Effect of Vermicompost Amendment on the Accumulation and Chemical Forms of Trace Metals in Leafy Vegetables Grown in Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Basic Properties of VRM and Soils
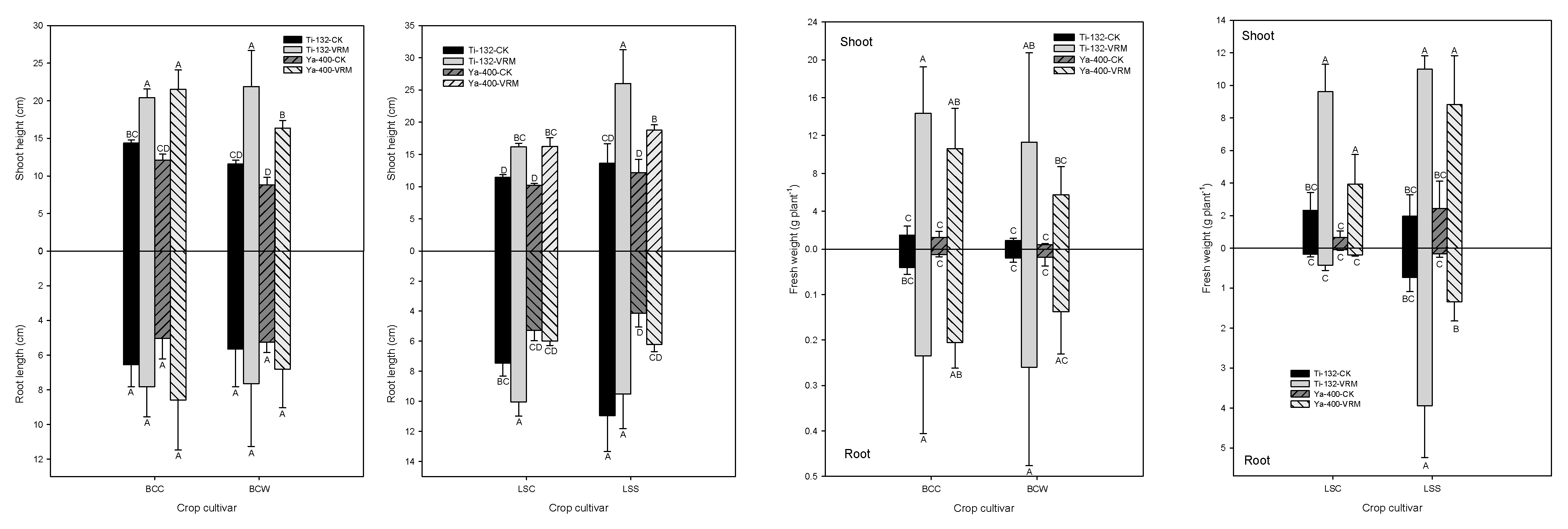
3.2. Growth Exhibitions
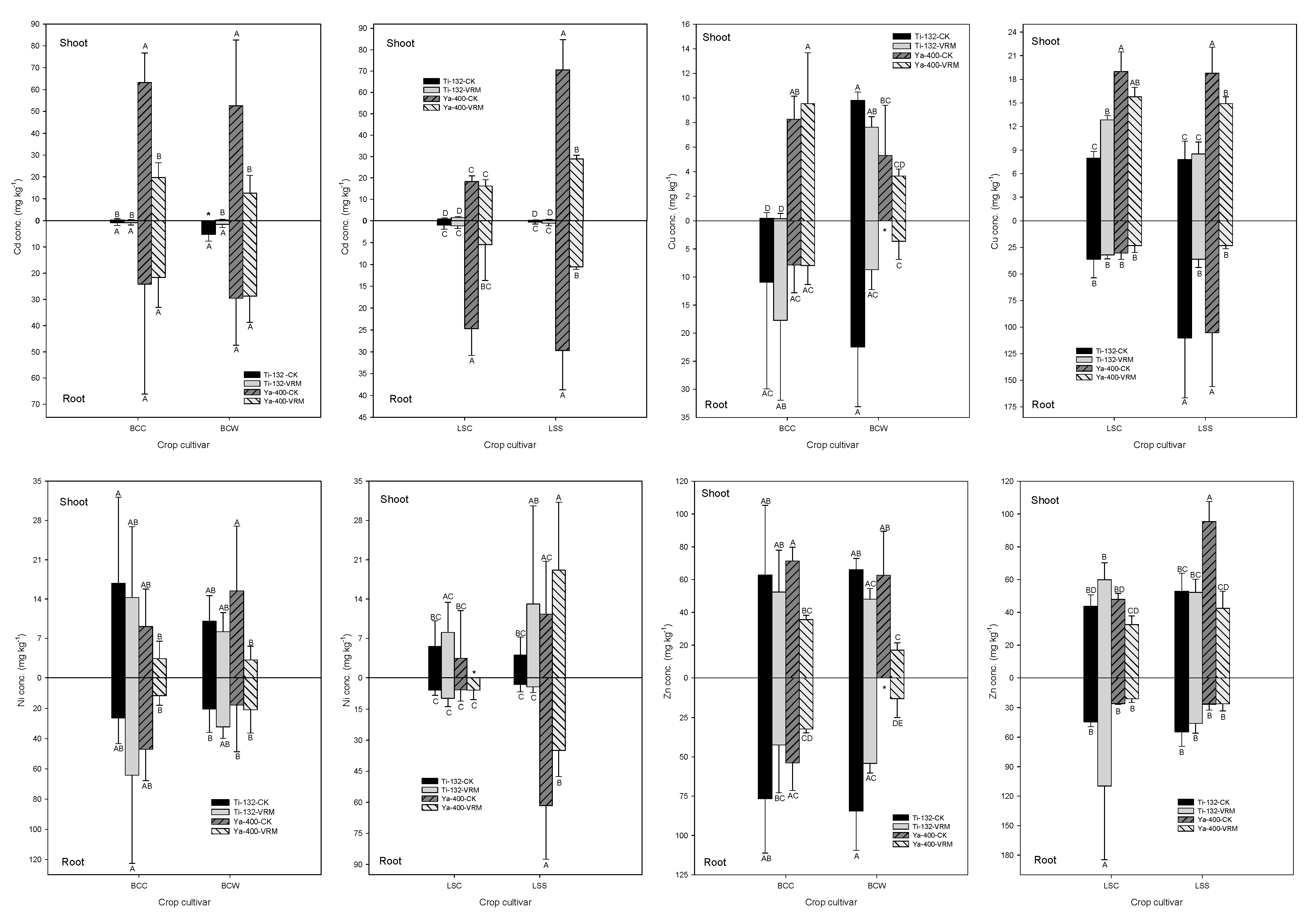
3.3. Accumulation of TMs
3.4. Chemical Forms
3.5. Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.Y.; Chen, S.K.; Wang, J.S.; Lu, C.J.; Lai, H.Y. Farmland trace metal contamination and management model—model development and a case study in central Taiwan. Sustainability 2020, 12, 10066. [Google Scholar] [CrossRef]
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Chen, B.C.; Lai, H.Y. Subcellular distribution of cadmium in two paddy rice varieties with different cooking methods. Agr. Sci. 2016, 7, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Cai, Q. Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ. Exp. Bot. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.T.; Chen, Z.S.; Hong, C.Y. Cadmium-induced physiological response and antioxidant enzyme changes in the novel cadmium accumulator, Tagetes patula. J. Hazard. Mater. 2011, 189, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Y.; Chen, Z.F.; Sun, K.; Yan, D.; Kang, M.J.; Zhao, Y. Effect of cadmium on the physiological parameters and the subcellular cadmium localization in the potato (Solanum tuberosum L.). Ecotox. Environ. Safe. 2013, 97, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.T.; Liu, C.; Geng, B. Phytotoxic effects of copper on nitrogen metabolism and plant growth in Brassica pekinensis Rupr. Ecotox. Environ. Safe. 2006, 64, 273–280. [Google Scholar] [CrossRef]
- Küpper, H.; Zhao, F.J.; McGrath, S.P. Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant. Physiol. 1999, 119, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Jiao, Y.Q.; Sun, B.L.; Qin, R.; Jiang, W.S.; Liu, D.H. Cadmium-mediated oxidative stress and ultrastructural changes in root cells of poplar cultivars. S. Afr. J. Bot. 2012, 83, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. Ion exchange properties of roots and ionic interactions within root apoplasm: Their role in ion accumulation by plants. Bot. Rev. 1980, 46, 75–99. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wu, J.F.; Shang, D.R.; Ning, J.S.; Zhai, Y.X.; Shend, X.F.; Ding, H.Y. Subcellular distribution and chemical forms of cadmium in the edible seaweed, Porphyra yezoensis. Food Chem. 2015, 168, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.L.; Huang, B.F.; Yang, Z.Y.; Yuan, J.G.; Zhang, Y.D. Comparison of cadmium subcellular distribution in different organs of two water spinach (Ipomoea aquatica Forsk.) cultivars. Plant. Soil 2013, 372, 431–444. [Google Scholar] [CrossRef]
