Refill Adherence Measures and Its Association with Economic, Clinical, and Humanistic Outcomes Among Pediatric Patients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
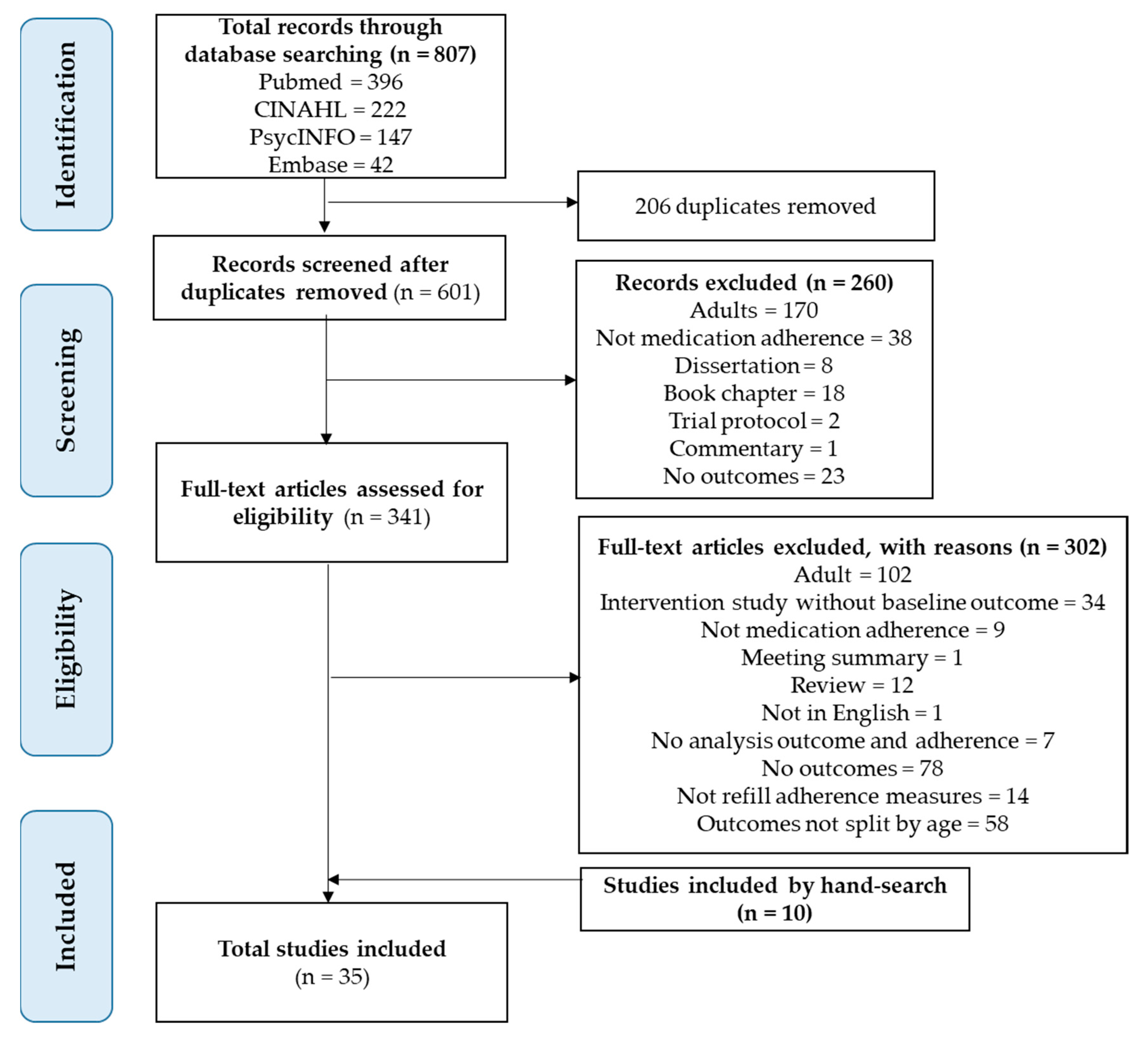
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Measures of Adherence
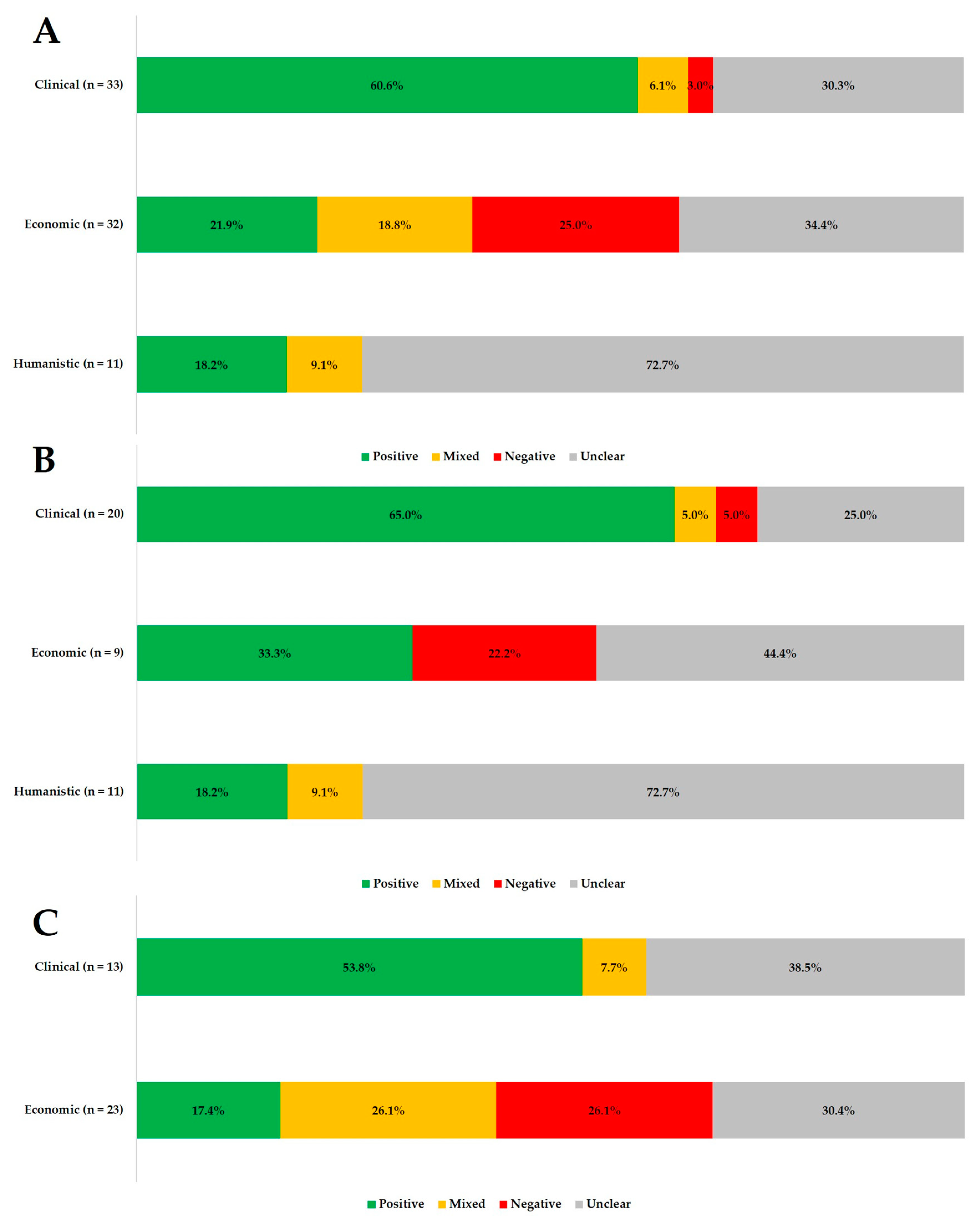
3.3. Overview of Adherence and Patient Outcomes
3.4. Adherence and Clinical Outcomes (n = 33)
3.5. Adherence and Economic Outcomes (n = 32)
3.6. Adherence and Humanistic Outcomes (n = 11)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McGrady, M.E.; Hommel, K.A. Medication adherence and health care utilization in pediatric chronic illness: A systematic review. Pediatrics 2013, 132, 730–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Rachidi, S.; Larochelle, J.M.; Morgan, J.A. Pharmacists and pediatric medication adherence: Bridging the gap. Hosp. Pharm. 2017, 52, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoff, M.A. Adherence to Pediatric Medical Regimens, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Adherence to Long-term Therapies: Evidence for Action. Available online: https://www.who.int/chp/knowledge/publications/adherence_report/en/ (accessed on 27 June 2019).
