Index Properties, Hydraulic Conductivity and Contaminant-Compatibility of CMC-Treated Sodium Activated Calcium Bentonite
Abstract
:1. Introduction
2. Materials and Methods
2.1. SACaB
2.2. CMC-SACaB Preparation
2.3. Chemical Solutions
2.4. Properties of CMC-SACaB
2.5. Swell Index with Chemical Solutions
2.6. Hydraulic Conductivity Via Modified Fluid Loss Tests
2.7. Hydraulic Conductivity Based on Flexible-Wall Permeameter Tests
3. Results and Discussion
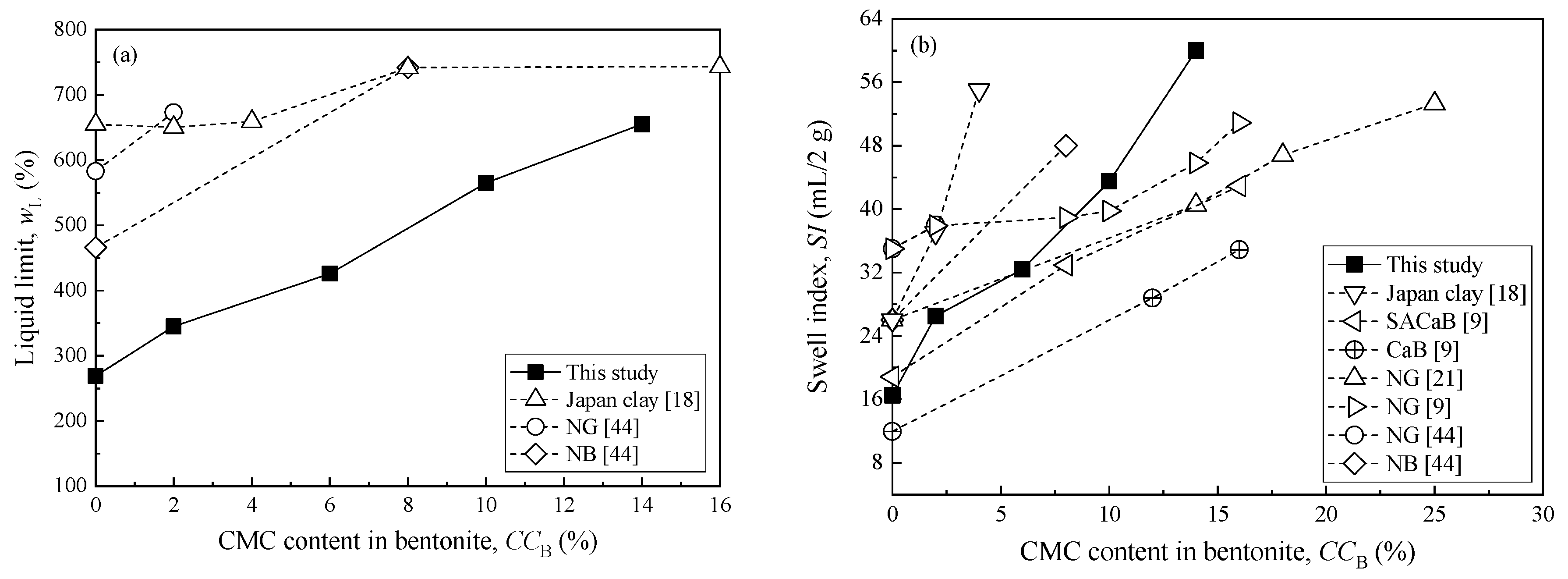
3.1. Properties of CMC-SACaB
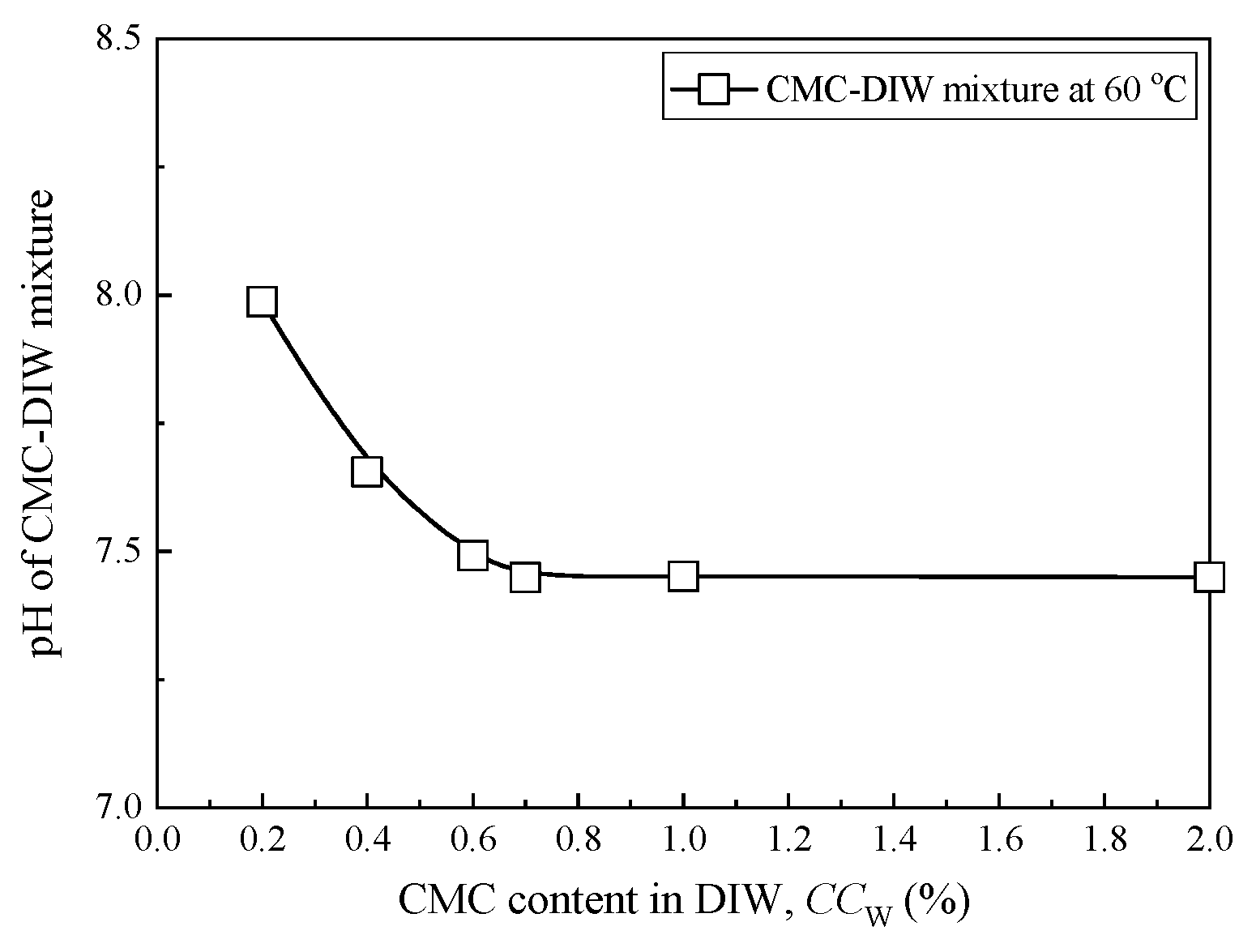
3.2. Swell Index Exposed to Chemical Solutions
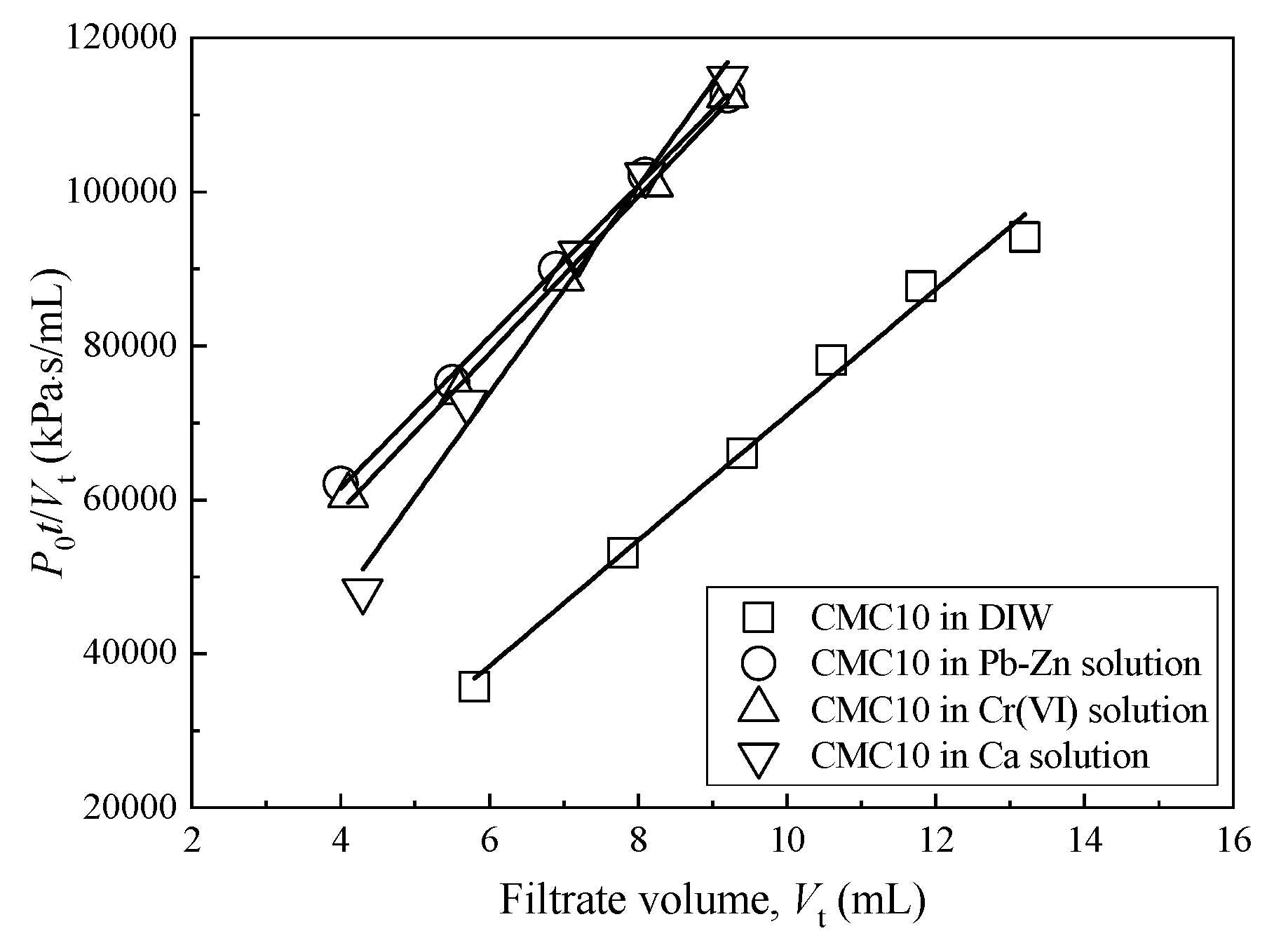
3.3. Hydraulic Conductivity
3.4. Study Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, H.D.; Reddy, K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Techonolgies, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Bai, X.; Song, K.; Liu, J.; Mohamed, A.K.; Mou, C.; Liu, D. Health risk assessment of groundwater contaminated by oil pollutants based on numerical modeling. Int. J. Environ. Res. Public Health. 2019, 16, 3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-L.; Du, Y.-J.; Yu, J.; Yang, Y.-L.; Li, V.C. Hydraulic conductivity and self-healing performance of engineered cementitious composites exposed to acid mine drainage. Sci. Total Environ. 2020, 716, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.D.; Du, Y.J.; Reddy, K.R.; Liu, S.Y.; Yang, Y.L. Compressibility and hydraulic conductivity of clayey soil mixed with calcium bentonite for slurry wall backfill: Initial assessment. Appl. Clay Sci. 2014, 101, 119–127. [Google Scholar] [CrossRef]
- Du, Y.J.; Fan, R.D.; Reddy, K.R.; Liu, S.Y.; Yang, Y.L. Impacts of presence of lead contamination in clayey soil–calcium bentonite cutoff wall backfills. Appl. Clay Sci. 2015, 108, 111–122. [Google Scholar] [CrossRef]
