L.; Caspar, F. Prospective effects of emotion-regulation skills on emotional adjustment. J. Couns. Psychol. 2008, 55, 485–494. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Hatzenbuehler, M.L.; Mennin, D.S.; Nolen-Hoeksema, S. Emotion dysregulation and adolescent psychopathology: A prospective study. Behav. Res. Ther. 2011, 49, 544–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorber, S.C.; Tremblay, M.; Moher, D.; Gorber, B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obes. Rev. 2007, 8, 307–326. [Google Scholar] [CrossRef] [PubMed]

| Variables | M | SD | Range | HF-HRV | REG | DERS | PSQI | BDI-II‘1 | BDI-II‘2 |
|---|---|---|---|---|---|---|---|---|---|
| Primary study variables | |||||||||
| CVC(HF-HRV; ms2) a | 7.36 | 1.25 | - | - | - | - | - | - | |
| Self-regulation (REG) b | 30.86 | 4.16 | 10–40 | 0.18 + | - | - | - | - | - |
| Emotion regulation (DERS) b | 70.62 | 16.89 | 36–180 | −0.19 * | −0.65 *** | - | - | - | - |
| Sleep quality (PSQI) b | 4.64 | 2.18 | 0–21 | −0.17 + | −0.28 ** | 0.36 *** | - | - | - |
| Depression T1 (BDI-II‘1) b | 5.45 | 5.32 | 0–57 | −0.11 | −0.50 *** | 0.64 *** | 0.57 *** | - | - |
| Depression T2 (BDI-II‘2) c | 5.23 | 5.77 | 0–57 | 0.01 | −0.32 ** | 0.43 *** | 0.36 *** | 0.62 *** | - |
| Control variables | |||||||||
| Age (years) b | 23.58 | 4.38 | −0.05 | −0.04 | −0.05 | −0.14 | −0.09 | −0.06 | |
| Body mass index (BMI, kg/m2) b | 21.94 | 3.09 | 0.15 | −0.10 | 0.04 | −0.01 | −0.04 | −0.04 |
| Predictors | b | SE (HC3) | t | p | 95%CI (l; u) |
|---|---|---|---|---|---|
| Cross-sectionala | |||||
| Model C1 | |||||
| CVC (HF-HRV) | −0.51 | 0.36 | −1.41 | 0.160 | −1.22; 0.20 |
| Sleep quality (PSQI) | 0.98 | 0.18 | 5.53 | <0.001 *** | 0.63; 1.33 |
| CVC x sleep quality | −0.24 | 0.15 | −1.57 | 0.120 | −0.54; 0.06 |
| Model C2 | |||||
| CVC (HF-HRV) | −1.01 | 0.49 | −2.06 | 0.042 * | −1.98; −0.04 |
| Sleep quality (PSQI) | 1.06 | 0.23 | 4.53 | <0.001 *** | 0.60; 1.52 |
| CVC x sleep quality | −0.62 | 0.25 | −2.55 | 0.012 * | −1.11; −0.14 |
| Sex | −0.99 | 0.77 | −1.29 | 0.201 | −2.51; 0.53 |
| Sex x CVC | 1.35 | 0.71 | 1.91 | 0.059 + | −0.05; 2.76 |
| Sex x PSQI | −0.05 | 0.41 | −0.13 | 0.899 | −0.86; 0.76 |
| Sex x CVC x PSQI | 0.74 | 0.39 | 1.91 | 0.059 + | −0.03; 1.50 |
| Longitudinalb | |||||
| Model L1 | |||||
| Depression T1 (BDI-II‘1) | 0.78 | 0.25 | 3.10 | 0.003 ** | 0.28; 1.28 |
| CVC (HF-HRV) | 0.54 | 0.44 | 1.23 | 0.222 | −0.34; 1.43 |
| Sleep quality (PSQI) | 0.07 | 0.35 | −0.20 | 0.842 | −0.63; 0.77 |
| CVC × sleep quality | 0.17 | 0.26 | 0.67 | 0.505 | −0.34; 0.68 |
| Model L2 | |||||
| Depression T1 (BDI-II‘1) | 0.72 | 0.25 | 2.86 | 0.006 ** | 0.22; 1.22 |
