Potentially Toxic Element Contaminations and Lead Isotopic Fingerprinting in Soils and Sediments from a Historical Gold Mining Site
Abstract
:1. Introduction
2. Materials and Methods
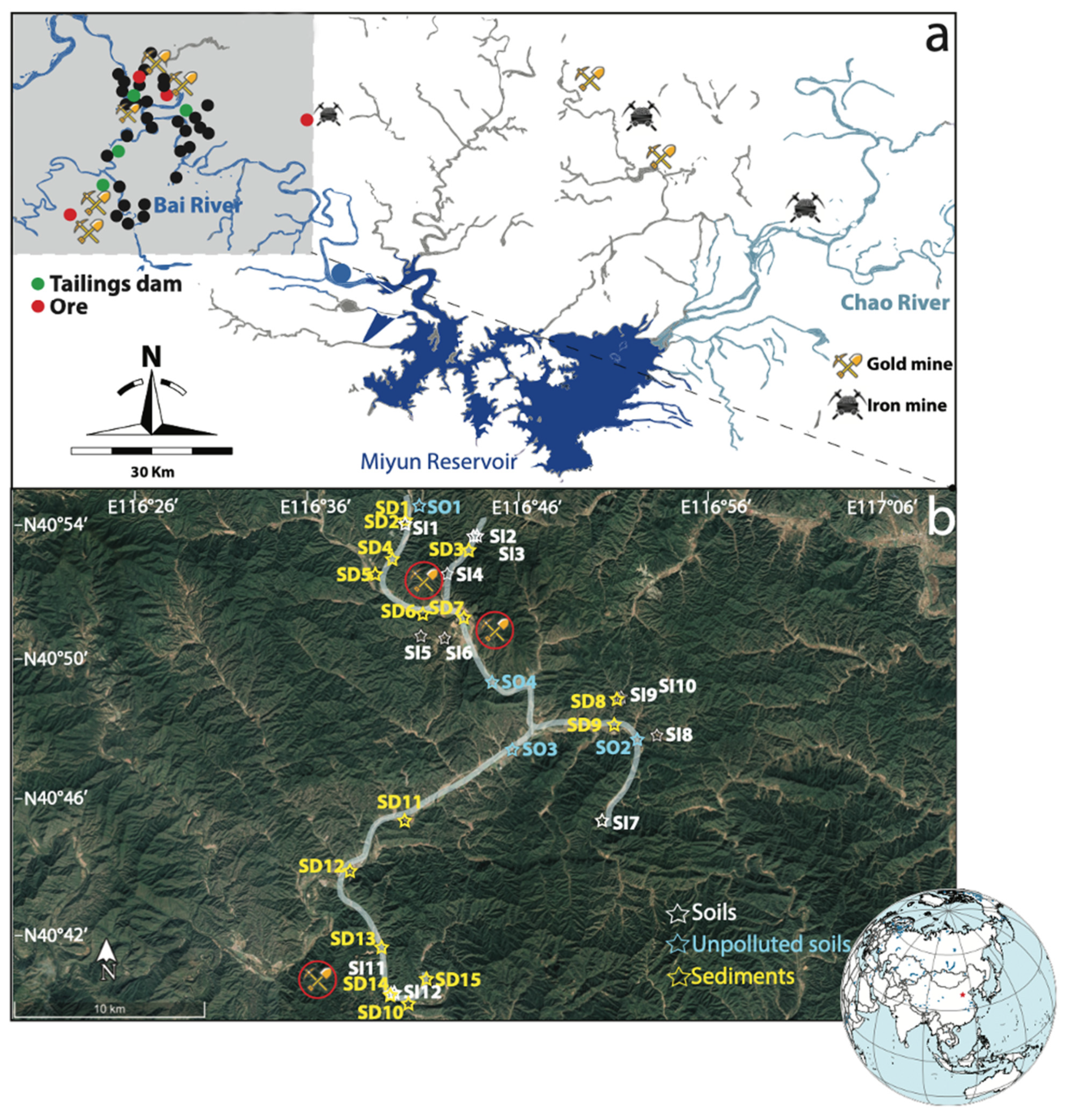