- Lehmann, A.; Aslani, P.; Ahmed, R.; Celio, J.; Gauchet, A.; Bedouch, P.; Bugnon, O.; Allenet, B.; Schneider, M.P. Assessing medication adherence: Options to consider. Int. J. Clin. Pharm. 2014, 36, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Fresco, P. Medication adherence measures: An overview. Biomed. Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Osterberg, L.; Blaschke, T. Adherence to medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Ivanovska, V.; Rademaker, C.M.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.L.; Mentrikoski, J.M.; Wu, Y.P.; Fredericks, E.M. Practice-based approach to assessing and treating nonadherence in pediatric regimens. Clin. Pract. Pediatr. Psychol. 2014, 2, 322. [Google Scholar] [CrossRef] [Green Version]
- Quittner, A.L.; Modi, A.C.; Lemanek, K.L.; Ievers-Landis, C.E.; Rapoff, M.A. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J. Pediatr. Psychol. 2007, 33, 916–936. [Google Scholar] [CrossRef] [Green Version]
- Harpe, S.E. Using secondary data sources for pharmacoepidemiology and outcomes research. Pharmacotherapy 2009, 29, 138–153. [Google Scholar] [CrossRef]
- Walsh, C.A.; Cahir, C.; Tecklenborg, S.; Byrne, C.; Culbertson, M.A.; Bennett, K.E. The association between medication non-adherence and adverse health outcomes in ageing populations: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2019, 85, 2464–2478. [Google Scholar] [CrossRef]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, e016982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Boven, J.F.; Chavannes, N.H.; van der Molen, T.; Rutten-van Mölken, M.P.; Postma, M.J.; Vegter, S. Clinical and economic impact of non-adherence in COPD: A systematic review. Respir. Med. 2014, 108, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunter, M.J. The role of the ECHO model in outcomes research and clinical practice improvement. Am. J. Manag. Care 1999, 5, S217–S224. [Google Scholar]
- Ingerski, L.M.; Hente, E.A.; Modi, A.C.; Hommel, K.A. Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. J. Pediatr. 2011, 159, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quittner, A.L.; Espelage, D.L.; Drotar, D. Measuring adherence to medical treatments in childhood chronic illness: Considering multiple methods and sources of information. J. Clin. Psychol. Med. Sett. 2000, 7, 41–54. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfield, S.L.; Zuckerman, A.; Anguiano, R.H.; Jolly, J.A.; DeClercq, J.; Wascher, M.; Choi, L.; Knox, S.; Mitchell, D.G. Navigating the wild west of medication adherence reporting in specialty pharmacy. J. Manag. Care Spec. Pharm. 2019, 25, 1073–1077. [Google Scholar] [CrossRef]
- The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 June 2019).
