In Plasma Catalytic Oxidation of Toluene Using Monolith CuO Foam as a Catalyst in a Wedged High Voltage Electrode Dielectric Barrier Discharge Reactor: Influence of Reaction Parameters and Byproduct Control
Abstract
:1. Introduction
2. Materials and Methods
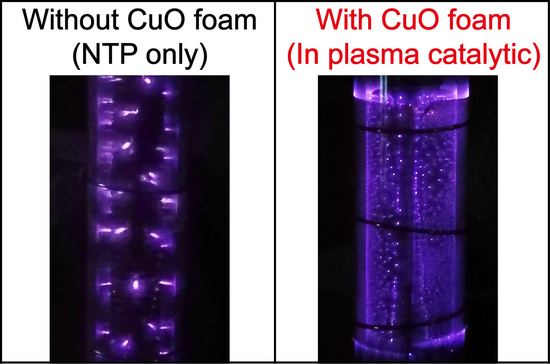
2.1. Experimental Setup of the IPC System
2.2. Preparation and Characterization of CuO Foam
2.3. Toluene Removal and Evaluation
3. Results and Discussion
3.1. Characterization of CuO Foam
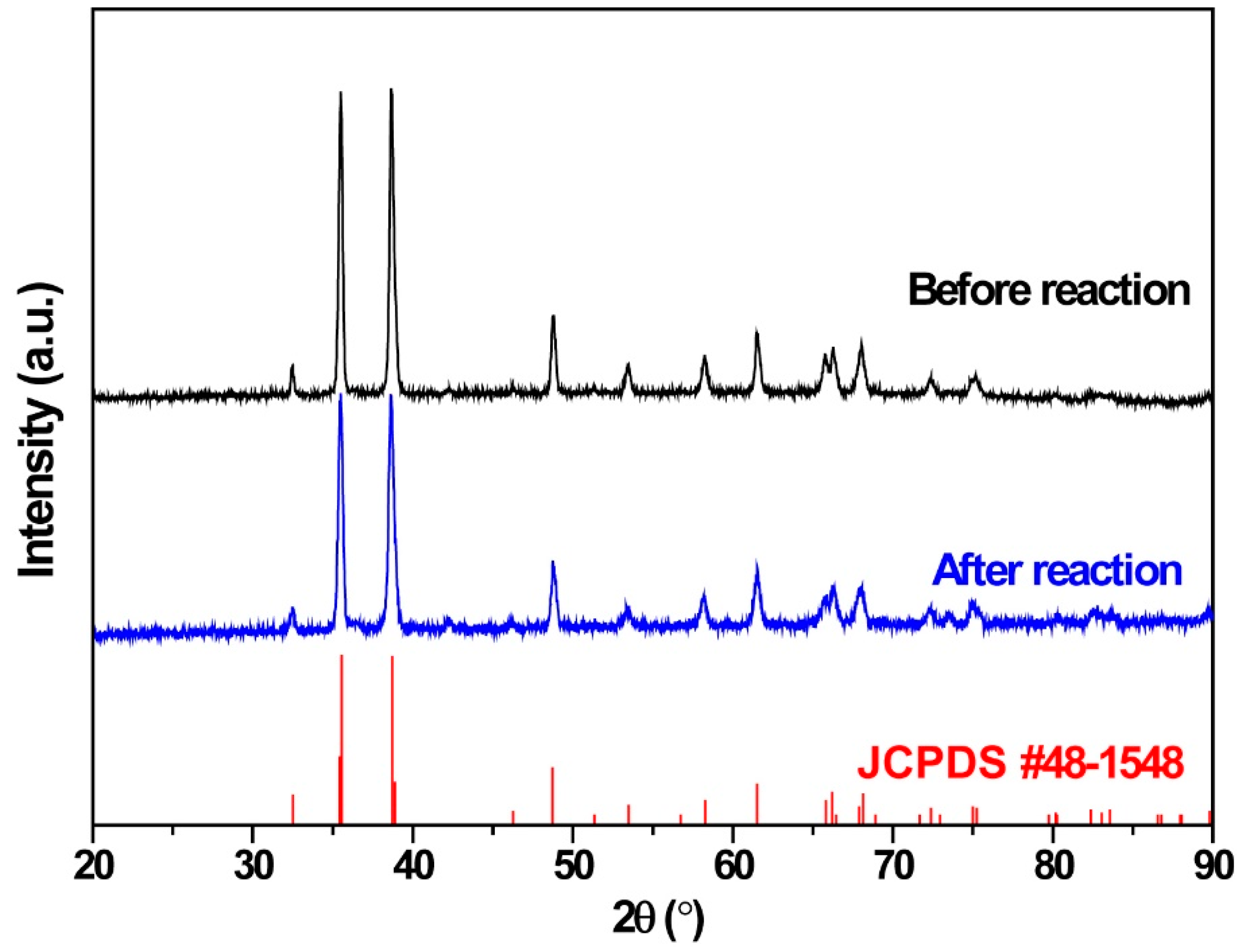
3.1.1. XRD of CuO Foam
3.1.2. FESEM of CuO Foam
3.2. Effect of Catalyst Loadings and Applied Power
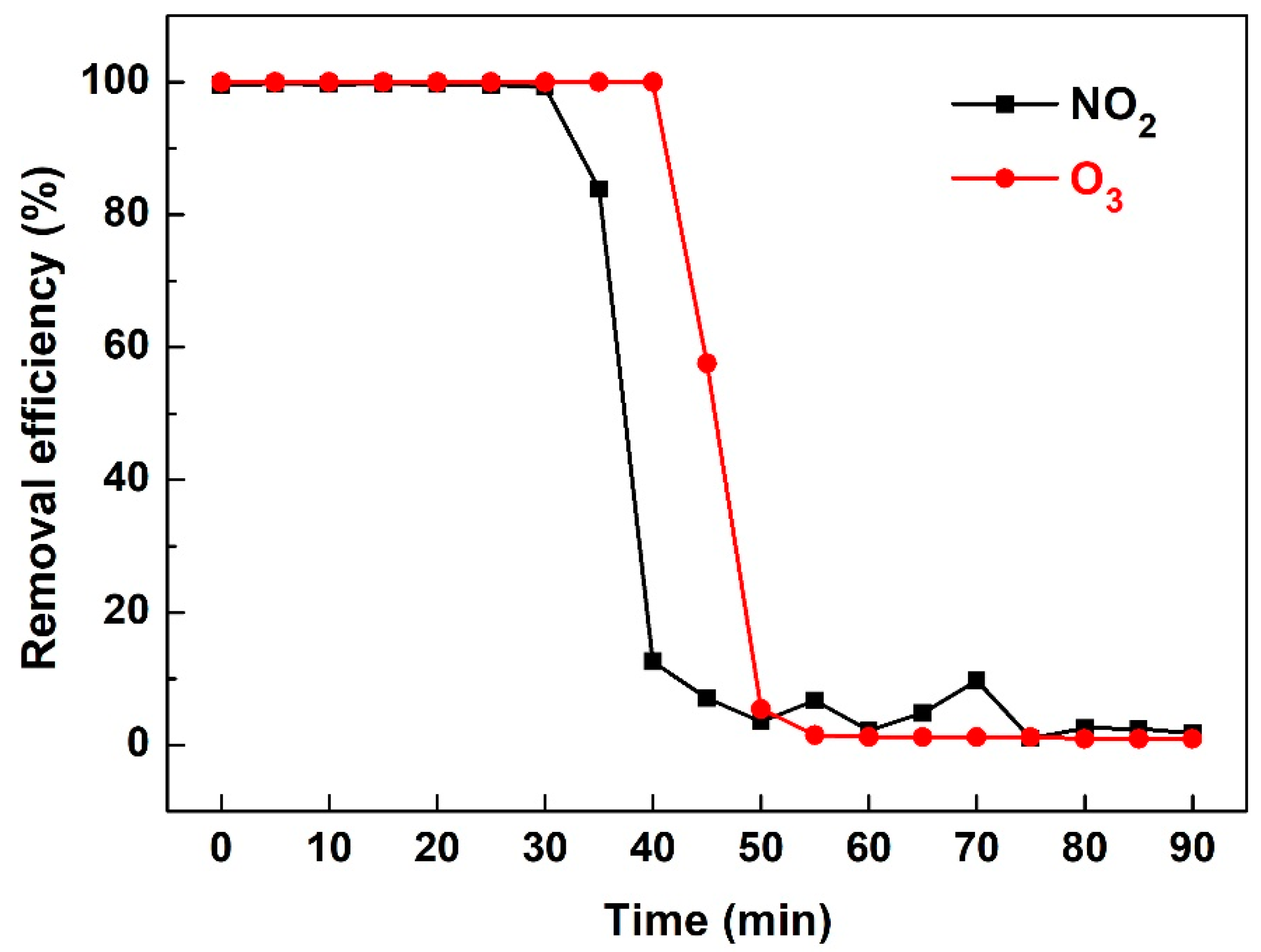
3.3. NOx and O3 Production
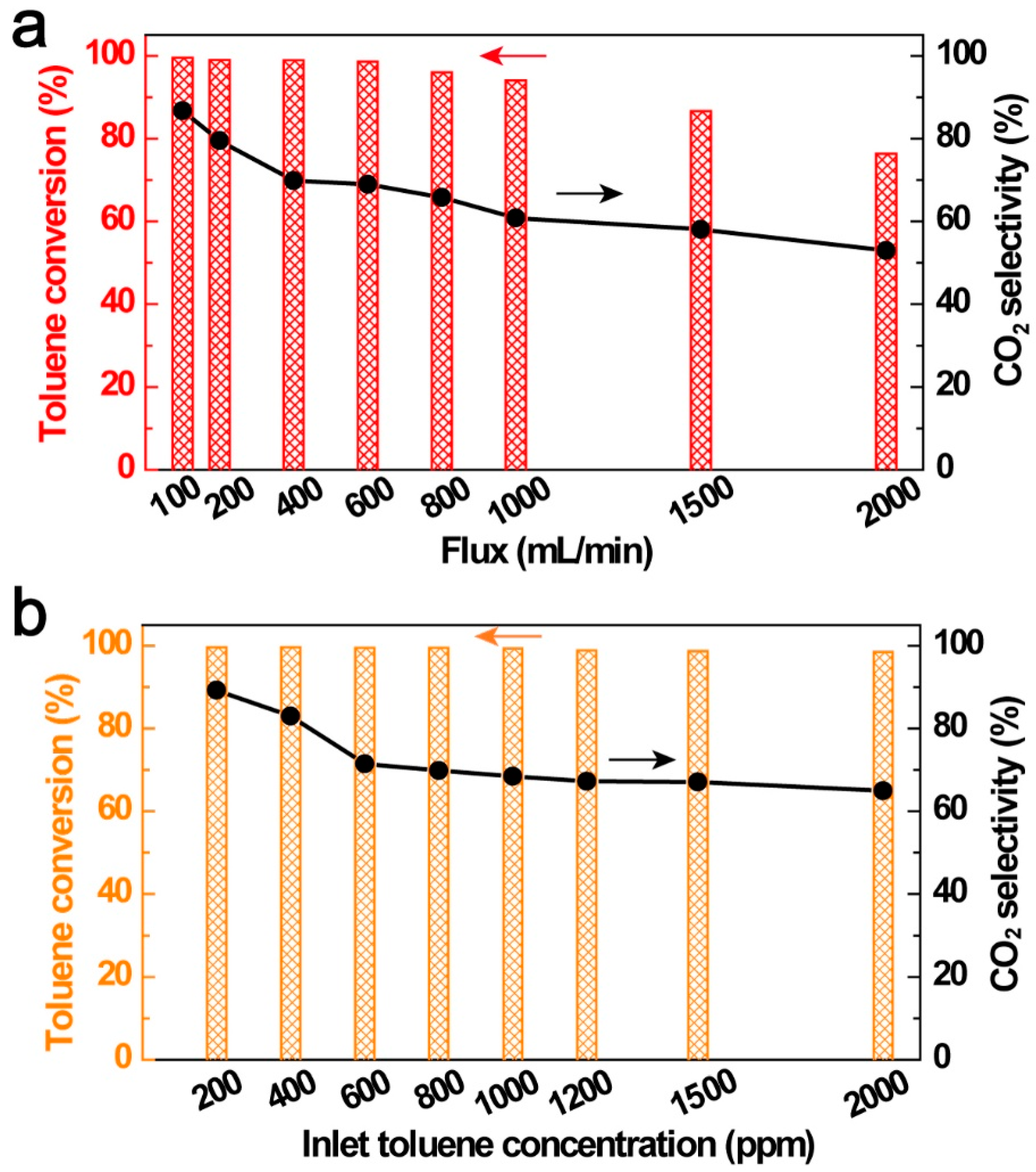
3.4. Influence of VOC Initial Concentration and Stream Flux
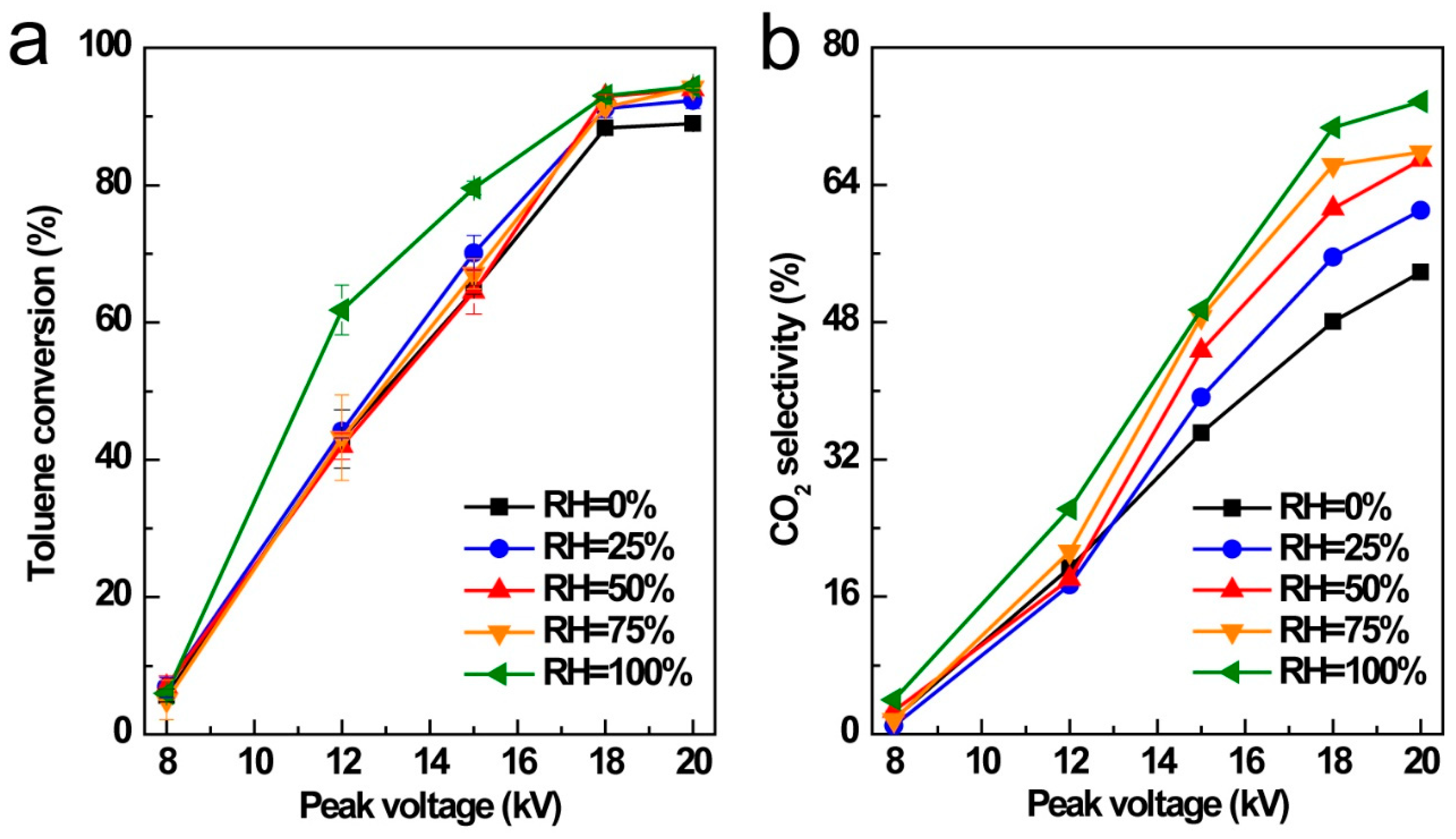
3.5. Influence of Relative Humidity (RH)
3.6. Degradation Mechanism
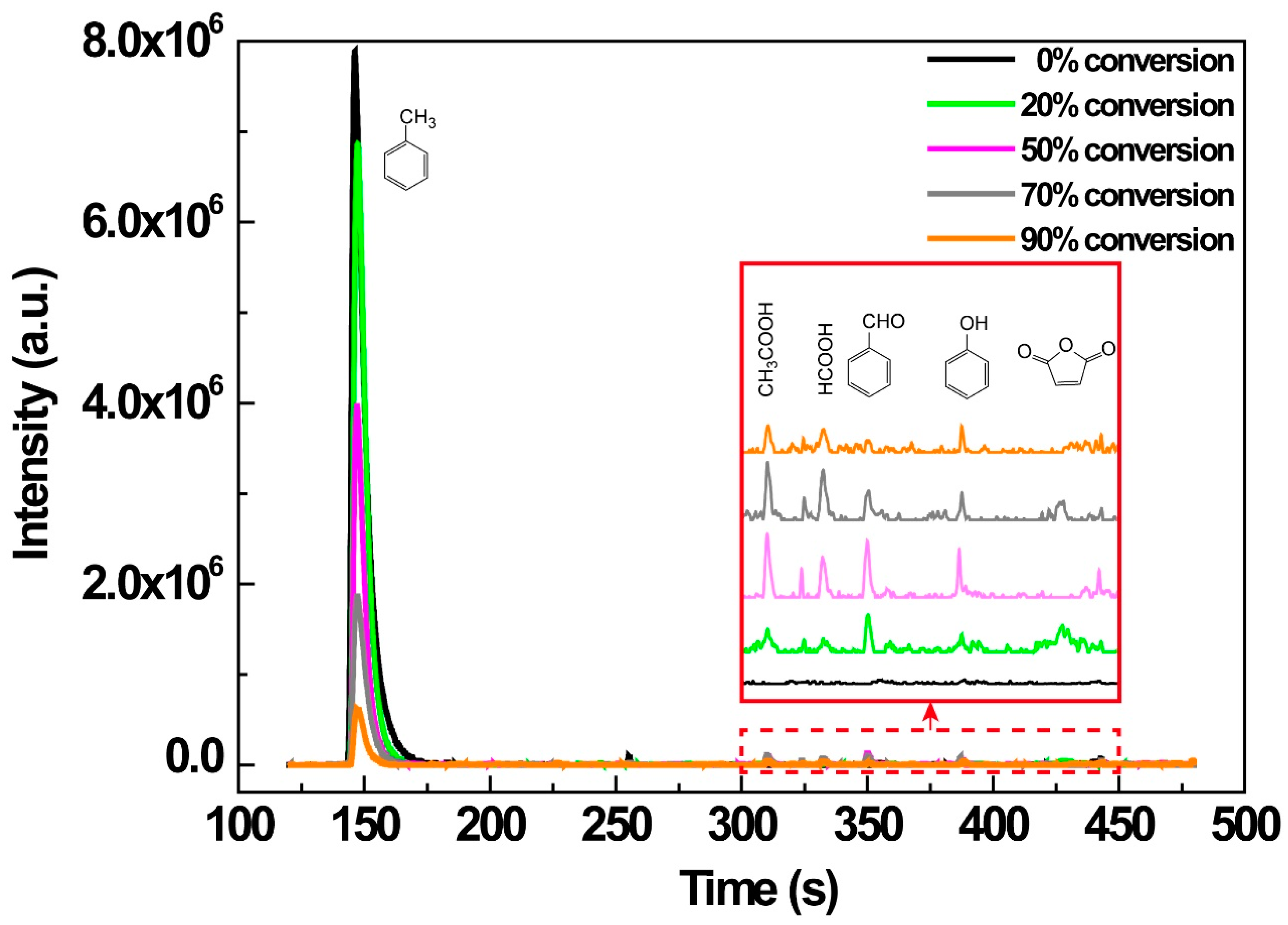
3.7. Byproducts Control
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Ciaparra, D.; Aries, E.; Booth, M.-J.; Anderson, D.R.; Almeida, S.M.; Harrad, S. Characterisation of volatile organic compounds and polycyclic aromatic hydrocarbons in the ambient air of steelworks. Atmos. Environ. 2009, 43, 2070–2079. [Google Scholar] [CrossRef]
- Shi, J.; Deng, H.; Bai, Z.; Kong, S.; Wang, X.; Hao, J.; Han, X.; Ning, P. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515-516, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.M.; Rohr, A.C.; Mikheev, V.B.; Munson, J.; Sabo-Attwood, T. Influence of airborne particulates on respiratory tract deposition of inhaled toluene and naphthalene in the rat. Inhalat. Toxicol. 