- Chen, Y.G.; Jia, L.Y.; Niu, L.H.; Ye, W.M.; Chen, B.; Cui, Y.J. Effect of dry density and pH on the diffusion behavior of lanthanum in compacted Chinese GMZ bentonite. J. Radioanal. Nucl. Chem. 2016, 310, 1303–1310. [Google Scholar] [CrossRef]
- Ye, W.M.; Zheng, Z.J.; Chen, B.; Chen, Y.G.; Cui, Y.J.; Wang, J. Effects of pH and temperature on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite. Appl. Clay Sci. 2014, 101, 192–198. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Bakır, S. Some properties and potential applications of the Na- and Ca-bentonites of ordu (N.E. Turkey). Appl. Clay Sci. 2011, 54, 159–165. [Google Scholar] [CrossRef]
- Janssen, J.; Emidio, G.D.; Flores, R.D.V.; Bezuijen, A. Hydraulic conductivity and swelling pressure of GCLs using polymer treated clays to high concentration CaCl2 solutions. In Proceedings of the XVI ECSMGE Geotechnical Engineering for Infrastructure and Development, Edinburgh, UK, 13–17 September 2015. [Google Scholar]
- Yang, Y.-L.; Reddy, K.; Du, Y.-J.; Fan, R.-D. Sodium hexametaphosphate (SHMP)-amended calcium bentonite for slurry trench cutoff walls: Workability and microstructure characteristics. Can. Geotech. J. 2018, 55, 528–537. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-L.; Reddy Krishna, R.; Du, Y.-J.; Fan, R.-D. Short-term hydraulic conductivity and consolidation properties of soil-bentonite backfills exposed to CCR-impacted groundwater. J. Geotech. Geoenviron. Eng. 2018, 144, 04018025. [Google Scholar] [CrossRef]
- Malusis, M.A.; McKeehan, M.D. Chemical compatibility of model soil-bentonite backfill containing multiswellable bentonite. J. Geotech. Geoenviron. Eng. 2013, 139, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Tian, K.; Benson, C.H.; Likos, W.J. Hydraulic conductivity of geosynthetic clay liners to low-level radioactive waste leachate. J. Geotech. Geoenviron. Eng. 2016, 142, 04016037. [Google Scholar] [CrossRef]
- Chen, J.N.; Benson, C.H.; Edil, T.B. Hydraulic conductivity of geosynthetic clay liners with sodium bentonite to coal combustion product leachates. J. Geotech. Geoenviron. Eng. 2018, 144, 04018008. [Google Scholar] [CrossRef]