- Watson, D.C.; Farley, J.J. Efficacy of and adherence to highly active antiretroviral therapy in children infected with human immunodeficiency virus type 1. Pediatr. Infect. Dis. J. 1999, 18, 682–689. [Google Scholar] [CrossRef]
- Katko, E.; Johnson, G.M.; Fowler, S.L.; Turner, R.B. Assessment of adherence with medications in human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 2001, 20, 1174–1176. [Google Scholar] [CrossRef]
- Farley, J.; Hines, S.; Musk, A.; Ferrus, S.; Tepper, V. Assessment of adherence to antiviral therapy in HIV-infected children using the medication event monitoring system, pharmacy refill, provider assessment, caregiver self-report, and appointment keeping. J Acquir Immune Defic Syndr 2003, 33, 211–218. [Google Scholar] [CrossRef]
- Bukstein, D.A.; Murphy, K.R.; Katz, L.M.; Ramachandran, S.; Doyle, J.J.; Stern, L.S. Outcomes among a young population of pediatric asthma patients using controller therapies: Results from a retrospective database analysis. Pediatr. Asthma Allergy Immunol. 2007, 20, 211–222. [Google Scholar] [CrossRef]
- Camargo, C.A., Jr.; Ramachandran, S.; Ryskina, K.L.; Lewis, B.E.; Legorreta, A.P. Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am. J. Health Syst. Pharm. 2007, 64, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.C.; Wan, G.J.; Zhang, H.F.; Olfson, M. Injury among stimulant-treated youth with ADHD. J. Atten. Disord. 2008, 12, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chisholm-Burns, M.; Spivey, C.; Rehfeld, R.; Zawaideh, M.; Roe, D.; Gruessner, R. Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. Am. J. Transplant 2009, 9, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Lasmar, L.; Camargos, P.; Champs, N.; Fonseca, M.; Fontes, M.; Ibiapina, C.; Alvim, C.; Moura, J. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy 2009, 64, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Collaco, J.M.; Kole, A.J.; Riekert, K.A.; Eakin, M.N.; Okelo, S.O.; McGrath-Morrow, S.A. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr. Pulmonol. 2012, 47, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Elkout, H.; Helms, P.J.; Simpson, C.R.; McLay, J.S. Adequate levels of adherence with controller medication is associated with increased use of rescue medication in asthmatic children. PLoS ONE 2012, 7, e39130. [Google Scholar] [CrossRef] [Green Version]
- Herndon, J.B.; Mattke, S.; Cuellar, A.E.; Hong, S.Y.; Shenkman, E.A. Anti-inflammatory medication adherence, healthcare utilization and expenditures among Medicaid and children’s health insurance program enrollees with asthma. Pharmacoeconomics 2012, 30, 397–412. [Google Scholar] [CrossRef]
- Robst, J.; Armstrong, M.; Dollard, N. After out-of-home mental health treatment: Atypical antipsychotic medication use and the probability of returning to treatment. Commun. Ment. Health J. 2012, 48, 284–293. [Google Scholar] [CrossRef]
- Eakin, M.N.; Brady, T.; Kandasamy, V.; Fivush, B.; Riekert, K.A. Disparities in antihypertensive medication adherence in adolescents. Pediatr. Nephrol. 2013, 28, 1267–1273. [Google Scholar] [CrossRef] [Green Version]
- Rust, G.; Zhang, S.; Reynolds, J. Inhaled corticosteroid adherence and emergency department utilization among Medicaid-enrolled children with asthma. J. Asthma 2013, 50, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickel, S.; Eid, S.; Eid, N. Prescription fill adherence in pediatric asthma: Are privately insured children at increased risk? Pediatr. Allergy Immunol. Pulmonol. 2016, 29, 125–129. [Google Scholar] [CrossRef]
- Engelkes, M.; Janssens, H.M.; de Jongste, J.C.; Sturkenboom, M.C.; Verhamme, K.M. Prescription patterns, adherence and characteristics of non-adherence in children with asthma in primary care. Pediatr. Allergy Immunol. 2016, 27, 201–208. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ah, Y.M.; Choi, Y.J.; Cho, Y.S.; Kim, K.J.; Lee, J.Y. Antiepileptic drug adherence and persistence in children with epilepsy attending a large tertiary care children’s hospital. Epileptic. Disord. 2016, 18, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Shetty, J.; Greene, S.A.; Mesalles-Naranjo, O.; Kirkpatrick, M. Adherence to antiepileptic drugs in children with epilepsy in a Scottish population cohort. Dev. Med. Child. Neurol. 2016, 58, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, C.M.; Mager, D.; Frazee, S.; Yu, F. Remission in pediatric inflammatory bowel disease correlates with prescription refill adherence rates. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 575–579. [Google Scholar] [CrossRef]
