New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review
Abstract
:1. Introduction
2. Principles of Soil Washing and Soil Flushing
- Pretreatment (removal of oversized materials from soil),
- Separation between coarse- and fine-grained soil particles in a scrubbing unit (separation of the coarse-grained soils is commonly done by using mechanical screening such as trommels, while the fine-grained soils are sorted using hydrocyclones or other methods),
- Coarse-grained treatment (usually coarse-grained soil particles >0.05 mm are likely unpolluted or minimally polluted; thus, they can be treated using surface attrition or washing with water),
- Fine-grained treatment (because fine soil particles <0.05 mm are highly contaminated, they should be treated with a suitable washing solution containing water and chemicals using sonication or mechanical agitation),
- SWS treatment (treatment of SWS from washing coarse and fine soil particles; necessary to reuse washing solutions or to dispose of them in sewers),
- Residual management (residual materials, i.e., treated soil, and the sludge of dispersed fine particles output during soil washing; if residuals are still considered polluted, they may require further treatment before disposal).
3. Novel WAs and Their Applicability for HMs Removal from Soil
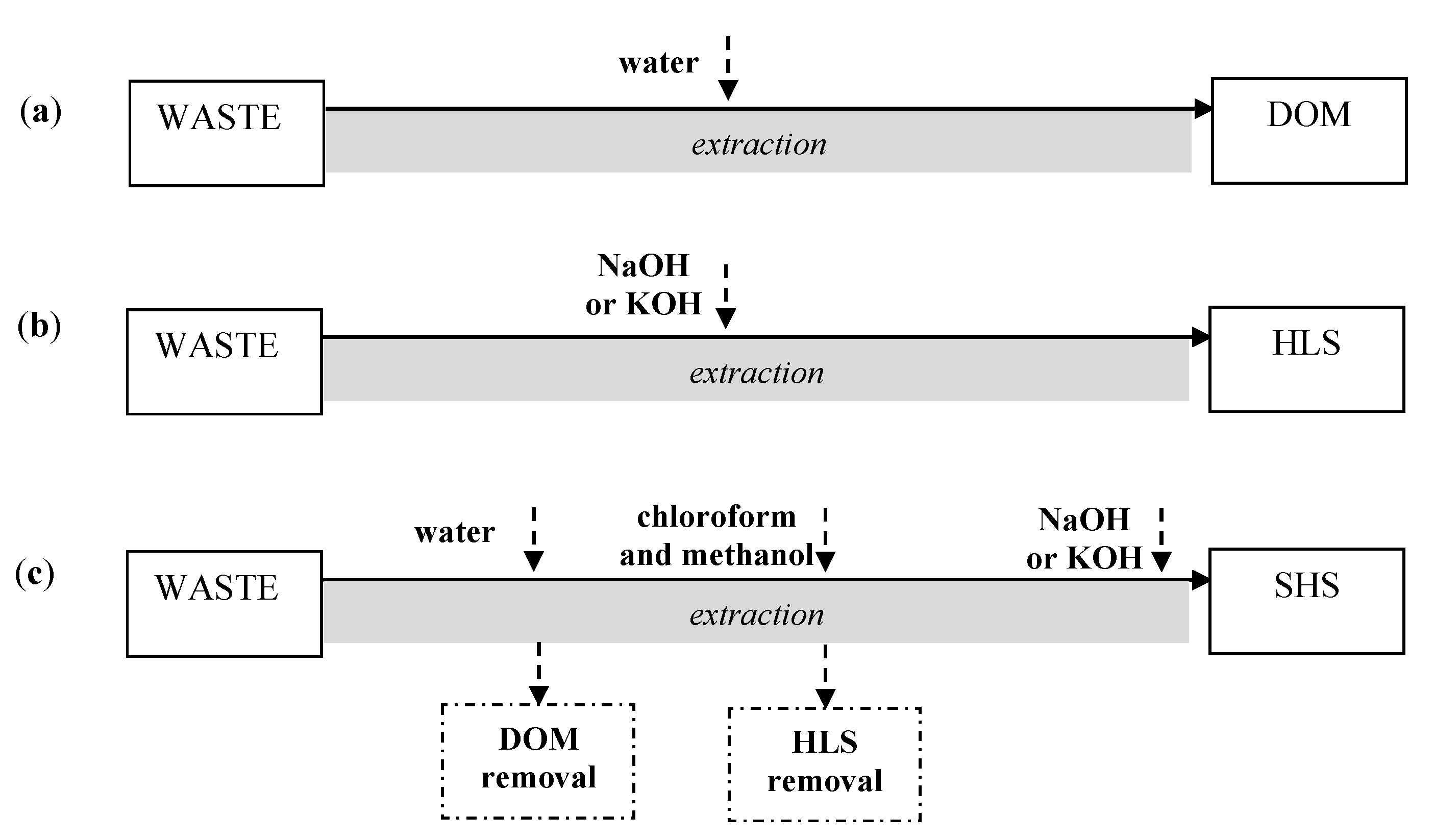
3.1. Sources of New-Generation WAs
3.1.1. Geochemical Resources
3.1.2. Unprocessed Wastes
3.1.3. Processed Waste
3.2. Examples of Chemical Substitutes for EDTA
| Type of WA | Source of WA | Selected Properties of Original WA | Soil Washing Efficiency (%) under Optimum Conditions | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (g TOC/L) | pH | Surface Tension (mN/m) | As | Cd | Cu | Ni | Pb | Zn | |||
| DOM | Municipal sewage sludge | 6.8 | 6.9 | 45.0 | – | – | 57 | – | 5 | 39 | [46] |
| Corn straw | 0.24 | 6.8 | – | – | 76 | – | – | 58 | – | [64] | |
| Liquid fertilizer from food-waste composting | 7.91 | 7.4 | – | – | – | – | – | – | 43 | [61] | |
| Municipal sewage sludge | 6.8 | 6.9 | 45.0 | – | 82–87 | – | – | – | – | [48] | |
| HLS | Wine-processing waste sludge | 2.0 | Alkaline | – | – | 80 | – | – | – | – | [54] |
| Wine-processing waste sludge | 2.5 | 12.0 | – | – | – | – | – | 86.5–93.0 | – | [55] | |
| Municipal sewage sludge | 9.7 | 11.7 | 40.4 | – | 79–81 | – | – | – | – | [48] | |
| SHS | Composted sewage sludge | 4.1 | 11.9 | 42.5 | 18–27 | – | – | – | – | – | [73] |
| Leonardite | 4.7 | Alkaline | 54.6 | – | 35.3–75.0 | – | – | – | – | [43] | |
| Composted sewage sludge | 2.2 | 12.7 | 51.7 | – | 79.1–82.6 | 51.5–71.8 | 35.4–46.1 | 44.8–47.6 | 27.9–35.8 | [74] | |