- Yong, R.N.; Ouhadi, V.R.; Goodarzi, A.R. Effect of Cu2+ ions and buffering capacity on smectite microstructure and performance. J. Geotech. Geoenviron. Eng. 2009, 135, 1981–1985. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Sample-Lord, K.M. Hydraulic conductivity and compatibility of bentonite for hydraulic containment barriers. In Proceedings of the Geo-Congress 2014: From Soil Behavior Fundamentals to Innovations in Geotechnical Engineering, Atlanta, GA, USA, 19–21 May 2014; ASCE: Reston, VA, USA, 2014. [Google Scholar]
- Bohnhoff, G.L.; Shackelford, C.D. Hydraulic conductivity of polymerized bentonite-amended backfills. J. Geotech. Geoenviron. Eng. 2014, 140, 04013028. [Google Scholar] [CrossRef]
- Di Emidio, G.; Mazzieri, F.; Verastegui-Flores, R.D.; Van Impe, W.; Bezuijen, A. Polymer-treated bentonite clay for chemical-resistant geosynthetic clay liners. Geosynth. Int. 2015, 22, 125–137. [Google Scholar] [CrossRef]
- Katsumi, T.; Ishimori, H.; Onikata, M.; Fukagawa, R. Long-term barrier performance of modified bentonite materials against sodium and calcium permeant solutions. Geotext. Geomembranes. 2008, 26, 14–30. [Google Scholar] [CrossRef]
- Scalia IV, J.; Benson, C.H. Hydraulic conductivity of geosynthetic clay liners exhumed from landfill final covers with composite barriers. J. Geotech. Geoenviron. Eng. 2011, 137, 1–13. [Google Scholar] [CrossRef]
- Hamer, D.A.D.; Emidio, G.D.; Bezuijen, A.; Flores, D.V. Preliminary test on modified clays for seawater resistant drilling fluids. In Proceedings of the XVI ECSMGE Geotechnical Engineering for Infrastructure and Development, Scotland, UK, 13–17 September 2015. [Google Scholar]
- Drilling and completion fluids, and well cements (ISO/TC 67/SC 3). In Petroleum and Natural Gas Industries—Drilling Fluid Materials—Specifications and Tests; ISO 13500:2008; Standards Norway: Oslo, Norway, 2014.