- Ying, C.L.; Shah, N.M. Adherence to insulin treatment in children with type I diabetes mellitus at a hospital in Malaysia. Asian J. Pharm. Clin. Res. 2017, 10, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.H.; Rascati, K.L.; Lopez, K.N.; Moffett, B.S. Increased fracture risk with furosemide use in children with congenital heart disease. J. Pediatr. 2018, 199, 92–98. [Google Scholar] [CrossRef]
- Wang, L.-J.; Lee, S.-Y.; Chou, M.-C.; Yang, K.-C.; Lee, T.-L.; Shyu, Y.-C. Impact of drug adherence on oppositional defiant disorder and conduct disorder among patients with attention-deficit/hyperactivity disorder. J. Clin. Psychiatr. 2018, 79, e1–e7. [Google Scholar] [CrossRef]
- Amarilyo, G.; Chodick, G.; Zalcman, J.; Koren, G.; Levinsky, Y.; Somekh, I.; Harel, L. Poor long-term adherence to secondary penicillin prophylaxis in children with history of rheumatic fever. Semin. Arthritis Rheum 2019, 48, 1019–1024. [Google Scholar] [CrossRef]
- Chua, B.W.B.; Lim, X.; Poh, K.; Caleb, J.S.; Cheen, M.H.H.; Lim, S.T.; Lek, N. Adherence to insulin in singaporean pediatric type 1 diabetes patients and its impact on glycemic control and health-care utilization. Asian J. Pharm. Clin. Res. 2019, 12, 176–182. [Google Scholar] [CrossRef]
- Madjar, N.; Shlosberg, D.; Leventer-Roberts, M.; Akriv, A.; Ghilai, A.; Hoshen, M.; Krivoy, A.; Zalsman, G.; Shoval, G. Childhood methylphenidate adherence as a predictor of antidepressants use during adolescence. Eur. Child. Adolesc. Psychiatr. 2019, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, M.; Whitten, S.; Bajaj, P.; Knight, A.; Spoudeas, H.A. Improved adherence and growth outcomes with jet-delivered growth hormone. J. Pediatr. Endocrinol. Metab. 2019, 32, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Burack, G.; Gaur, S.; Marone, R.; Petrova, A. Adherence to antiretroviral therapy in pediatric patients with human immunodeficiency virus (HIV-1). J. Pediatr. Nurs. 2010, 25, 500–504. [Google Scholar] [CrossRef]
- Elliott, V.; Morgan, S.; Day, S.; Mollerup, L.S.; Wang, W. Parental health beliefs and compliance with prophylactic penicillin administration in children with sickle cell disease. J. Pediatr. Hematol. Oncol. 2001, 23, 112–116. [Google Scholar] [CrossRef]
- Marhefka, S.; Farley, J.; Rodrigue, J.; Sandrik, L.; Sleasman, J.; Tepper, V. Clinical assessment of medication adherence among HIV-infected children: Examination of the Treatment Interview Protocol (TIP). AIDS Care 2004, 16, 323–337. [Google Scholar] [CrossRef]
- Marhefka, S.L.; Tepper, V.J.; Farley, J.J.; Sleasman, J.W.; Mellins, C.A. Brief report: Assessing adherence to pediatric antiretroviral regimens using the 24-hour recall interview. J. Pediatr. Psychol. 2006, 31, 989–994. [Google Scholar] [CrossRef]
- Modi, A.C.; Quittner, A.L. Barriers to treatment adherence for children with cystic fibrosis and asthma: What gets in the way? J. Pediatr. Psychol. 2006, 31, 846–858. [Google Scholar] [CrossRef] [Green Version]
- Witherspoon, D.; Drotar, D. Correlates of adherence to prophylactic penicillin therapy in children with sickle cell disease. Child Health Care 2006, 35, 281–296. [Google Scholar] [CrossRef]
- Oliva-Hemker, M.M.; Abadom, V.; Cuffari, C.; Thompson, R.E. Nonadherence with thiopurine immunomodulator and mesalamine medications in children with Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 180–184. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Calatroni, A.; Telen, M.; Kemper, A.R. Adherence to hydroxyurea therapy in children with sickle cell anemia. J. Pediatr. 2010, 156, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faint, N.R.; Staton, J.M.; Stick, S.M.; Foster, J.M.; Schultz, A. Investigating self-efficacy, disease knowledge and adherence to treatment in adolescents with cystic fibrosis. J. Paediatr. Child Health 2017, 53, 488–493. [Google Scholar] [CrossRef] [PubMed]
- World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 27 June 2019).