| Composted sewage sludge | 4.0 | 13.0 | 48.0 | – | 36.5–69.1 | 53.2–80.7 | – | – | – | [75] | |
| Compost park and garden waste (as percolate) | 100 * | 6.0 | – | 16.0–61.0 | – | 61.0–95.0 | – | – | – | [76] | |
| Municipal sewage sludge | 5.0 | 12.3 | 42.4 | – | 75–80 | – | – | – | – | [48] | |
4. Treatment of SWSs after Soil Washing/Soil Flushing
4.1. Adsorption
4.2. Precipitation
4.3. Advanced Oxidation Processes (AOPs)
4.4. Membrane Technologies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AOP | advanced oxidation process |
| CHA | commercial humic acid |
| DOM | dissolved organic matter |
| DTPA | diethylenetriaminepentaacetic acid |
| EAOP | electrochemical advanced oxidation process |
| EDDS | ethylenediaminedisuccinic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FA | fulvic acid |
| FCS | ferric chloride solution |
| GAA | granular activated alumina |
| GAC | granular activated carbon |
| GLDA | N,N-bis(carboxymethyl)-l-glutamic acid |
| HA | humic acid |
| HLS | soluble humic-like substances |
| HM | heavy metal |
| HPMA | hydrolytic polymaleic anhydride |
| HS | humic substances |
| IDA | iminodiacetic acid |
| KLS | potassium lignosulfonate |
| NF | nanofiltration |
| NOM | natural organic matter |
| NTA | nitrilotriacetic acid |
| PBTCA | 2-phosphonobutane-1,2,4-tricarboxylic acid |
| RO | reserve osmosis |
| SHLA | synthetic humic-like acids |
| SHS | soluble humic substances |
| SHS | soluble humic substances |
| SMZ | surfactant-modified zeolite |
| SWS | spent washing solution |
| TOC | total organic carbon |
| UF | ultrafiltration |
| WA | washing agent |
| WPWS | wine-processing waste sludge |
References
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Ray, S.; Kim, K.-H.; Yoon, H.-O.; Jeon, E.-C.; Kim, Y.S.; Cho, Y.-S.; Yun, S.-T.; Brown, R.J.C. Current Status of Trace Metal Pollution in Soils Affected by Industrial Activities. Sci. World J. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–25. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- ATSDR Priority List of Hazardous Substances. Available online: https://www.atsdr.cdc.gov/SPL/index.html (accessed on 28 February 2020).
- Gorospe, J. Growing Greens and Soiled Soil; San Jose State University: San Jose, CA, USA, 2012. [Google Scholar]
- USEPA. Cleaning Up the Nation’s Waste Sites Markets and Technology Trends, 4th ed.; United States Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- EEA Progress in Management of Contaminated Sites. Available online: https://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment (accessed on 28 February 2020).