- Qiu, H.; Yu, J. Polyacrylate/(carboxymethylcellulose modified montmorillonite) superabsorbent nanocomposite: Preparation and water absorbency. J. Appl. Polym. Sci. 2008, 107, 118–123. [Google Scholar] [CrossRef]
- Chapuis, R.P. Predicting the saturated hydraulic conductivity of soils: A review. Bullt. Eng. Geol. Environ. 2012, 71, 401–434. [Google Scholar] [CrossRef]
- Lee, J.M.; Shackelford, C.D.; Benson, C.H.; Jo, H.Y.; Edil, T.B. Correlating index properties and hydraulic conductivity of geosynthetic clay liners. J. Geotech. Geoenviron. Eng. 2005, 131, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- API. Recommended Practice for Field Testing Water-Based Drilling Fluids; 13B-1; American Petroleum Institute: Washington, DC, USA, 2009. [Google Scholar]
- Chung, J.; Daniel, D.E. Modified fluid loss test as an improved measure of hydraulic conductivity for bentonite. Geotech. Test J. 2008, 31, 243–251. [Google Scholar] [CrossRef]
- Fehervari, A.; Gates, W.P.; Patti, A.F.; Turney, T.W.; Bouazza, A.; Rowe, R.K. Potential hydraulic barrier performance of cyclic organic carbonate modified bentonite complexes against hyper-salinity. Geotext. Geomembranes 2016, 44, 748–760. [Google Scholar] [CrossRef]
- Liu, Y.; Gates, W.P.; Bouazza, A.; Rowe, R.K. Fluid loss as a quick method to evaluate hydraulic conductivity of geosynthetic clay liners under acidic conditions. Can. Geotech. J. 2013, 51, 158–163. [Google Scholar] [CrossRef]
- Rosin-Paumier, S.; Touze-Foltz, N.; Pantet, A. Impact of a synthetic leachate on permittivity of GCLs measured by filter press and oedopermeameter tests. Geotext. Geomembranes 2011, 29, 211–221. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis; D7928-17; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; D2974-14; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; D4318-17; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; D854-10; ASTM: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); D2487-17; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test Method for pH of Soils; D4972-19; ASTM: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM. Standard Test Method for Swell Index of Clay Mineral Component of Geosynthetic Clay Liners; D5890-18; ASTM: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM. Standard Test Method for Measuring the Exchange Complex and Cation Exchange Capacity of Inorganic Fine-Grained Soils; D7503-18; ASTM: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Benson, C.H.; Chen, J.N.; Edil, T.B.; Likos, W.J. Hydraulic conductivity of compacted soil liners permeated with coal combustion product leachates. J. Geotech. Geoenviron. Eng. 2018, 144, 04018011. [Google Scholar] [CrossRef] [Green Version]