- Khandekar, S.; Leisner, S. Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. J. Plant. Physiol. 2011, 168, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Censi, V.; Di Girolamo, V.; De Paolis, A.; Di Toppi, L.S.; Aromolo, R.; Costantino, P.; Cardarelli, M. Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 2006, 223, 180–190. [Google Scholar] [CrossRef]
- Lai, H.Y. Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere 2015, 138, 370–376. [Google Scholar] [CrossRef]
- Wu, F.B.; Dong, J.; Qian, Q.Q.; Zhang, G.P. Subcellular distribution and chemical form of Cd and Ca-Zn interaction in different barley genotypes. Chemosphere 2005, 60, 1437–1446. [Google Scholar] [CrossRef]
- Fu, X.; Dou, C.; Chen, Y.; Chen, X.; Shi, J. Subcellular distribution and chemical forms of cadmium in Phytplacca americana L. J. Hazard. Mater. 2011, 186, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.M.; Lai, H.Y. Effect of inoculation with arbuscular mycorrhizal fungi and blanching on the bioaccessibility of heavy metals in water spinach (Ipomoea aquatica Forsk.). Ecotox. Environ. Safe. 2018, 162, 563–570. [Google Scholar] [CrossRef]
- Domínguez, J.; Aira, M.; Gómez-Brandón, M. Vermicomposting: Earthworms enhance the work of microbes. In Microbes at Work: From Wastes to Resources; Insam, H., Franke-Whittle, I., Goberna, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 93–114. [Google Scholar]
- Suleiman, H.; Rorat, A.; Grobelak, A.; Grosser, A.; Milczarek, M.; Płytycz, B.; Kacprzak, M.; Vandenbulcke, F. Determination of the performance of vermicomposting process applied to sewage sludge by monitoring of the compost quality and immune responses in three earthworm species: Eisenia fetida, Eisenia andrei and Dendrobaena veneta. Bioresour. Technol. 2017, 241, 103–112. [Google Scholar] [CrossRef]
- Da Silva, E.N.; Heerdt, G.; Cidade, M.; Pereira, C.D.; Morgon, N.H.; Cadore, S. Use of in vitro digestion method and theoretical calculations to evaluate the bioaccessibility of Al, Cd, Fe and Zn in lettuce and cole by inductivity coupled plasma mass spectrometry. Microchem. J. 2015, 119, 152–158. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V.K. Recycling of organic wastes by employing Eisenia fetida. Bioresour. Technol. 2011, 102, 2874–2880. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Karmegam, N.; Tripathi, S.; Kannan, S.; Thangaraj, R. Environment-friendly management of textile mill wastewater sludge using epigeic earthworms: Bioaccumulation of heavy metals and metallothionein production. J. Environ. Manag. 2020, 254, 109813. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, Z.M.; Li, X.; Liu, H.Y.; Li, N.; Wei, S.Q. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 11. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef]