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Janoš, P.; Vávrová, J.; Herzogová, L.; Pilařová, V. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 2010, 159, 335–341. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mackay, A.K.; Hudson-Edwards, K.A.; Holz, E. Soil Cd, Cu, Pb and Zn contaminants around Mount Isa city, Queensland, Australia: Potential sources and risks to human health. Appl. Geochem. 2010, 25, 841–855. [Google Scholar] [CrossRef]
- Parizanganeh, A.; Hajisoltani, P.; Zamani, A. Assessment of heavy metal pollution in surficial soils surrounding Zinc Industrial Complex in Zanjan-Iran. Procedia Environ. Sci. 2010, 2, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Ahlawat Sainger, P.; Dhankhar, R.; Sainger, M.; Kaushik, A.; Pratap Singh, R. Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol. Environ. Saf. 2011, 74, 2284–2291. [Google Scholar] [CrossRef]
- Ettler, V.; Mihaljevič, M.; Kříbek, B.; Majer, V.; Šebek, O. Tracing the spatial distribution and mobility of metal/metalloid contaminants in Oxisols in the vicinity of the Nkana copper smelter, Copperbelt province, Zambia. Geoderma 2011, 164, 73–84. [Google Scholar] [CrossRef]
- Nannoni, F.; Protano, G.; Riccobono, F. Fractionation and geochemical mobility of heavy elements in soils of a mining area in northern Kosovo. Geoderma 2011, 161, 63–73. [Google Scholar] [CrossRef]
- Yaylalı-Abanuz, G. Heavy metal contamination of surface soil around Gebze industrial area, Turkey. Microchem. J. 2011, 99, 82–92. [Google Scholar] [CrossRef]
- Nakayama, S.M.M.; Ikenaka, Y.; Hamada, K.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Metal and metalloid contamination in roadside soil and wild rats around a Pb–Zn mine in Kabwe, Zambia. Environ. Pollut. 2011, 159, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medyńska-Juraszek, A.; Kabała, C. Heavy metal pollution of forest soils affected by the copper industry. J. Elem. 2012, 17, 441–451. [Google Scholar] [CrossRef]
- Torres, L.G.; Lopez, R.B.; Beltran, M. Removal of As, Cd, Cu, Ni, Pb, and Zn from a highly contaminated industrial soil using surfactant enhanced soil washing. Phys. Chem. Earth Parts ABC 2012, 37–39, 30–36. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Wang, Y.; Luo, G.; Chen, X.; Yang, X.; Hall, M.H.P.; Guo, R.; Wang, H.; Cui, J.; et al. Heavy metal contamination of urban soil in an old industrial city (Shenyang) in Northeast China. Geoderma 2013, 192, 50–58. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Pearl, M. Understanding Soil Washing. CL AIRE Tech. Bull. 2007, 13, 1–4. [Google Scholar]
- Hubler, J.; Metz, K. Soil Washing. Available online: https://www.geoengineer.org/education/web-class-projects/cee-549-geoenvironmental-engineering-winter-2013/assignments/soil-washing (accessed on 29 May 2020).