- ASTM. Standard Specification for Reagent Water; D1193-06; ASTM: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Fan, R.D.; Liu, S.Y.; Du, Y.J.; Reddy, K.R.; Yang, Y.L. Impacts of presence of lead contamination on settling behavior and microstructure of clayey soil - calcium bentonite blends. Appl. Clay Sci. 2017, 142, 109–119. [Google Scholar] [CrossRef]
- Liu, S.Y.; Fan, R.D.; Du, Y.J.; Yang, Y.L. Modified fluid loss test to measure the hydraulic conductivity of heavy metal-contaminated bentonite filter cakes. In Proceedings of the Geo-Chicago 2016: Sustainable Geoenvironmental Systems, Chicago, IL, USA, 14–18 August 2016; ASCE: Reston, VA, USA, 2016. [Google Scholar]
- ASTM. Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Materials Using a Flexible Wall Permeameter; D5084-16a; ASTM: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Malusis, M.A.; Emidio, G.D. Hydraulic conductivity of sand-bentonite backfills containing HYPER clay. In Proceedings of the Geo-Congress 2014: From Soil Behavior Fundamentals to Innovations in Geotechnical Engineering, Atlanta, GA, USA, 23–26 February 2014; ASCE: Reston, VA, USA, 2014. [Google Scholar]
- Fedderse, R.L.; Thorp, S.N. Sodium carboxymethylcellulose. In Industrial Gums: Polysaccharides and Their Derivatives, 3rd ed.; Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: London, UK, 1993; pp. 538–578. [Google Scholar]
- Nara, M.; Torii, H.; Tasumi, M. Correlation between the vibrational frequencies of the carboxylate group and the types of its coordination to a metal ion: An ab initio molecular orbital study. J. Phys. Chem. 1996, 100, 19812–19817. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, G.; An, C.; Wei, J.; Yao, Y. Enhancement of soil retention for phenanthrene in binary cationic gemini and nonionic surfactant mixtures: Characterizing two-step adsorption and partition processes through experimental and modeling approaches. J. Hazard Mater. 2015, 286, 144–151. [Google Scholar] [CrossRef]