- García, A.C.; Izquierdo, F.G.; de Amaral Sobrinho, N.M.B.; Castro, R.N.; Santos, L.A.; de Souza, L.G.A.; Berbara, R.L.L. Humified insoluble solid for efficient decontamination of nickel and lead in industrial effluents. J. Environ. Chem. Eng. 2013, 1, 916–924. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tian, Y.; Hu, D.F.; Fan, J.S.; Shen, M.C.; Zeng, G.M. Is vermicompost the possible in situ sorbent? Immobilization of Pb, Cd and Cr in sediment with sludge derived vermicompost, a column study. J. Hazard. Mater. 2019, 367, 83–90. [Google Scholar] [CrossRef]
- Paltseva, A.; Cheng, Z.Q.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in garden-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640, 273–283. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.X.; Shen, M.C.; Zeng, G.M.; Zhou, M.C.; Li, M.R. Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour. Technol. 2016, 218, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.Y.; Xu, Z.L.; Wu, J.Y.; Tian, G.M. Bioaccumulation of heavy metals in the earthworm Eisenia fetida in relation to bioavailable metal concentrations in pig manure. Bioresour. Technol. 2010, 101, 3430–3436. [Google Scholar] [CrossRef]
- Sizmur, T.; Hodson, M.E. Do earthworms impact metal mobility and availability in soil? A review. Environ. Pollut. 2009, 157, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suthar, S.; Singh, S. Feasibility of vermicomposting in biostabilization of sludge from a distillery industry. Sci. Total Environ. 2008, 394, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Lo, S.L. Effect of composting on characterization and leaching of copper, manganese, and zinc from swine manure. Environ. Pollut. 2001, 114, 119–127. [Google Scholar] [CrossRef]
- Rorat, A.; Suleiman, H.; Grobelak, A.; Grosser, A.; Kacprzak, M.; Pytycz, B.; Vandenbulcke, F. Interactions between sewage sludge-amended soil and earthworms-comparison between Eisenia fetida and Eisenia andrei composting species. Environ. Sci. Pollut. Res. 2016, 23, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Song, X.C.; Liu, M.Q.; Wu, D.; Qi, L.; Ye, C.L.; Jiao, J.G.; Hu, F. Heavy metal and nutrient changes during vermicomposting animal manure spiked with mushroom residues. Waste Manage. 2014, 34, 1977–1983. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, C.H.; Xing, M.Y.; Lin, Y.A. Enhancement stabilization of heavy metals (Zn, Pb, Cr and Cu) during vermifiltration of liquid-state sludge. Bioresour. Technol. 2013, 146, 649–655. [Google Scholar] [CrossRef]