- Anderson, W.C. Soil Washing/Soil Flushing; American Academy of Environmental Engineers: Annapolis, MD, USA, 1993. [Google Scholar]
- Griffiths, R.A. Soil-washing technology and practice. J. Hazar. Mater. 1995, 40, 175–189. [Google Scholar] [CrossRef]
- Dekonta Transportable Soil Washing Plant. Available online: https://www.dekonta.cz/en/about-us/download.html (accessed on 29 May 2020).
- USEPA. A Citizen’s Guide to In Situ Soil Flushing; United States Environmental Protection Agency: Washington, DC, USA, 1996. [Google Scholar]
- Mulligan, C.; Yong, R.; Gibbs, B. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Boskalis Environmental Enhanced Soil Washing. Available online: https://environmental.boskalis.com/activities/enhanced-soil-washing.html (accessed on 29 May 2020).
- Water Front Toronto Pilot Soil Recycling Facility Fact Sheet. Available online: https://www.waterfrontoronto.ca/nbe/wcm/connect/waterfront/40e110c0-8580-485d-a555-88b10e0c79d0/soil_recycling_pilot_facility_fact_sheet___april_2012___clean_1.pdf?MOD=AJPERES&CACHEID=40e110c0-8580-485d-a555-88b10e0c79d0 (accessed on 29 May 2020).
- FRTR Remediation Technologies Screening Matrix and Reference Guide, Version 4.0. Available online: https://frtr.gov/matrix2/top_page.html (accessed on 29 May 2020).
- Ko, I.; Chang, Y.-Y.; Lee, C.-H.; Kim, K.-W. Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J. Hazard. Mater. 2005, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boskalis Environmental. Available online: https://environmental.boskalis.com/ (accessed on 29 May 2020).
- ART Engineering LLC. Available online: https://www.art-engineering.com/index.html (accessed on 29 May 2020).
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, Y.; Tang, J.; Chen, D.; Li, X.; Lin, Q.; Yin, G.; Zhang, M.; Hu, H. Potassium lignosulfonate as a washing agent for remediating lead and copper co-contaminated soils. Sci. Total Environ. 2019, 658, 836–842. [Google Scholar] [CrossRef]
- Ali, M.; Mindari, W. Effect of Humic Acid on Soil Chemical and Physical Characteristics of Embankment. MATEC Web Conf. 2016, 58, 01028. [Google Scholar] [CrossRef]
- Damian, G.E.; Micle, V.; Sur, I.M. Mobilization of Cu and Pb from multi-metal contaminated soils by dissolved humic substances extracted from leonardite and factors affecting the process. J. Soils Sediment 2019, 19, 2869–2881. [Google Scholar] [CrossRef]
- Tsang, D.C.W.; Olds, W.E.; Weber, P. Residual leachability of CCA-contaminated soil after treatment with biodegradable chelating agents and lignite-derived humic substances. J. Soils Sediment 2013, 13, 895–905. [Google Scholar] [CrossRef]
- Meng, F.; Yuan, G.; Wei, J.; Bi, D.; Ok, Y.S.; Wang, H. Humic substances as a washing agent for Cd-contaminated soils. Chemosphere 2017, 181, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Conte, P.; Agretto, A.; Spaccini, R.; Piccolo, A. Soil remediation: Humic acids as natural surfactants in the washings of highly contaminated soils. Environ. Pollut. 2005, 135, 515–522. [Google Scholar] [CrossRef]
- Gusiatin, Z.M. Novel and Eco-Friendly Washing Agents to Remove Heavy Metals from Soil by Soil Washing. Environ. Anal. Ecol. Stud. 2018, 2, 1–4. [Google Scholar]