- Du, Y.J.; Fan, R.D.; Liu, S.Y.; Reddy, K.R.; Jin, F. Workability, compressibility and hydraulic conductivity of zeolite-amended clayey soil/calcium-bentonite backfills for slurry-trench cutoff walls. Eng Geol. 2015, 195, 258–268. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Reddy, K.R.; Zhang, W.-J.; Fan, R.-D.; Du, Y.-J. SHMP-amended Ca-bentonite/sand backfill barrier for containment of lead contamination in groundwater. Int. J. Environ. Res. Public Health. 2020, 17, 370. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Li, C.-P.; Yan, X.-L.; Arulrajah, A.; Wang, F.; Song, D.-J. In-situ solidification/stabilization of heavy metals contaminated site soil using a dry jet mixing method and new hydroxyapatite based binder. J. Hazard. Mater. 2019, 369, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Jin, F.; Bo, Y.-L.; Du, Y.-J.; Zheng, J.-X. Leaching and microstructural properties of lead contaminated kaolin stabilized by GGBS-MgO in semi-dynamic leaching tests. Constr. Build. Mater. 2018, 172, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.Y.; Du, Y.J.; Li, F.S.; Guo, G.L.; Yan, X.L.; Li, C.P.; Arulrajah, A.; Wang, F.; Wang, S. Field evaluation of a new hydroxyapatite based binder for ex-situ solidification/stabilization of a heavy metal contaminated site soil around a Pb-Zn smelter. Constr. Build. Mater. 2019, 210, 278–288. [Google Scholar] [CrossRef]
- Du, Y.-J.; Wu, J.; Bo, Y.-L.; Jiang, N.-J. Effects of acid rain on physical, mechanical and chemical properties of GGBS–MgO-solidified/stabilized Pb-contaminated clayey soil. Acta Geotech. 2019. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Du, Y.-J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]
- Dong, Q.; Zhao, X.; Chen, X.; Ma, X.; Cui, X. Long-term mechanical properties of in situ semi-rigid base materials. Road Mater. Pavement. 2020. [Google Scholar] [CrossRef]
- Dong, Q.; Gao, J.L.; Chen, X.Q.; Wang, X. Development of a turpentine cutback asphalt mixture for porous pavement pothole repair. J. Mater. Civ. Eng. 2020, 32, 05020001. [Google Scholar] [CrossRef]
- Tang, F.L.; Ma, T.; Guan, Y.S.; Zhang, Z.X. Parametric modeling and structure verification of asphalt pavement based on BIM-ABAQUS. Auto. Constr. 2020, 111, 103066. [Google Scholar] [CrossRef]
- Chen, T.; Luan, Y.C.; Ma, T.; Zhu, J.Q.; Huang, X.M.; Ma, S.J. Mechanical and microstructural characteristics of different interfaces in cold recycled mixture containing cement and asphalt emulsion. J. Clean. Prod. 2020, 258, 120674. [Google Scholar] [CrossRef]