2018, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.B.; Tagiyeva, N.; Semple, S.; Devereux, G.; Sheikh, A. Volatile organic compounds and risk of asthma and allergy: A systematic review. Eur. Respir. Rev. 2015, 24, 92–101. [Google Scholar] [CrossRef] [PubMed]
- El-Hashemy, M.A.; Ali, H.M. Characterization of BTEX group of VOCs and inhalation risks in indoor microenvironments at small enterprises. Sci. Total Environ. 2018, 645, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Fakhri, Y.; Oliveri Conti, G.; Ferrante, M.; Taghavi, M.; Tavakoli, J.; Heshmati, A.; Keramati, H.; Moradi, B.; Amanidaz, N.; et al. The Concentration of BTEX in the Air of Tehran: A Systematic Review-Meta Analysis and Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1837. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Li, Q.; Zhou, B.; Huang, J.; Liang, G.; Zhang, L.e.; Ma, S.; Qing, L.; Liang, L.; Su, J.; et al. Oxidative Stress and Genotoxicity of Long-Term Occupational Exposure to Low Levels of BTEX in Gas Station Workers. Int. J. Environ. Res. Public Health 2016, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, J.; Hao, Y.; Li, Y.; Zeng, L.; Xie, S. Evolution process and sources of ambient volatile organic compounds during a severe haze event in Beijing, China. Sci. Total Environ. 