- Bramley, T.J.; Nightengale, B.S.; Frech-Tamas, F.; Gerbino, P.P. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J. Manag. Care Pharm. 2006, 12, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pladevall, M.; Williams, L.K.; Potts, L.A.; Divine, G.; Xi, H.; Lafata, J.E. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care 2004, 27, 2800–2805. [Google Scholar] [CrossRef] [Green Version]
- Vreeman, R.C.; Wiehe, S.E.; Pearce, E.C.; Nyandiko, W.M. A systematic review of pediatric adherence to antiretroviral therapy in low-and middle-income countries. Pediatr. Infect. Dis. J. 2008, 27, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Sangeda, R.Z.; Mosha, F.; Prosperi, M.; Aboud, S.; Vercauteren, J.; Camacho, R.J.; Lyamuya, E.F.; Van Wijngaerden, E.; Vandamme, A.-M. Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in resource-limited settings. BMC Public Health 2014, 14, 1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellad, W.F.; Thorpe, C.T.; Steiner, J.F.; Voils, C.I. The myths of medication adherence. Pharmacoepidemiol. Drug. Saf. 2017, 26, 1437–1441. [Google Scholar] [CrossRef]
- Karve, S.; Cleves, M.A.; Helm, M.; Hudson, T.J.; West, D.S.; Martin, B.C. Good and poor adherence: Optimal cut-point for adherence measures using administrative claims data. Curr. Med. Res. Opin. 2009, 25, 2303–2310. [Google Scholar] [CrossRef]
- Baumgartner, P.C.; Haynes, R.; Hersberger, K.E.; Arnet, I. A systematic review of medication adherence thresholds dependent of clinical outcome. Front. Pharmacol. 2018, 9, 1290. [Google Scholar] [CrossRef]
- Lo-Ciganic, W.-H.; Donohue, J.M.; Thorpe, J.M.; Perera, S.; Thorpe, C.T.; Marcum, Z.A.; Gellad, W.F. Using machine learning to examine medication adherence thresholds and risk of hospitalization. Med. Care 2015, 53, 720. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.M.; Nau, D.P.; Cramer, J.A.; Benner, J.; Gwadry-Sridhar, F.; Nichol, M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007, 10, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buono, E.W.; Vrijens, B.; Bosworth, H.B.; Liu, L.Z.; Zullig, L.L.; Granger, B.B. Coming full circle in the measurement of medication adherence: Opportunities and implications for health care. Patient Prefer. Adher. 2017, 11, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.E.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author (year) | Study Setting | Medication Refill Data Source | Study Design (Duration/ Time Scale) | Medication Refill Indicator/ Definition of Adequate Level of Adherence | Sample Size | Study Participants’ Age (Years) |
|---|---|---|---|---|---|---|
| Asthma | ||||||
| Elkout et al. (2012) | UK | Claims database | Retrospective cohort study (5 years) | MPR: 0.80–1.20 | 3172 | Mean ICS only: 6 LI: 6 LABA/ICS: 9 LABA + ICS: 8 |
| Herndon et al. (2012) | USA | Claims database | Retrospective cohort study (3 years) | MPR ≥ 0.50 | 18,456 | Range (number of patients) 2–4: (4203) 5–11: (9998) 12–18: (4255) |
| Camargo et al. (2007) | USA | Claims database | Retrospective cohort study (4.5 years) | BIS: MPR > 0.08 Non-nebulized ICS: MPR >0.08 LI: MPR > 0.16 | 10,976 | Mean (SD) 3.8 (2.2) |
| Lasmar et al. (2009) | Brazil | Individual pharmacy | Prospective cohort study (1 year) | Adherence rate1: no specific threshold defined | 122 | Median 6 (range: 3.1–12.2) |
| Bickel et al. (2016) | USA | Individual pharmacy | Prospective cohort study (6 months) | First prescription filled within 14 days | 77 | Mean 6.35 (4.58) |