- Kulikowska, D.; Klik, B.K.; Gusiatin, Z.M.; Hajdukiewicz, K. Characteristic of humic substances from municipal sewage sludge: A case study. Desalin. Water Treat. 2019, 144, 57–64. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Zou, S.; Li, C. Extracting humic acids from digested sludge by alkaline treatment and ultrafiltration. J. Mater. Cycles Waste Manag. 2014, 16, 93–100. [Google Scholar] [CrossRef]
- Klik, B.; Kulikowska, D.; Gusiatin, Z.M.; Pasieczna-Patkowska, S. Washing agents from sewage sludge: Efficiency of Cd removal from highly contaminated soils and effect on soil organic balance. J. Soils Sediments 2020, 20, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Zhang, S.; Li, L.; Wang, G.; Xu, X.; Li, T.; Zhong, Q. Feasibility of four wastes to remove heavy metals from contaminated soils. J. Environ. Manag. 2018, 212, 258–265. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Hansen, H.C.B.; Holm, P.E.; Jensen, J.K.; Rasmussen, S.B.; Sabiene, N.; Steponkaite, L.; Strobel, B.W. Experimental Assessment of Using Soluble Humic Substances for Remediation of Heavy Metal Polluted Soils. Soil Sediment Contam. 2009, 18, 369–382. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Chen, Y.-M.; Chen, L.-F.; Wang, M.-K.; Liu, C.-C. Remediation of Arsenic-Contaminated Soil Using Alkaline Extractable Organic Carbon Solution Prepared from Wine-Processing Waste Sludge. Soil Sediment Contam. 2017, 26, 569–583. [Google Scholar] [CrossRef]
- Liu, C.C.; Wang, M.K.; Chiou, C.S.; Li, Y.S.; Yang, C.Y.; Lin, Y.A. Biosorption of chromium, copper and zinc by wine-processing waste sludge: Single and multi-component system study. J. Hazard. Mater. 2009, 171, 386–392. [Google Scholar] [CrossRef]
- Liu, C.C.; Lin, Y.C. Reclamation of copper-contaminated soil using EDTA or citric acid coupled with dissolved organic matter solution extracted from distillery sludge. Environ. Pollut. 2013, 178, 97–101. [Google Scholar] [CrossRef]
- Liu, C.-C.; Chen, G.-B. Reclamation of cadmium-contaminated soil using dissolved organic matter solution originating from wine-processing waste sludge. J. Hazard. Mater. 2013, 244–245, 645–653. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Lin, W.-H.; Lin, Y.-A.; Liu, C.-C.; Wang, M.-K. Remediation of lead-contaminated soil using dissolved organic carbon solutions prepared by wine-processing waste sludge. Geoderma 2014, 235–236, 233–239. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Y.; Zhao, W.; Yang, T.; Zhang, X.; Xie, X.; Cui, H.; Wei, Z. Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour. Tech. 2017, 226, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Gusiatin, Z.M.; Kulikowska, D. Influence of compost maturation time on Cu and Zn mobility (MF) and redistribution (IR) in highly contaminated soil. Environ. Earth Sci. 2015, 74, 6233–6246. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Masaguer, A.; Moliner, A. Effects of sheep and horse manure and pine bark amendments on metal distribution and chemical properties of contaminated mine soils. Eur. J. Soil Sci. 2012, 63, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil washing with solutions of humic substances from manure compost removes heavy metal contaminants as a function of humic molecular composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef]