2016, 560-561, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Garmash, O.; Bianchi, F.; Zheng, J.; Yan, C.; Kontkanen, J.; Junninen, H.; Mazon, S.B.; Ehn, M.; Paasonen, P.; et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science 2018, 361, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, F.; Hu, B.; Tang, G.; Zhang, J.; Wang, Y. VOC characteristics, emissions and contributions to SOA formation during hazy episodes. Atmos. Environ. 2016, 141, 560–570. [Google Scholar] [CrossRef]
- Ahlberg, E.; Falk, J.; Eriksson, A.; Holst, T.; Brune, W.H.; Kristensson, A.; Roldin, P.; Svenningsson, B. Secondary organic aerosol from VOC mixtures in an oxidation flow reactor. Atmos. Environ. 2017, 161, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.-D.-H.; Lin, C.; Weng, C.-E.; Yuan, C.-S.; Lee, C.-W.; Hung, C.-H.; Bui, X.-T.; Lo, K.-C.; Lin, J.-X. Vertical stratification of volatile organic compounds and their photochemical product formation potential in an industrial urban area. J. Environ. Manag. 2018, 217, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Liu, T.; Zhang, Y.; Situ, S.; Hu, Q.; He, Q.; Zhang, Z.; Lü, S.; Bi, X.; Wang, X.; et al. Secondary organic aerosol formation from photo-oxidation of toluene with NOx and SO2: Chamber simulation with purified air versus urban ambient air as matrix. Atmos. Environ. 2017, 150, 67–76. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; White, S.J.; Han, K.; Lv, B.; Bao, K.; Wu, X.; Gao, X.; Azzi, M.; Cen, K. Effect of nitrogen oxides (NO and NO2) and toluene on SO2 photooxidation, nucleation and growth: A smog chamber study. Atmos. Res. 2017, 192, 38–47. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J.; Hupka, J. Treatment of malodorous air in biotrickling filters: A review. Biochem. Eng. J. 2019, 141, 146–162. [Google Scholar] [CrossRef]