| Engelkes et al. (2016) | Netherlands | Claims database | Retrospective cohort study (12 years) | MPR > 0.87 | 14,303 | 10.2 (4.0) |
| Bukstein et al. (2007) | USA | Claims database | Retrospective cohort study (9 years) | Number of prescriptions filled: ≥ 2 post-index date | 11,407 | Mean (SD) 2.2 (0.92) |
| Rust et al. (2013) | USA | Claims database | Retrospective cohort study (1 year) | Proportion of prescribed days covered2 ≥ 0.50 | 43,156 | Mean (SD) Controller-to-total asthma medication ratio ≥ 0.5: 8.1 (2.4) Controller-to-total asthma medication ratio < 0.5: 7.8 (2.3) |
| Human immunodeficiency virus infection | ||||||
| Watson et al. (1999) | USA | Individual pharmacy | Retrospective cohort study (6 months) | Pharmacy refill rate3 ≥ 0.75 | 72 | Range (number of patients) 3–23 months: 11 2–5 years: 28 6–12 year: 33 |
| Marhefka et al. (2004) | USA | Individual pharmacy | Prospective cross-sectional study (3 months) | Pharmacy refill rate 1 ≥ 0.90 | 51 | Mean age (SD) 8.76 (3.06) |
| Burack et al. (2010) | USA | Individual pharmacy | Prospective cross-sectional study (3 years) | Number of prescriptions filled: No missing refill in 6 months | 46 | Mean (SD) Viral load ≤ 400 copies/ml: 11.2 (3.4) Viral load > 400 copies/ml: 11.5 (3.1) |
| Marhefka et al. (2006) | USA | Individual pharmacy | Prospective cross-sectional study (not specified) | Pharmacy refill rate3 = 1.00 | 51 | Mean (range) 8 (2–12) |
| Farley et al. (2003) | USA | Individual pharmacy | Prospective cohort study (2 years) | Pharmacy refill rate1: no specific threshold defined | 26 | Mean (SD) 6.9 (3.2) |
| Katko et al. (2001) | USA | Individual pharmacy | Prospective cohort study (1 year) | Pharmacy refill rate3 ≥ 0.90 | 34 | Median (range) Adherent: 7.5 (1.50–16.3) Non-adherent: 8.9 (2.90–19.9) |
| Attention deficit disorder | ||||||
| Wang et al. (2018) | Taiwan | Claims database | Retrospective cohort study (12 years) | MPR ≥ 0.50 | ODD cohort: 32168CD cohort: 32676 | Mean (SD) ODD cohort: 9.13 (2.87) CD cohort: 9.15 (2.86) |
| Marcus et al. (2008) | USA | Claims database | Retrospective cohort study (4 years) | Low: MPR < 0.30 Medium: MPR 0.30–0.70 High: MPR > 0.70 | 11,770 | Range (number of patients) 6–12: 9,916 13–17: 1854 |
| Inflammatory bowel disease | ||||||
| Oliva-Hemker et al. (2007) | USA | Individual pharmacy | Prospective cross-sectional study (2 years) | Refill score3 ≥ 0.80 | 51 | Mean 14.2 (3.2) |
| Samson et al. (2017) | USA | Claims database | Retrospective cohort study (1 year) | MPR ≥ 0.80 | 228 | Mean (SD) MPR < 0.80: 16.7 (3.3) MPR ≥ 0.80: 15.7 (3.3) |
| Sickle cell disease | ||||||
| Thornburg et al. (2010) | USA | Individual pharmacy | Prospective cross-sectional study (3 years) | Duration of supply: ≥ 5 months in 6 months period | 75 | Mean (range) 11.2 (3.5–17.8) |
| Witherspoon et al. (2006) | USA | Individual pharmacy | Prospective cross-sectional study (not defined) | Duration without medication: ≤ 7 days per month | 30 | Mean (SD) 2.95 (1.48) |
| Elliot et al. (2001) | USA | Individual pharmacy | Prospective cross-sectional study (5 months) | Prescription filled within 14 days | 50 | Mean month (SD) 28.3 (16.0) |
| Epilepsy | ||||||
| Lee et al. (2016) | Korea | Individual pharmacy | Retrospective cohort study (3.5 years) | MPR ≥ 0.80 | 1172 | Range (number of patients): 1 (51) 2–5 (208) 6–11 (486) 12–18 (427) |
| Shetty et al. (2016) | UK | Claims database | Retrospective cohort study (2 years) | Adherence Index1 > 0.90 | 320 | Median (IQR) 10 (7–14) |