- Chiang, P.-N.; Tong, O.-Y.; Chiou, C.-S.; Lin, Y.-A.; Wang, M.-K.; Liu, C.-C. Reclamation of zinc-contaminated soil using a dissolved organic carbon solution prepared using liquid fertilizer from food-waste composting. J. Hazard. Mater. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Evolution of humic substances during anaerobic sludge digestion. Environ. Eng. Manag. J. 2017, 16, 1577–1582. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.J.; Verstraten, J.M. Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: Influence of solution pH and metal/organic carbon ratios. Geoderma 2003, 113, 323–340. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, Y. Environmentally friendly remediation of lead/cadmium co-contaminated loess soil in northwestern China using a humificated straw solution. Environ. Sci. Pollut. Res. 2018, 25, 25243–25254. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Holm, P.E.; Strobel, B.W. Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review. Geoderma 2019, 343, 235–246. [Google Scholar] [CrossRef]
- Strobel, B.W. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—A review. Geoderma 2001, 99, 169–198. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Gusiatin, Z.M. Tannic acid and saponin for removing arsenic from brownfield soils: Mobilization, distribution and speciation. J. Environ. Sci. 2014, 26, 855–864. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhong, H.; Zeng, G.; Liang, Y.; Chen, M.; Wu, Z.; Zhou, Y.; Yu, M.; Shao, B. Recent advances in the environmental applications of biosurfactant saponins: A review. J. Environ. Chem. Eng. 2017, 5, 6030–6038. [Google Scholar] [CrossRef]
- Fukuchi, S.; Fukushima, M.; Nishimoto, R.; Qi, G.; Sato, T. Fe-loaded zeolites as catalysts in the formation of humic substance-like darkcoloured polymers in polycondensation reactions of humic precursors. Clay Miner. 2012, 47, 355–364. [Google Scholar] [CrossRef]
- Yang, T.; Hodson, M.E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci. Total Environ. 2019, 647, 290–300. [Google Scholar] [CrossRef]
- Polymer Properties Database, Lignin and Its Derivatives. Available online: http://polymerdatabase.com/polymer%20classes/Lignin%20type.html (accessed on 29 May 2020).
- Gusiatin, Z.M.; Kulikowska, D.; Klik, B. Suitability of humic substances recovered from sewage sludge to remedy soils from a former as mining area—A novel approach. J. Hazard. Mater. 2017, 338, 160–166. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Klik, B. Feasibility of using humic substances from compost to remove heavy metals (Cd, Cu, Ni, Pb, Zn) from contaminated soil aged for different periods of time. J. Hazard. Mater. 2015, 300, 882–891. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Jensen, J.K.; Borggaard, O.K. A laboratory test of NOM-assisted remediation of arsenic and copper contaminated soils. J. Environ. Chem. Eng. 2015, 3, 3020–3023. [Google Scholar] [CrossRef]
- Akzo Nobel. Dissolvine® GL Technical brochure; Nouryon: Arnhem, The Netherlands, 2010. [Google Scholar]
- Wang, G.; Zhang, S.; Xu, X.; Zhong, Q.; Zhang, C.; Jia, Y.; Li, T.; Deng, O.; Li, Y. Heavy metal removal by GLDA washing: Optimization, redistribution, recycling, and changes in soil fertility. Sci. Total Environ. 2016, 569–570, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Shandong Taihe Water Treatment Technologies, Co. 2-Phosphonobutane-1,2,4-Tricarboxylic Acid (PBTCA). Available online: http://thwater.net/01-PBTCA.htm (accessed on 29 May 2020).