- Dobslaw, D.; Schöller, J.; Krivak, D.; Helbich, S.; Engesser, K.-H. Performance of different biological waste air purification processes in treatment of a waste gas mix containing tert-butyl alcohol and acetone: A comparative study. Chem. Eng. J. 2019, 355, 572–585. [Google Scholar] [CrossRef]
- Mokhtari, M.; Hajizadeh, Y.; Ebrahimi, A.A.; Shahi, M.A.; Jafari, N.; Abdolahnejad, A. Enhanced biodegradation of n-hexane from the air stream using rhamnolipid in a biofilter packed with a mixture of compost, scoria, sugar beet pulp and poplar tree skin. Atmos. Pollut. Res. 2019, 10, 115–122. [Google Scholar] [CrossRef]
- Du, C.; Lu, S.; Wang, Q.; Buekens, A.G.; Ni, M.; Debecker, D.P. A review on catalytic oxidation of chloroaromatics from flue gas. Chem. Eng. J. 2018, 334, 519–544. [Google Scholar] [CrossRef] [Green Version]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Favre, E. Energy efficiency of a hybrid membrane/condensation process for VOC (Volatile Organic Compounds) recovery from air: A generic approach. Energy 2016, 95, 291–302. [Google Scholar] [CrossRef]
- Dobslaw, D.; Schulz, A.; Helbich, S.; Dobslaw, C.; Engesser, K.-H. VOC removal and odor abatement by a low-cost plasma enhanced biotrickling filter process. J. Environ. Chem. Eng. 2017, 5, 5501–5511. [Google Scholar] [CrossRef]