| Congenital heart disease | ||||||
| Heo et al. (2018) | USA | Claims database | Retrospective cohort study(7 years) | MPR ≥ 0.70 | Propensity score matched cohort: 3912 | Mean age (SD) MPR ≥ 0.70: 1.67 (2.30) MPR < 0.70: 1.51 (2.06) Not on furosemide: 1.58 (2.08) |
| Type 1 diabetes mellitus | ||||||
| Ying et al. (2017) | Malaysia | Individual pharmacy | Retrospective cohort study (5 years) | MPR ≥ 0.80 | 57 | Mean (SD) 14.4 (3.41) |
| Chua et al. (2019) | Singapore | Individual pharmacy | Retrospective cohort study (5 years) | MPR ≥ 1.00 | 206 | Mean (SD) Adherent: 11.6 (3.7) Non-adherent: 12.4 (4.1) |
| Renal transplant | ||||||
| Chisholm-Burns et al. (2009) | USA | Claims database | Retrospective cohort study (6 years) | MPR ≥ 0.92 | 877 | Mean (SD) 11.9 (5.35) |
| Hypertension | ||||||
| Eakin et al. (2013) | USA | Individual pharmacy | Prospective cohort study (not specified) | MPR ≥ 0.65 | 21 | Mean (SD) 14.7 (2.0) |
| Chronic lung disease | ||||||
| Collaco et al. (2010) | USA | Claims database | Prospective cohort study (2.5 years) | MPR: no specific threshold defined | 194 of which 33 had prescription claims | Mean month (SD) Patients with prescription claims: - At discharge: 4.3 (2.5) - At first clinic visit: 7.0 (3.1) |
| Patients receiving psychiatric residential/foster care | ||||||
| Robst et al. (2012) | USA | Claims database | Retrospective cohort study (3 years) | MPR: no specific threshold defined | 2304 treatment episodes | Range (number of patients) 6–12: 749 13–17: 1,555 |
| Patients on methylphenidate | ||||||
| Madjar et al. (2019) | Israel | Claims database | Prospective cohort study (12 years) | MPR ≥ 0.50 | 6834 | Non-ADM, age, (number of patients): 6: 1555 7: 2295 8: 2414 ADM, age, (number of patients): 6: 156 7: 227 8: 187 |
| Cystic fibrosis | ||||||
| Faint et al. (2017) | Australia | Individual pharmacy | Prospective cohort study (6 months) | MPR: no specific threshold defined | 39 | Median 14 (range: 12–17) |
| Cystic fibrosis or asthma | ||||||
| Modi et al. (2006) | USA | Individual pharmacy | Prospective cross-section study (3 months) | Prescription refill rate3: no specific threshold defined | 73 | Mean Cystic fibrosis: 10.1 Asthma: 9.7 |
| Patients on growth hormones | ||||||
| Michaelidou et al. (2019) | UK | Individual pharmacy | Retrospective cohort study (3 years) | PDC > 0.80 | 52 | Mean (SD) 8.50 (3.78) |
| Rheumatic fever | ||||||
| Amarilyo et al. (2019) | Israel | Claims database | Retrospective cohort study (19 years 5 months) | PDC > 0.80 | 842 | Mean (SD) Oral: 8.6 (3.7) Intramuscular: 10.9 (3.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, B.; Morgan, J.; Yap, K.Z. Refill Adherence Measures and Its Association with Economic, Clinical, and Humanistic Outcomes Among Pediatric Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2133. https://doi.org/10.3390/ijerph17062133
Chua B, Morgan J, Yap KZ. Refill Adherence Measures and Its Association with Economic, Clinical, and Humanistic Outcomes Among Pediatric Patients: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(6):2133. https://doi.org/10.3390/ijerph17062133
Chicago/Turabian StyleChua, Brandon, James Morgan, and Kai Zhen Yap. 2020. "Refill Adherence Measures and Its Association with Economic, Clinical, and Humanistic Outcomes Among Pediatric Patients: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 6: 2133. https://doi.org/10.3390/ijerph17062133