- Santana, N.A.; Ferreira, P.A.A.; Soriani, H.H.; Brunetto, G.; Nicoloso, F.T.; Antoniolli, Z.I.; Jacquesa, R.J.S. Interaction between arbuscular mycorrhizal fungi and vermicompost on copper phytoremediation in a sandy soil. Appl. Soil Ecol. 2015, 96, 172–182. [Google Scholar] [CrossRef]
- Luo, X.S.; Yu, S.; Zhu, Y.G.; Li, X.D. Trace metal contamination in urban soils of China. Sci. Total Environ. 2012, 421, 17–30. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, K.K. Risk assessment of soil, water and crops in abandoned Geumryeong mine in South Korea. J. Geochem. Explor. 2013, 128, 117–123. [Google Scholar] [CrossRef]
- Horner, N.S.; Beauchemin, D. The effect of cooking and washing rice on the bio-accessibility of As, Cu, Fe, V and Zn using an on-line continuous leaching method. Anal. Chim. Acta. 2013, 758, 28–35. [Google Scholar] [CrossRef]
- Llorente-Mirandes, T.; Llorens-Munoz, M.; Funes-Collado, V.; Sahuquillo, A.; Lopez-Sanchez, J.F. Assessment of arsenic bioaccessibility in raw and cooked edible mushrooms by a PBET method. Food Chem. 2016, 194, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Pai, C.Y.; Lai, H.Y. Amendment of husk biochar on accumulation and chemical form of cadmium in lettuce and pak-choi grown in contaminated soil. Water 2020, 12, 868. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Mulvaney, R.L. Nitrogen—inorganic forms. In Methods of soil analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3; SSSA Book Series No. 5, SSSA and ASA; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc. and ASA Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Murer, E.J.; Baumgarten, A.; Eder, G.; Gerzabek, M.H.; Kandeler, E.; Rampazzo, N. An improved sieving machine for estimation of soil aggregate stability (sas). In Soil Structure/Soil Biota Interrelationships; Brussaardm, L., Kooistra, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 539–547. [Google Scholar]
- Jones, J.B.; Case, V.W. Sampling, handling and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA Inc.: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- European Food Safety Authority (EFSA). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- Mania, M.; Rebeniak, M.; Postupolski, J. Food as a source of exposure to nickel. Roczniki Państwowego Zakładu Higieny 2019, 70, 393–399. [Google Scholar] [PubMed]
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives-Copper (JECFA). Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2824&fbclid=IwAR17B0rQPBhh9lKnWMRX01NSqBqiWu0YD8PovIrVPooOx0dRA2GghI4RrGY (accessed on 16 June 2021).
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)-Zinc. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=4197&fbclid=IwAR1D-IveiS8lBjm-8__7KlxpDiw2o_fYKfPPgchNVM1g5Wu7m9JgebAvbK8 (accessed on 16 June 2021).