- Wu, H.-L.; Jin, F.; Du, Y.-J. Influence of wet-dry cycles on vertical cutoff walls made of reactive magnesia-slag-bentonite-soil mixtures. J. Zhejiang Univ.-Sci. A. 2019, 20, 948–960. [Google Scholar] [CrossRef]



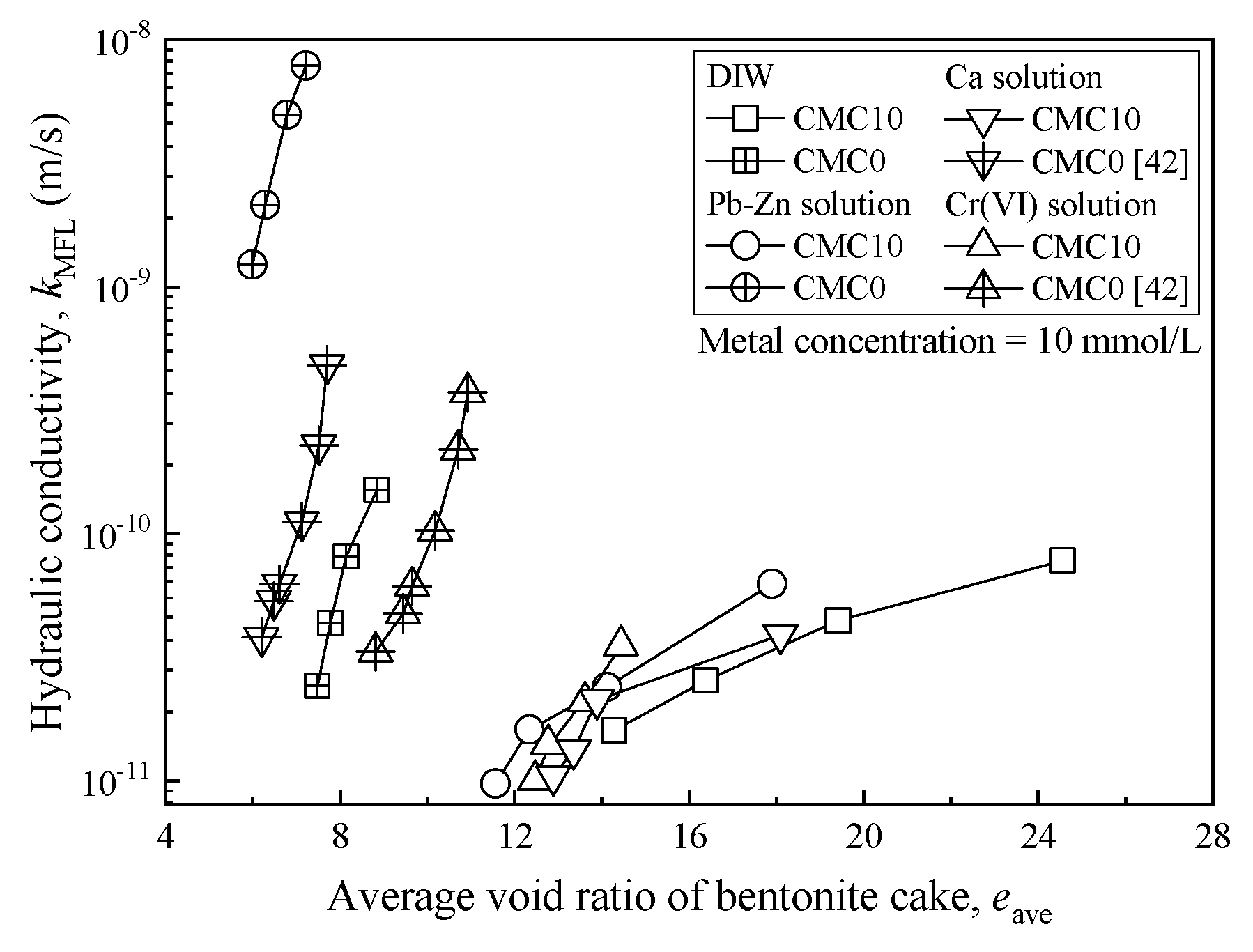
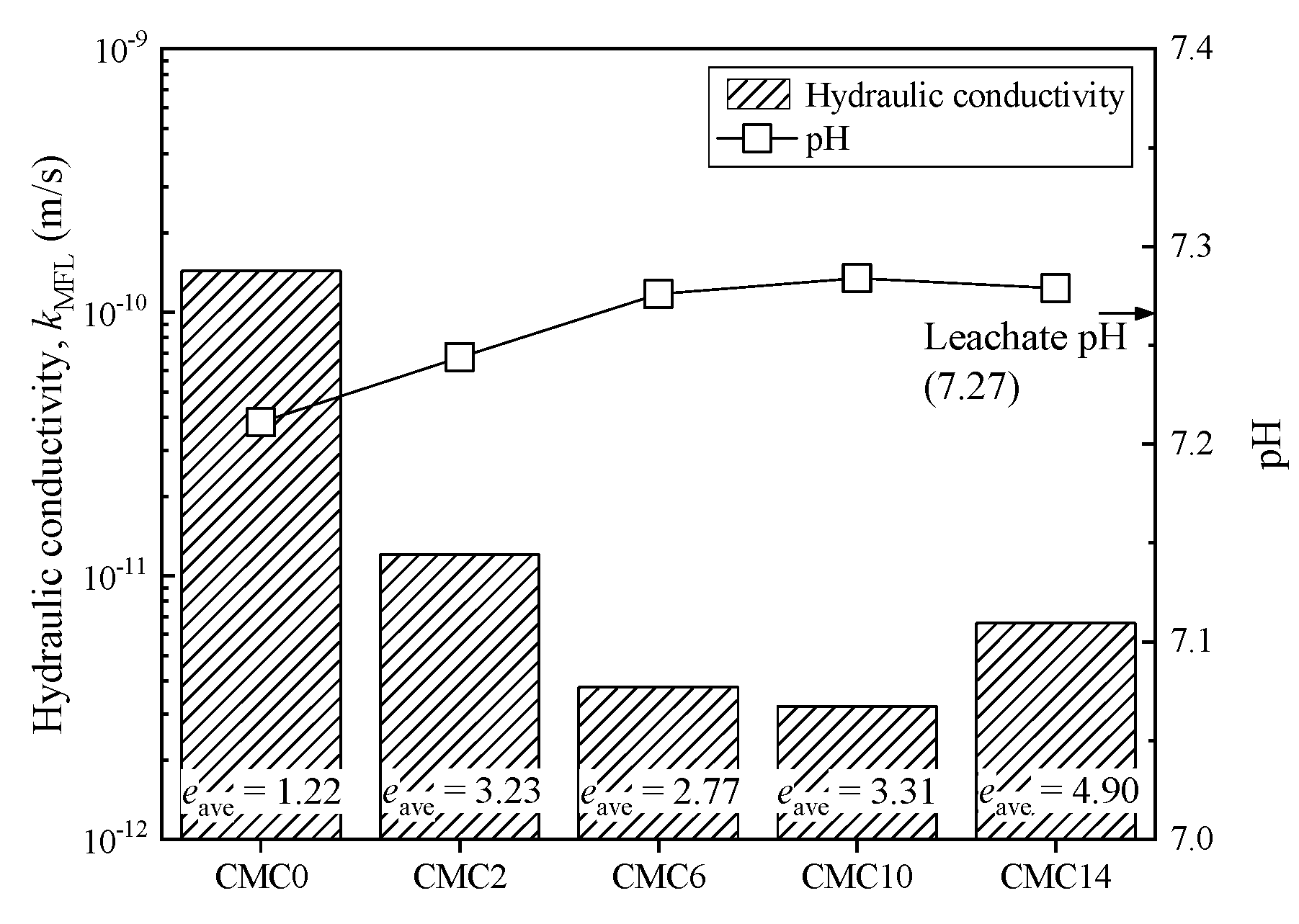
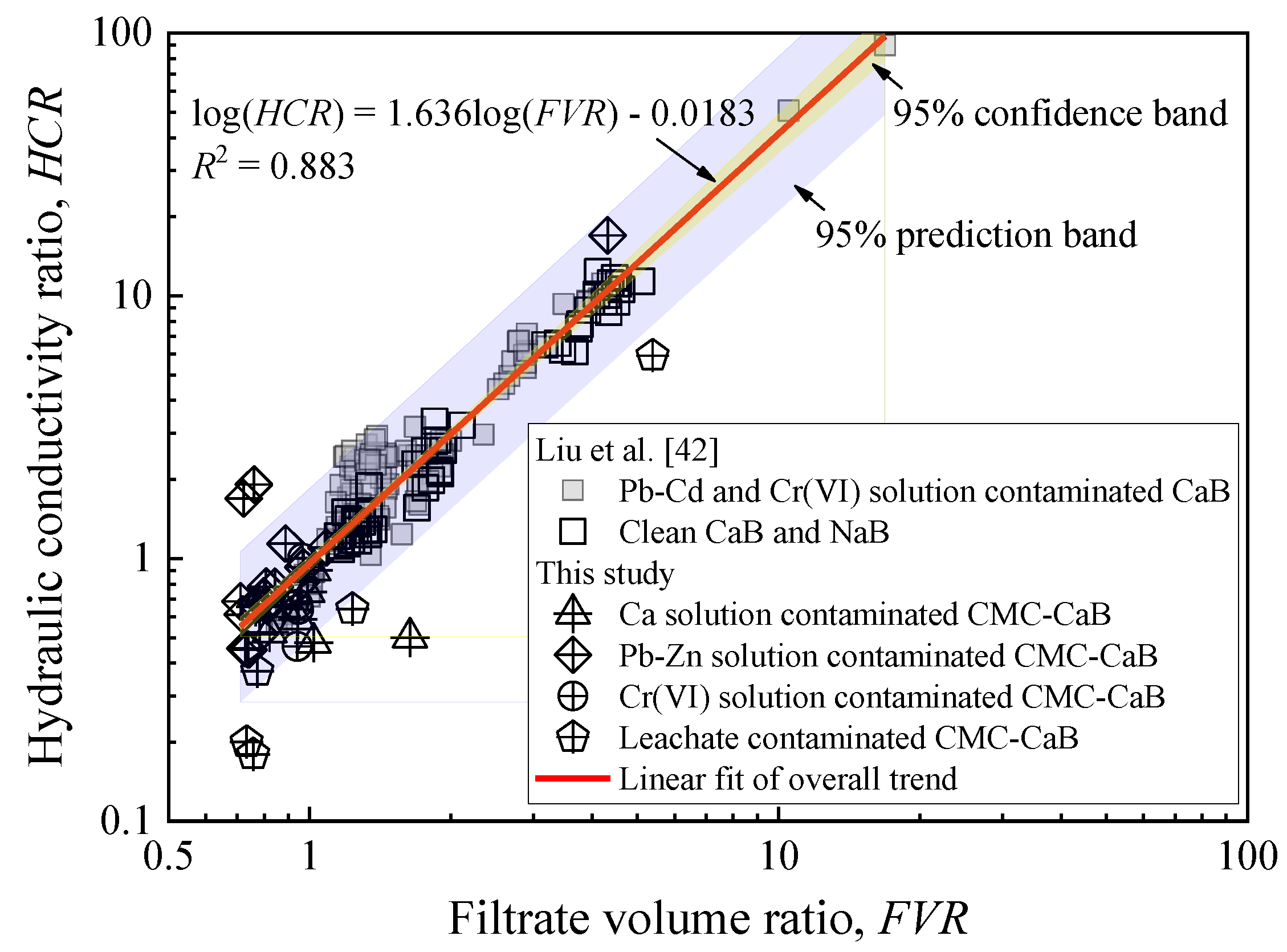
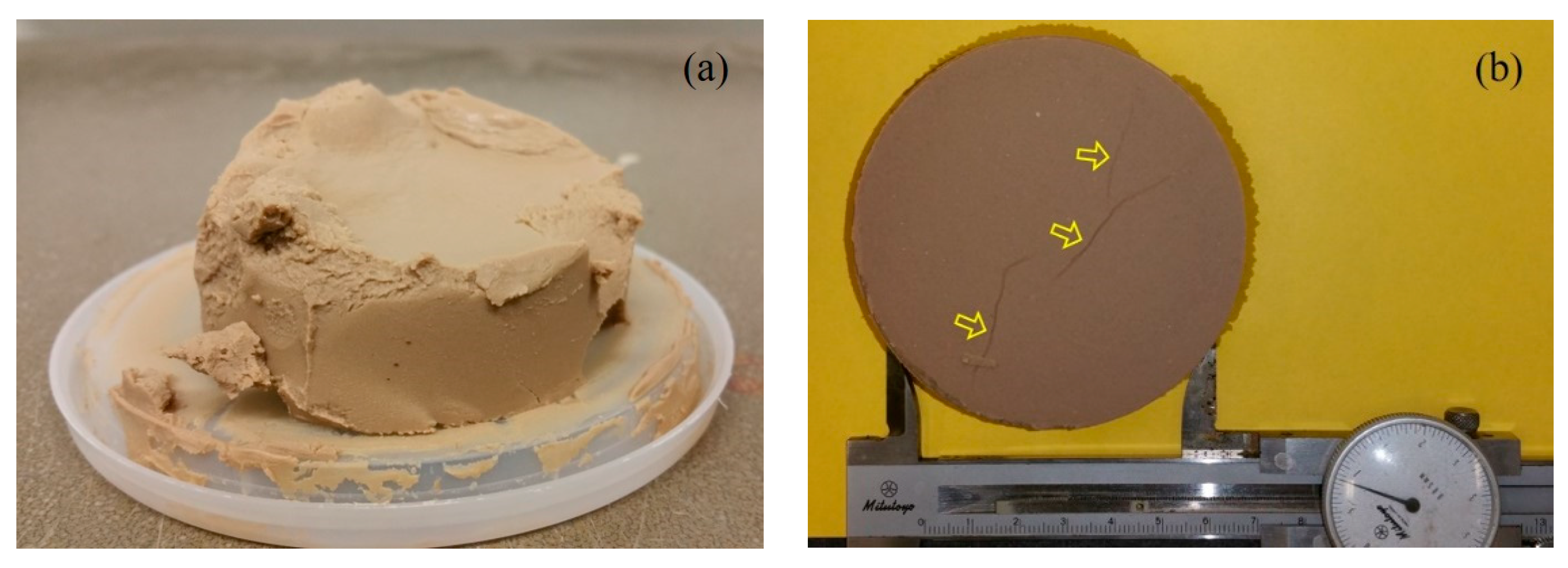
| Property | Testing Method | Value |
|---|---|---|
| Fines Content, FC (%) | [31] | 100 |
| Clay Content, CC (%) | [31] | 49 |
| Organic Content, OC (%) | [32] | 1.18 |
| Liquid Limit, wL (%) | [33] | 269 |
| Plastic Limit, wP (%) | [33] | 34.0 |
| Specific Gravity, Gs | [34] | 2.66 |
| Classification | [35] | CH |
| pH | [36] | 10.33 |
| Swell index, SI (mL/2 g) | [37] | 16.5 |
| Exchangeable metals (cmolc/kg): | [38] | |
| Ca2+ | 14.5 | |
| Mg2+ | 0.6 | |
| Na+ | 32.7 | |
| K+ | 0.3 |
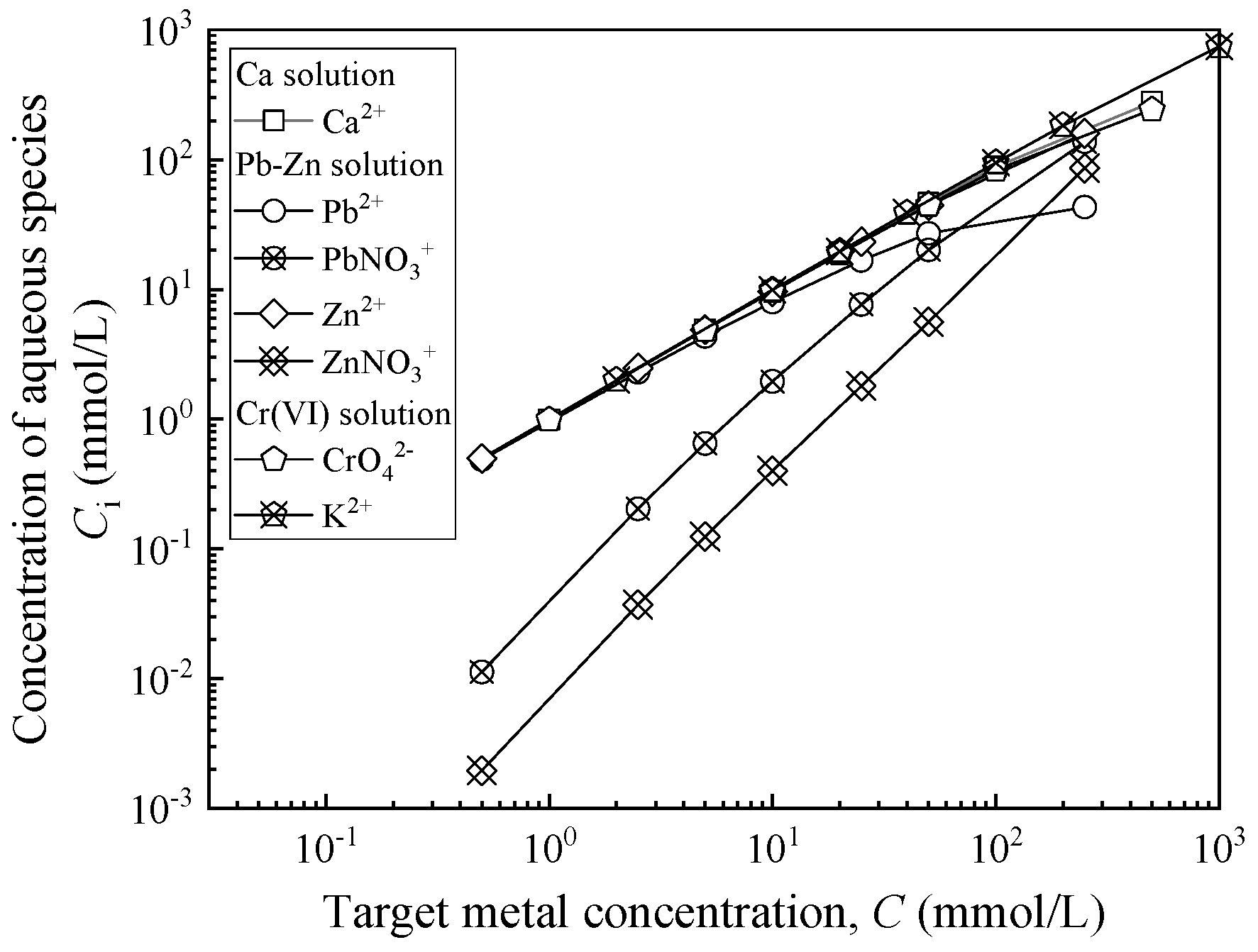
| Chemical Solution | Total Metal Concentration, C (mmol/L) | Ionic Strength, I (mmol/L) | Absolute Viscosity, μ a (mPa·s) | Unit Weight, γL (kN/m3) | pH | Electrical Conductivity, EC (mS/cm) |
|---|---|---|---|---|---|---|
| DIW | N.D. | - | 1.00 | 9.79 | 6.90 | 0.004 |
| Ca solution | 1 | 2.99 | 1.02 | 9.79 | 6.80 | 0.27 |
| Ca solution | 5 | 14.80 | 1.03 | 9.81 | 6.79 | 1.21 |
| Ca solution | 10 | 29.50 | 1.03 | 9.85 | 6.78 | 2.36 |
| Ca solution | 20 | 58.40 | 1.04 | 9.90 | 6.76 | 4.24 |
| Ca solution | 50 | 142.50 | 1.05 | 9.94 | 6.61 | 10.52 |
| Ca solution | 100 | 275.80 | 1.07 | 9.99 | 6.46 | 16.45 |
| Ca solution | 500 | 1444.83 | 1.15 | 10.46 | 5.90 | 72.8 |
| Pb-Zn solution | 1 | 2.97 | 1.26 | 9.79 | 5.49 | 0.31 |
| Pb-Zn solution | 5 | 14.50 | 1.28 | 9.81 | 5.39 | 1.22 |
| Pb-Zn solution | 10 | 28.40 | 1.31 | 9.85 | 5.31 | 2.20 |
| Pb-Zn solution | 20 | 55.00 | 1.38 | 9.90 | 5.21 | 3.86 |
| Pb-Zn solution | 50 | 129.10 | 1.43 | 9.93 | 5.09 | 8.28 |
| Pb-Zn solution | 100 | 239.60 | 1.54 | 10.01 | 4.94 | 15.66 |
| Pb-Zn solution | 500 | 829.00 | 1.69 | 10.87 | 4.33 | 51.15 |
| Cr(VI) solution | 1 | 2.98 | 1.08 | 9.79 | 7.69 | 0.29 |
| Cr(VI) solution | 5 | 14.80 | 1.09 | 9.85 | 8.26 | 1.38 |
| Cr(VI) solution | 10 | 29.20 | 1.10 | 9.88 | 8.40 | 2.58 |
| Cr(VI) solution | 20 | 57.60 | 1.10 | 9.92 | 8.69 | 4.76 |
| Cr(VI) solution | 50 | 139.30 | 1.14 | 9.99 | 8.87 | 9.71 |
| Cr(VI) solution | 100 | 266.60 | 1.18 | 10.07 | 9.20 | 16.32 |
| Cr(VI) solution | 500 | 984.70 | 1.35 | 10.59 | 9.33 | 78.4 |
| CMC Content in Bentonite, CCB (%) | Chemical Solution | Total Metal Concentration, C (mmol/L) | Applied Overall Pressure, P0 (kPa) |