- Wei, Z.S.; Li, H.Q.; He, J.C.; Ye, Q.H.; Huang, Q.R.; Luo, Y.W. Removal of dimethyl sulfide by the combination of non-thermal plasma and biological process. Bioresou. Technol. 2013, 146, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhu, R.; Mao, Y.; Chen, J.; Zhang, L. Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment. Int. J. Environ. Res. Public Health 2015, 12, 1334–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Zhao, Y.; Yang, J.; Zhang, S.; Zhang, J.; Zheng, C. Research progress of pollutants removal from coal-fired flue gas using non-thermal plasma. Renew. Sustain. Energy Rev. 2017, 67, 791–810. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
- Anaghizi, S.J.; Talebizadeh, P.; Rahimzadeh, H.; Ghomi, H. The Configuration Effects of Electrode on the Performance of Dielectric Barrier Discharge Reactor for NOx Removal. IEEE Trans. Plasma Sci. 2015, 43, 1944–1953. [Google Scholar] [CrossRef]
- Trinh, H.Q.; Mok, Y.S. Plasma-catalytic oxidation of acetone in annular porous monolithic ceramic-supported catalysts. Chem. Eng. J. 2014, 251, 199–206. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Yang, Y.; Zheng, C.; Zhou, J.; Gao, X.; Tu, X. Enhanced performance for plasma-catalytic oxidation of ethyl acetate over La1-xCexCoO3+δ catalysts. Appl. Catal. B Environ. 2017, 213, 97–105. [Google Scholar] [CrossRef]
- Zhang, H.; Li, K.; Li, L.; Liu, L.; Meng, X.; Sun, T.; Jia, J.; Fan, M. High efficient styrene mineralization through novel NiO-TiO2-Al2O3 packed pre-treatment/treatment/post-treatment dielectric barrier discharge plasma. Chem. Eng. J. 2018, 343, 759–769. [Google Scholar] [CrossRef]