- Wu, Z.C.; Xu, S.J.; Shi, H.Z.; Zhao, P.H.; Liu, X.X.; Li, F.R.; Deng, T.H.B.; Du, R.Y.; Wang, X.; Wang, F.H. Comparison of foliar silicon and selenium on cadmium absorption, compartmentation, translocation and the antioxidant system in Chinese flowering cabbage. Ecotox. Environ. Safe. 2018, 166, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, K.; Garg, V.K. Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia foetida (Sav.). Bioresour. Technol. 2018, 250, 708–715. [Google Scholar] [CrossRef]
- Salati, S.; Quadri, G.; Tambone, F.; Adani, F. Fresh organic matter of municipal solid waste enhances phytoextraction of heavy metals from contaminated soil. Environ. Pollut. 2010, 158, 1899–1906. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, G.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [Green Version]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Filipović, L.; Romić, M.; Romić, D.; Filipović, V.; Ondrašek, G. Organic matter and salinity modify cadmium soil (phyto)availability. Ecotox. Environ. Safe. 2018, 147, 824–831. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Pevicharova, G.; Ivanov, K. Effect of organic amendments on heavy metals uptake by potato plants. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, QLD, Australia, 1–6 August 2010. [Google Scholar]
- Alam, M.; Hussain, Z.; Khan, A.; Khan, M.A.; Rab, A.; Asif, M.; Shah, M.A.; Muhammad, A. The effects of organic amendments on heavy metals bioavailability in mine impacted soil and associated human health risk. Sci. Hortic. 2020, 262, 109067. [Google Scholar] [CrossRef]
- Mignardi, S.; Corami, A.; Ferrini, V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 2012, 86, 354–360. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Rosa, D.J.; Prado, J.P.C.; Borghezan, M.; de Melo, G.W.B.; Soares, C.; Comin, J.J.; Simao, D.G.; Brunetto, G. Reduction of copper phytotoxicity by liming: A study of the root anatomy of young vines (Vitis labrusca L.). Plant. Phyiol. Biochem. 2015, 96, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshadri, B.; Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.D.; Tsang, D.C.W.; Ok, Y.S.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef]
- Valipour, M.; Shahbazi, K.; Khanmirzaei, A. Chemical immobilization of lead, cadmium, copper, and nickel in contaminated soils by phosphate amendments. Clean Soil Air Water 2016, 44, 572–578. [Google Scholar] [CrossRef]
- He, S.Y.; Wu, Q.L.; He, Z.L. Synergetic effects of DA-6/GA3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 2014, 117, 132–138. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission of cadmium in food. ESFA J. 2009, 980, 1–139. [Google Scholar]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary cadmium intake and sources in the US. Nutrients 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Crop and Treatment 1 | pH (w/v = 1/5) | EC | OM | Avail N | Avail P | Exch Ca | Exch Mg | Exch K | |