- Cao, Y.; Zhang, S.; Wang, G.; Li, T.; Xu, X.; Deng, O.; Zhang, Y.; Pu, Y. Enhancing the soil heavy metals removal efficiency by adding HPMA and PBTCA along with plant washing agents. J. Hazard. Mater. 2017, 339, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wasay, S.A.; Barrington, S.; Tokunaga, S. Efficiency of GAC for Treatment of Leachate from Soil Washing Process. Water Air Soil Pollut. 1999, 116, 449–460. [Google Scholar] [CrossRef]
- Meunier, N.; Blais, J.-F.; Tyagi, R.D. Selection of a natural sorbent to remove toxic metals from acidic leachate produced during soil decontamination. Hydrometallurgy 2002, 67, 19–30. [Google Scholar] [CrossRef]
- Sullivan, E.J.; Bowman, R.S.; Legiec, I.A. Sorption of arsenic from soil-washing leachate by surfactant-modified zeolite. J. Environ. Qual. 2003, 32, 2387–2391. [Google Scholar] [CrossRef]
- Gusiatin, Z.M. Fe-modified Clinoptilolite is Effective to Recover Plant Biosurfactants Used for Removing Arsenic from Soil. Clean Soil Air Water 2015, 43, 1224–1231. [Google Scholar] [CrossRef]
- Hong, K.-J.; Tokunaga, S.; Kajiuchi, T. Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 2002, 49, 379–387. [Google Scholar] [CrossRef]
- Di Palma, L.; Ferrantelli, P.; Merli, C.; Biancifiori, F. Recovery of EDTA and metal precipitation from soil flushing solutions. J. Hazard. Mater. 2003, 103, 153–168. [Google Scholar] [CrossRef]
- Lo, I.M.; Zhang, W. Study on Optimal Conditions for Recovery of EDTA from Soil Washing Effluents. J. Environ. Eng. 2005, 131, 1507–1513. [Google Scholar] [CrossRef]
- Gao, L.; Kano, N.; Sato, Y.; Li, C.; Zhang, S.; Imaizumi, H. Behavior and Distribution of Heavy Metals Including Rare Earth Elements, Thorium, and Uranium in Sludge from Industry Water Treatment Plant and Recovery Method of Metals by Biosurfactants Application. Bioinorg. Chem. Appl. 2012. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Mukherjee, S.; Hashim, M.A.; Sen Gupta, B. Application of colloidal gas aphron suspensions produced from Sapindus mukorossi for arsenic removal from contaminated soil. Chemosphere 2015, 119, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiraroj, D.; Unob, F.; Hagège, A. Degradation of Pb–EDTA complex by a H2O2/UV process. Water Res. 2006, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Finžgar, N.; Leštan, D. Advanced Oxidation for Treatment of Aqueous Extracts from EDTA Extraction of Pb and Zn Contaminated Soil. J. Environ. Eng. 2006, 132, 1376–1380. [Google Scholar] [CrossRef]
- Finžgar, N.; Leštan, D. Heap leaching of Pb and Zn contaminated soil using ozone/UV treatment of EDTA extractants. Chemosphere 2006, 63, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Finzgar, N.; Lestan, D. The two-phase leaching of Pb, Zn and Cd contaminated soil using EDTA and electrochemical treatment of the washing solution. Chemosphere 2008, 73, 1484–1491. [Google Scholar] [CrossRef]
- Lestan, D.; Finzgar, N. Leaching of Pb Contaminated Soil using Ozone/UV Treatment of EDTA Extractants. Sep. Sci. Technol. 2007, 42, 1575–1584. [Google Scholar] [CrossRef]
- Satyro, S.; Race, M.; Marotta, R.; Dezotti, M.; Guida, M.; Clarizia, L. Photocatalytic processes assisted by artificial solar light for soil washing effluent treatment. Environ. Sci. Pollut. Res. 2017, 24, 6353–6360. [Google Scholar] [CrossRef]
- Volchek, K.; Velicogna, D.; Obenauf, A.; Somers, A.; Wong, B.; Tremblay, A.Y. Novel applications of membrane processes in soil cleanup operations. Desalination 2002, 147, 123–126. [Google Scholar] [CrossRef]
- Ortega, L.M.; Lebrun, R.; Blais, J.-F.; Hausler, R. Removal of metal ions from an acidic leachate solution by nanofiltration membranes. Desalination 2008, 227, 204–216. [Google Scholar] [CrossRef]