|---|---|---|---|
| 0, 2 | Pb-Zn | 0, 1, 5, 10, 20 | 400 |
| 6, 10, 14 | Pb-Zn | 0, 1, 5, 10, 20 | 400 |
| Optimized CCB | Cr(VI), Ca | 0, 1, 5, 10, 20 | 400 |
| 0, Optimized CCB | landfill leachate | - | 400 |
| 0 | Pb-Zn | 0, 10 | 50, 100, 200, 400 |
| Optimized CCB | Pb-Zn, Cr(VI), Ca | 0, 10 | 50, 100, 200, 400 |
| Sample ID | CMC Content in Bentonite, CCB (%) | Organic Content, OC (%) | Plastic Limit, wP (%) | Specific Gravity, Gs | pH | References |
|---|---|---|---|---|---|---|
| CMC2 | 2 | 2.88 | 38 | 2.63 | 9.55 | This study |
| CMC6 | 6 | 5.77 | 52 | 2.61 | 9.43 | This study |
| CMC10 | 10 | 10.39 | 55 | 2.61 | 9.26 | This study |
| CMC14 | 14 | 13.99 | 56 | 2.59 | 9.21 | This study |
| HC2 | 2 | N.A. | 73 | N.A. | N.A. | [44] |
| HC8 | 8 | N.A. | 61 | N.A. | N.A. | [44] |
| HC2% | 2 | N.A. | 56.22 | 2.53 | N.A. | [18] |
| HC4% | 4 | N.A. | 58.62 | 2.47 | N.A. | [18] |
| HC8% | 8 | N.A. | 61.03 | 2.25 | N.A. | [18] |
| HC16% | 16 | N.A. | 70.55 | 2.40 | N.A. | [18] |
| Sample ID | MFL Test | FWP Test | |||||
|---|---|---|---|---|---|---|---|
| eave1 | ρd3 | kMFL4 | ef2 | ρd3 | i5 | kFWP6 | |
| CMC0 | 7.4 | 0.32 | 2.5 × 10−11 | 6.6 | 0.35 | 94 | 6.1 × 10−11 |
| CMC2 | 9.7 | 0.25 | 1.9 × 10−11 | 9.1 | 0.26 | 135 | 1.6 × 10−11 |
| CMC6 | 16.7 | 0.15 | 2.1 × 10−11 | 15.3 | 0.16 | 172 | 1.0 × 10−11 |
| CMC10 | 14.3 | 0.17 | 1.6 × 10−11 | 13.1 | 0.19 | 119 | 9.6 × 10−12 |
| CMC14 | 18.9 | 0.13 | 1.8 × 10−11 | 17.8 | 0.14 | 137 | 8.6 × 10−12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.-D.; Reddy, K.R.; Yang, Y.-L.; Du, Y.-J. Index Properties, Hydraulic Conductivity and Contaminant-Compatibility of CMC-Treated Sodium Activated Calcium Bentonite. Int. J. Environ. Res. Public Health 2020, 17, 1863. https://doi.org/10.3390/ijerph17061863
Fan R-D, Reddy KR, Yang Y-L, Du Y-J. Index Properties, Hydraulic Conductivity and Contaminant-Compatibility of CMC-Treated Sodium Activated Calcium Bentonite. International Journal of Environmental Research and Public Health. 2020; 17(6):1863. https://doi.org/10.3390/ijerph17061863
Chicago/Turabian StyleFan, Ri-Dong, Krishna R. Reddy, Yu-Ling Yang, and Yan-Jun Du. 2020. "Index Properties, Hydraulic Conductivity and Contaminant-Compatibility of CMC-Treated Sodium Activated Calcium Bentonite" International Journal of Environmental Research and Public Health 17, no. 6: 1863. https://doi.org/10.3390/ijerph17061863