|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | % | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||
| BCC | CK | 8.55 ± 0.02 A 2 | 0.10 ± 0.01 B | 1.59 ± 0.09 D | 2.25 ± 3.89 A | 19.77 ± 0.21 D | 4463.87 ± 51.38 AC | 188.42 ± 15.99 C | 142.46 ± 20.89 D |
| VRM | 8.23 ± 0.05 DE | 0.11 ± 0.01 AB | 2.87 ± 0.26 B | 6.74 ± 6.74 A | 89.00 ± 5.96 B | 4362.40 ± 15.27 BD | 368.68 ± 15.93 A | 612.03 ± 53.35 B | |
| BCW | CK | 8.49 ± 0.06 AB | 0.13 ± 0.02 AB | 1.50 ± 0.03 D | 4.49 ± 3.89 A | 20.48 ± 0.18 D | 4671.46 ± 183.82 A | 202.17 ± 19.40 C | 168.04 ± 5.88 D |
| VRM | 8.02 ± 0.12 F | 0.15 ± 0.06 A | 3.30 ± 0.43 A | 8.95 ± 10.25 A | 96.93 ± 8.38 A | 4157.62 ± 76.85 D | 394.32 ± 46.48 A | 826.88 ± 61.36 A | |
| LSC | CK | 8.37 ± 0.09 CD | 0.13 ± 0.00 AB | 1.66 ± 0.04 D | 8.99 ± 7.79 A | 20.17 ± 1.92 D | 4493.56 ± 41.67 AB | 187.68 ± 23.60 C | 140.02 ± 7.52 D |
| VRM | 8.13 ± 0.07 EF | 0.11 ± 0.00 AB | 1.81 ± 0.10 D | 8.99 ± 3.91 A | 59.25 ± 1.81 C | 4249.37 ± 102.52 CD | 300.57 ± 10.60 B | 365.66 ± 17.58 C | |
| LSS | CK | 8.38 ± 0.05 BC | 0.12 ± 0.01 AB | 1.52 ± 0.04 D | 4.47 ± 3.87 A | 21.92 ± 1.06 D | 4313.42 ± 126.94 BD | 197.49 ± 8.59 C | 149.51 ± 20.33 D |
| VRM | 8.29 ± 0.10 CD | 0.11 ± 0.00 AB | 2.21 ± 0.32 C | 6.73 ± 0.02 A | 60.30 ± 4.39 C | 4269.85 ± 255.57 BD | 296.06 ± 19.55 B | 346.35 ± 8.89 C | |
| Crop and Treatment 1 | pH (w/v = 1/5) | EC | OM | Avail N | Avail P | Exch Ca | Exch Mg | Exch K | |
|---|---|---|---|---|---|---|---|---|---|
| dS m−1 | % | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||
| BCC | CK | 7.18 ± 0.09 C 2 | 0.03 ± 0.00 D | 1.48 ± 0.04 D | 11.23 ± 3.91 BC | 5.62 ± 0.23 DE | 2059.09 ± 66.78 A | 355.36 ± 15.93 BC | 57.78 ± 2.73 E |
| VRM | 7.65 ± 0.55 A | 0.07 ± 0.01 B | 2.60 ± 0.55 B | 8.98 ± 3.89 BC | 49.28 ± 1.58 B | 2247.68 ± 88.71 A | 484.96 ± 13.35 A | 155.75 ± 12.43 B | |
| BCW | CK | 7.16 ± 0.06 C | 0.04 ± 0.01 D | 1.57 ± 0.10 D | 8.98 ± 3.87 BC | 4.88 ± 0.36 E | 1914.34 ± 46.21 A | 327.96 ± 7.82 C | 65.62 ± 9.06 DE |
| VRM | 7.54 ± 0.05 AB | 0.10 ± 0.00 A | 3.07 ± 0.35 A | 13.45 ± 0.03 BC | 54.83 ± 4.85 A | 2385.32 ± 101.12 A | 517.24 ± 20.74 A | 176.74 ± 13.93 A | |
| LSC | CK | 7.17 ± 0.06 C | 0.05 ± 0.01 C | 1.50 ± 0.10 D | 22.43 ± 3.87 A | 4.88 ± 0.40 E | 2436.52 ± 941.56 A | 355.85 ± 11.64 BC | 61.57 ± 2.98 E |
| VRM | 7.26 ± 0.03 BC | 0.07 ± 0.01 BC | 2.25 ± 0.07 BC | 11.22 ± 3.91 BC | 25.59 ± 2.01 C | 2011.13 ± 312.84 A | 393.16 ± 79.67 BC | 76.60 ± 7.02 CD | |
| LSS | CK | 7.19 ± 0.04 C | 0.04 ± 0.00 D | 1.38 ± 0.05 D | 6.72 ± 6.72 C | 9.54 ± 4.72 D | 1992.70 ± 1222.34 A | 336.26 ± 25.15 C | 63.85 ± 3.80 DE |
| VRM | 7.21 ± 0.07 BC | 0.07 ± 0.01 BC | 2.01 ± 0.17 C | 13.47 ± 0.03 B | 24.52 ± 0.50 C | 2246.69 ± 66.84 A | 406.15 ± 59.01 B | 84.93 ± 6.65 C | |
| Treatment 1 | Bioconcentration Factor (BCF) | Translocation Factor (TF) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Ni | Zn | Cd | Cu | Ni | Zn | ||