- Huang, H.; Ye, D.; Leung, D.Y.C.; Feng, F.; Guan, X. Byproducts and pathways of toluene destruction via plasma-catalysis. J. Mol. Catal. A Chem. 2011, 336, 87–93. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Ye, D.-Q.; Chen, K.-F.; He, J.-C.; Chen, W.-L. Toluene decomposition using a wire-plate dielectric barrier discharge reactor with manganese oxide catalyst in situ. J. Mol. Catal. A Chem. 2006, 245, 93–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, X.; Lü, S.; Huang, Z.; Huang, X.; Yang, W.; Wang, Y.; Zhang, Q. Spatiotemporal patterns and source implications of aromatic hydrocarbons at six rural sites across China’s developed coastal regions. J. Geophys. Res. Atmos. 2016, 121, 6669–6687. [Google Scholar] [CrossRef]
- Mustafa, M.F.; Fu, X.; Liu, Y.; Abbas, Y.; Wang, H.; Lu, W. Volatile organic compounds (VOCs) removal in non-thermal plasma double dielectric barrier discharge reactor. J. Hazard. Mater. 2018, 347, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ayesh, A.I.; Ahmed, R.E.; Al-Rashid, M.A.; Alarrouqi, R.A.; Saleh, B.; Abdulrehman, T.; Haik, Y.; Al-Sulaiti, L.A. Selective gas sensors using graphene and CuO nanorods. Sens. Actuators A Phys. 2018, 283, 107–112. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Ye, D.-Q.; Chen, K.-F.; He, J.-C. Toluene removal by a DBD-type plasma combined with metal oxides catalysts supported by nickel foam. Catal. Today 2007, 126, 328–337. [Google Scholar] [CrossRef]
- Xu, N.; Fu, W.; He, C.; Cao, L.; Liu, X.; Zhao, J.; Pan, H. Benzene Removal Using Non-thermal Plasma with CuO/AC Catalyst: Reaction Condition Optimization and Decomposition Mechanism. Plasma Chem. Plasma Process. 2014, 34, 1387–1402. [Google Scholar] [CrossRef]