- Ortega, L.M.; Lebrun, R.; Blais, J.-F.; Hausler, R.; Drogui, P. Effectiveness of soil washing, nanofiltration and electrochemical treatment for the recovery of metal ions coming from a contaminated soil. Water Res. 2008, 42, 1943–1952. [Google Scholar] [CrossRef] [PubMed]



| Source/Location | As | Cd | Cr | Cu | Ni | Pb | Zn | Ref. |
|---|---|---|---|---|---|---|---|---|
| Area around Pb and Zn mine (farmland, pasture, mining waste); Spain | – | – | – | – | – | 368–18,260 | 449–9489 | [10] |
| Area around former smelter; Czech Republic | – | 5.7 | – | 21.7 | – | 1160 | 233 | [11] |
| Urban area, Pb–Zn–Ag and Cu mines; Australia | – | 1.0–9.8 | – | 34–2730 | – | 1–2020 | 49–15,600 | [12] |
| Zinc industrial complex; Iran | – | 0.2–18.5 | – | – | – | 4.1–630 | 146.6–3066 | [13] |
| Electroplating industry; India | – | – | 1172–3240 | 263–374 | 234–335 | 1376–3112 | [14] | |
| Metallurgical factory; Zambia | 0.04–255 | – | – | 34–27,410 | – | 4.0–480 | 4.0–450 | [15] |
| Industrial and mining complex with battery factory; Kosovo | 16–110 | 0.3–11.8 | – | 17.8–134 | – | 53.4–5536 | 86.1–1553 | [16] |
| Industrial area (e.g., paint, plastic, metal industry); Turkey | 1.5–66 | 0.05–176 | 10–1161 | 8–725 | – | 17–8469 | 29.5–10,000 | [17] |
| Pb–Zn mining; Zambia | 0.04–141 | 0.01–139 | 3–110 | 2–5727 | 2–74 | 9–51188 | 5–91,595 | [18] |
| Smelting industry; Poland | – | – | – | 855–13,143 | – | 585–9181 | 718–3363 | [19] |
| Metallurgical industry; Mexico | 4019 | 14.4 | – | 35,582 | 2063 | 70 | 261.4 | [20] |
| Urban soils, Tiexi Industrial District (heavy machinery and manufacturing industry); China | – | 0.01–9.6 | 4.8–207 | 7.6–430 | – | 1.9–940 | 25–1140 | [21] |
| Feature | Soil Washing | Soil Flushing |
|---|---|---|
| Applicability | Pollutant(s): broad range, e.g., HMs, gasoline, fuel oils, and pesticidesSoils: permeable with low content of clay and organic matter, moderate to high contamination | |
| Site: ex situ (on site or off site) | Site: in situ | |
| Scale: small | Scale: wider | |
| Status: regularly practiced | Status: limited number of applications | |
| Pre-treatment | Soil excavation is required; reduction of soil volume by physical separation with special equipment (e.g., trommels, attrition or flotation machines, screens, hydrocyclones) | Soil excavation is not required; installation of barriers in ground; injection of washing solution into ground |
| Soil treatment | Pollutant removal from separated soil fractions by extraction with washing solutions | Pollutant removal by percolation of washing solutions through contaminated zone |
| Post-treatment | Stabilization and disposal of concentrated soil fraction, treatment of SWSs, pollutant recovery | Treatment of SWSs, groundwater treatment |
| Advantages | Permanently removes pollutants from soil; a rapid method; effective even for highly polluted soils | Minimal soil disturbance; short to medium treatment time; lower cost |
| Limitations | Deterioration of soil structure and soil composition (e.g., nutrients removal); a relatively high cost | Effective only for permeable, coarse-textured soils; a potential risk of groundwater pollution |
| Cost ($USD/ton) | 25–300 | 100–200 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusiatin, Z.M.; Kulikowska, D.; Klik, B. New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6220. https://doi.org/10.3390/ijerph17176220
Gusiatin ZM, Kulikowska D, Klik B. New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review. International Journal of Environmental Research and Public Health. 2020; 17(17):6220. https://doi.org/10.3390/ijerph17176220
Chicago/Turabian StyleGusiatin, Zygmunt M., Dorota Kulikowska, and Barbara Klik. 2020. "New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review" International Journal of Environmental Research and Public Health 17, no. 17: 6220. https://doi.org/10.3390/ijerph17176220