| CVC (HF-HRV) | −0.07 | 0.50 | −0.14 | 0.893 | −1.05; 0.91 |
| Sleep quality (PSQI) | 0.09 | 0.45 | 0.19 | 0.848 | −0.81; 0.98 |
| CVC × sleep quality | 0.07 | 0.55 | 0.12 | 0.902 | −1.03; 1.16 |
| Sex | −0.93 | 1.61 | −0.58 | 0.567 | −4.14; 2.29 |
| Sex × CVC | 1.67 | 1.61 | 1.04 | 0.301 | −1.53; 4.89 |
| Sex × sleep quality | 0.23 | 1.05 | 0.22 | 0.829 | −1.86; 2.32 |
| Sex × CVC × sleep quality | 0.22 | 0.94 | 0.23 | 0.818 | −1.66; 2.10 |
| Predictors | b | SE (HC3) | t | P | 95% CI(l; u) |
|---|---|---|---|---|---|
| Cross-sectional a | |||||
| Model C3 | |||||
| Self-regulation (REG) | −0.67 | 0.13 | −4.96 | <0.001 *** | −0.93; −0.40 |
| Sleep quality (PSQI) | 0.91 | 0.19 | 4.83 | <0.001 *** | 0.53; 1.28 |
| Self-regulation × sleep quality | −0.16 | 0.06 | −2.87 | 0.005 ** | −0.27; −0.05 |
| Model C4 | |||||
| Emotion regulation (DERS) | 0.19 | 0.03 | 6.27 | <0.001 *** | 0.13; 0.25 |
| Sleep quality (PSQI) | 0.72 | 0.16 | 4.53 | <0.001 *** | 0.41; 1.04 |
| Emotion regulation × sleep quality | 0.03 | 0.01 | 2.64 | 0.009 ** | 0.01; 0.05 |
| Longitudinal b | |||||
| Model L3 | |||||
| Depression T1 (BDI-II’1) | 0.74 | 0.19 | 3.87 | <0.001 *** | 0.36; 1.12 |
| Self-regulation (REG) | −0.04 | 0.14 | −0.31 | 0.759 | −0.33; 0.24 |
| Sleep quality (PSQI) | −0.06 | 0.33 | −0.18 | 0.855 | −0.71; 0.59 |
| Self-regulation × sleep quality | 0.06 | 0.07 | 0.91 | 0.364 | −0.07; 0.20 |
| Model L4 | |||||
| Depression T1 (BDI-II’1) | 0.69 | 0.20 | 3.44 | <0.001 *** | 0.29; 1.09 |
| Emotion regulation (DERS) | 0.04 | 0.04 | 0.83 | 0.412 | −0.05; 0.13 |
| Sleep quality (PSQI) | −0.03 | 0.33 | −0.10 | 0.922 | −0.68; 0.62 |
| Emotion regulation × sleep quality | −0.02 | 0.02 | −0.95 | 0.347 | −0.05; 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danböck, S.K.; Werner, G.G. Cardiac Vagal Control, Regulatory Processes and Depressive Symptoms: Re-Investigating the Moderating Role of Sleep Quality. Int. J. Environ. Res. Public Health 2019, 16, 4067. https://doi.org/10.3390/ijerph16214067
Danböck SK, Werner GG. Cardiac Vagal Control, Regulatory Processes and Depressive Symptoms: Re-Investigating the Moderating Role of Sleep Quality. International Journal of Environmental Research and Public Health. 2019; 16(21):4067. https://doi.org/10.3390/ijerph16214067
Chicago/Turabian StyleDanböck, Sarah K., and Gabriela G. Werner. 2019. "Cardiac Vagal Control, Regulatory Processes and Depressive Symptoms: Re-Investigating the Moderating Role of Sleep Quality" International Journal of Environmental Research and Public Health 16, no. 21: 4067. https://doi.org/10.3390/ijerph16214067