- Ondarts, M.; Hajji, W.; Outin, J.; Bejat, T.; Gonze, E. Non-Thermal Plasma for indoor air treatment: Toluene degradation in a corona discharge at ppbv levels. Chem. Eng. Res. Des. 2017, 118, 194–205. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Li, J.; Jin, Y. Abatement of toluene from gas streams via ferro-electric packed bed dielectric barrier discharge plasma. J. Hazard. Mater. 2009, 170, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Shen, Z.; Huang, Y.; Lu, J.; Ren, D.; Sun, J.; Cao, J.; Liu, H. Post-plasma-catalytic removal of toluene using MnO2–Co3O4 catalysts and their synergistic mechanism. Chem. Eng. J. 2018, 348, 15–25. [Google Scholar] [CrossRef]
- Yoon, H.J.; Park, H.-W.; Park, D.-W. Simultaneous Oxidation and Absorption of NOx and SO2 in an Integrated O3 Oxidation/Wet Atomizing System. Energy Fuels 2016, 30, 3289–3297. [Google Scholar] [CrossRef]
- Karuppiah, J.; Karvembu, R.; Subrahmanyam, C. The catalytic effect of MnOx and CoOx on the decomposition of nitrobenzene in a non-thermal plasma reactor. Chem. Eng. J. 2012, 180, 39–45. [Google Scholar] [CrossRef]
- Ramaraju, B.; Subrahmanyam, C. Catalytic non-thermal plasma reactor for stripping the VOCs from air. Compos. Interfaces 2014, 21, 651–658. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Chen, X.; Xu, W.; Xu, Z.; Jia, H.; Chen, J. Homogeneous introduction of CeOy into MnOx-based catalyst for oxidation of aromatic VOCs. Appl. Catal. B Environ. 2018, 224, 825–835. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, H.; Li, L.; Zhou, P.; Meng, X.; Guo, M.; Jia, J.; Sun, T. In situ fabrication of highly active γ-MnO2/SmMnO3 catalyst for deep catalytic oxidation of gaseous benzene, ethylbenzene, toluene, and o-xylene. J. Hazard. Mater. 2019, 362, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hong, X.; Zhu, T.; Guo, X.; Xie, D. The Chemical Behaviors of Nitrogen Dioxide Absorption in Sulfite Solution. Appl. Sci. 2017, 7, 377. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, H.; Ying, D.; Wang, Y.; Sun, T.; Jia, J. In Plasma Catalytic Oxidation of Toluene Using Monolith CuO Foam as a Catalyst in a Wedged High Voltage Electrode Dielectric Barrier Discharge Reactor: Influence of Reaction Parameters and Byproduct Control. Int. J. Environ. Res. Public Health 2019, 16, 711. https://doi.org/10.3390/ijerph16050711
Li J, Zhang H, Ying D, Wang Y, Sun T, Jia J. In Plasma Catalytic Oxidation of Toluene Using Monolith CuO Foam as a Catalyst in a Wedged High Voltage Electrode Dielectric Barrier Discharge Reactor: Influence of Reaction Parameters and Byproduct Control. International Journal of Environmental Research and Public Health. 2019; 16(5):711. https://doi.org/10.3390/ijerph16050711
Chicago/Turabian StyleLi, Juexiu, Hongbo Zhang, Diwen Ying, Yalin Wang, Tonghua Sun, and Jinping Jia. 2019. "In Plasma Catalytic Oxidation of Toluene Using Monolith CuO Foam as a Catalyst in a Wedged High Voltage Electrode Dielectric Barrier Discharge Reactor: Influence of Reaction Parameters and Byproduct Control" International Journal of Environmental Research and Public Health 16, no. 5: 711. https://doi.org/10.3390/ijerph16050711