| Ti-132 | |||||||||
| BCC | CK | 0.11 | 0.003 | 0.32 | 0.19 | 0.51 | 0.02 | 0.64 | 0.82 |
| VRM | 0.05 | 0.002 | 0.28 | 0.16 | 0.26 | 0.01 | 0.22 | 1.23 | |
| BCW | CK | NA 2 | 0.08 | 0.19 | 0.25 | NA | 0.44 | 0.50 | 0.78 |
| VRM | 0.08 | 0.11 | 0.16 | 0.15 | 0.19 | 0.88 | 0.25 | 0.89 | |
| LSC | CK | 0.24 | 0.10 | 0.11 | 0.14 | 0.82 | 0.22 | 0.93 | 0.98 |
| VRM | 0.41 | 0.14 | 0.15 | 0.20 | 1.24 | 0.40 | 0.82 | 0.55 | |
| LSS | CK | 0.04 | 0.10 | 0.08 | 0.16 | 0.41 | 0.07 | 1.24 | 0.97 |
| VRM | 0.09 | 0.07 | 0.24 | 0.16 | 0.59 | 0.24 | 2.97 | 1.13 | |
| Ya-400 | |||||||||
| BCC | CK | 5.57 | 0.40 | 0.06 | 0.66 | 2.62 | 1.05 | 0.19 | 1.32 |
| VRM | 1.66 | 0.47 | 0.02 | 0.32 | 0.91 | 1.20 | 0.29 | 1.10 | |
| BCW | CK | 4.70 | 0.26 | 0.10 | 0.53 | 1.78 | NA | 0.87 | NA |
| VRM | 1.01 | 0.17 | 0.02 | 0.17 | 0.44 | 1.00 | 0.15 | 1.28 | |
| LSC | CK | 1.64 | 0.90 | 0.02 | 0.48 | 0.74 | 0.63 | 0.59 | 1.82 |
| VRM | 1.45 | 0.75 | NA | 0.34 | 2.96 | 0.68 | NA | 1.53 | |
| LSS | CK | 6.56 | 0.91 | 0.07 | 0.81 | 2.37 | 0.18 | 0.18 | 3.59 |
| VRM | 2.62 | 0.71 | 0.12 | 0.42 | 2.73 | 0.64 | 0.55 | 1.61 | |
| Treatment 1 | Hazard Quotient (HQv) | ||||
|---|---|---|---|---|---|
| Cd | Cu | Ni | Zn | ||
| Ti-132 | |||||
| BCC | CK | 0.20 | 0.00 | 1.05 | 0.05 |
| VRM | 0.10 | 0.00 | 1.00 | 0.04 | |
| BCW | CK | 0.00 | 0.01 | 0.85 | 0.06 |
| VRM | 0.13 | 0.00 | 0.57 | 0.03 | |
| LSC | CK | 0.43 | 0.00 | 0.40 | 0.03 |
| VRM | 0.73 | 0.01 | 0.57 | 0.04 | |
| LSS | CK | 0.07 | 0.00 | 0.31 | 0.04 |
| VRM | 0.17 | 0.00 | 0.99 | 0.04 | |
| Ya-400 | |||||
| BCC | CK | 23.8 | 0.00 | 0.57 | 0.03 |
| VRM | 10.1 | 0.00 | 0.24 | 0.03 | |
| BCW | CK | 25.7 | 0.00 | 1.12 | 0.04 |
| VRM | 6.84 | 0.00 | 0.24 | 0.01 | |
| LSC | CK | 8.67 | 0.01 | 0.26 | 0.03 |
| VRM | 8.36 | 0.01 | 0.00 | 0.02 | |
| LSS | CK | 33.3 | 0.01 | 0.78 | 0.06 |
| VRM | 15.6 | 0.01 | 1.45 | 0.03 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-S.; Chen, K.-S.; Yang, H.-Y.; Lai, H.-Y. Effect of Vermicompost Amendment on the Accumulation and Chemical Forms of Trace Metals in Leafy Vegetables Grown in Contaminated Soils. Int. J. Environ. Res. Public Health 2021, 18, 6619. https://doi.org/10.3390/ijerph18126619
Yen Y-S, Chen K-S, Yang H-Y, Lai H-Y. Effect of Vermicompost Amendment on the Accumulation and Chemical Forms of Trace Metals in Leafy Vegetables Grown in Contaminated Soils. International Journal of Environmental Research and Public Health. 2021; 18(12):6619. https://doi.org/10.3390/ijerph18126619
Chicago/Turabian StyleYen, Yu-Shan, Kuei-San Chen, Hsin-Yi Yang, and Hung-Yu Lai. 2021. "Effect of Vermicompost Amendment on the Accumulation and Chemical Forms of Trace Metals in Leafy Vegetables Grown in Contaminated Soils" International Journal of Environmental Research and Public Health 18, no. 12: 6619. https://doi.org/10.3390/ijerph18126619