2.1. Sample Collection and Preparation
2.2. Chemical Treatments and Analysis
2.3. Quality Assurance and Quality Control (QA/QC)
2.4. Pollution Risk Assessment
2.4.1. Contamination Factor (CF)
2.4.2. Pollution Level Index (PLI)
2.4.3. Geo-Accumulation Index (Igeo)
2.4.4. Potential Ecological Risk Assessment
2.5. Data Analysis
3. Results and Discussion
3.1. Current PTEs Contamination in Soils and Sediments
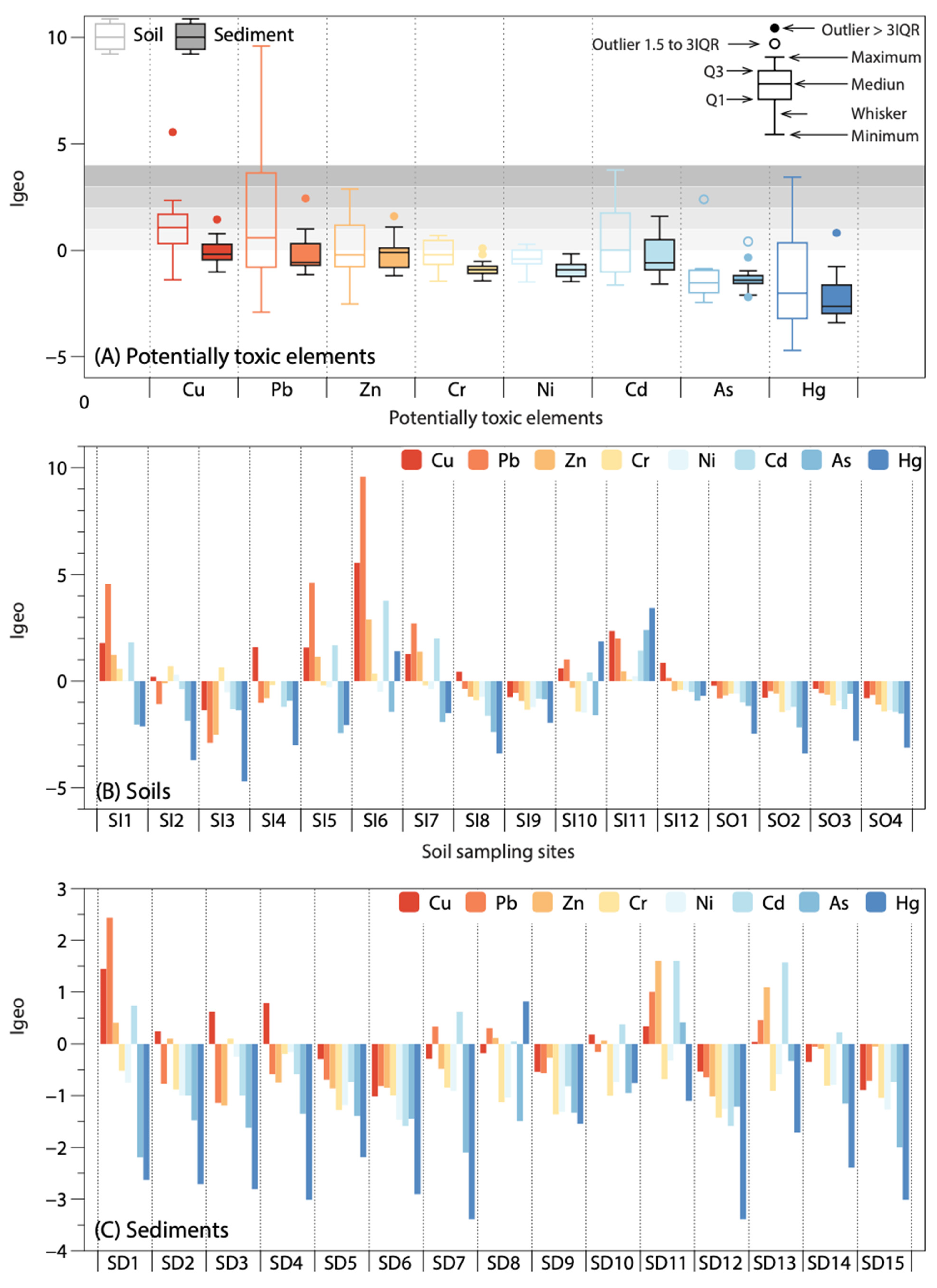
3.2. Pollution Assessment of PTEs in Soils and Sediments
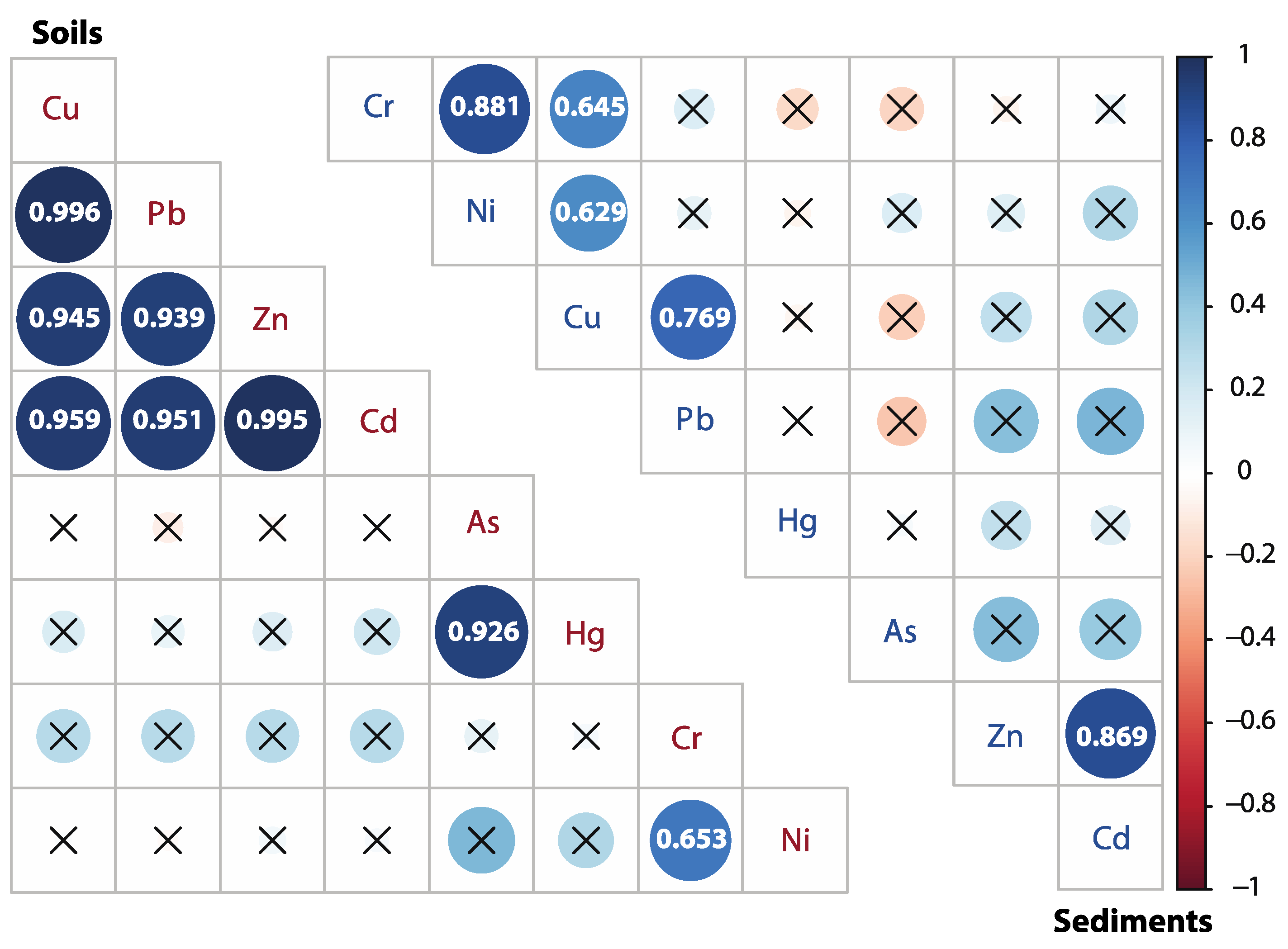
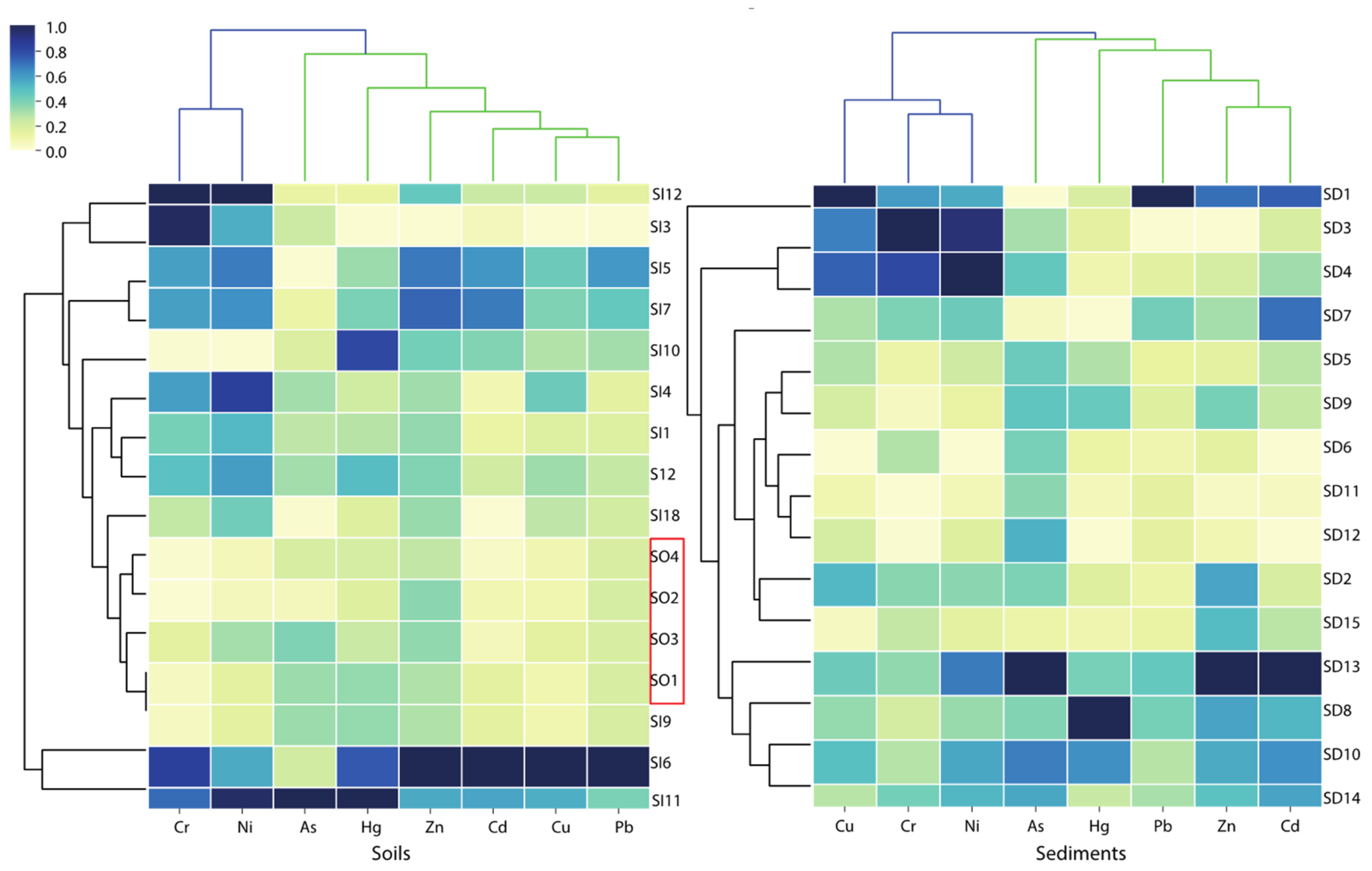
3.3. Source Identification
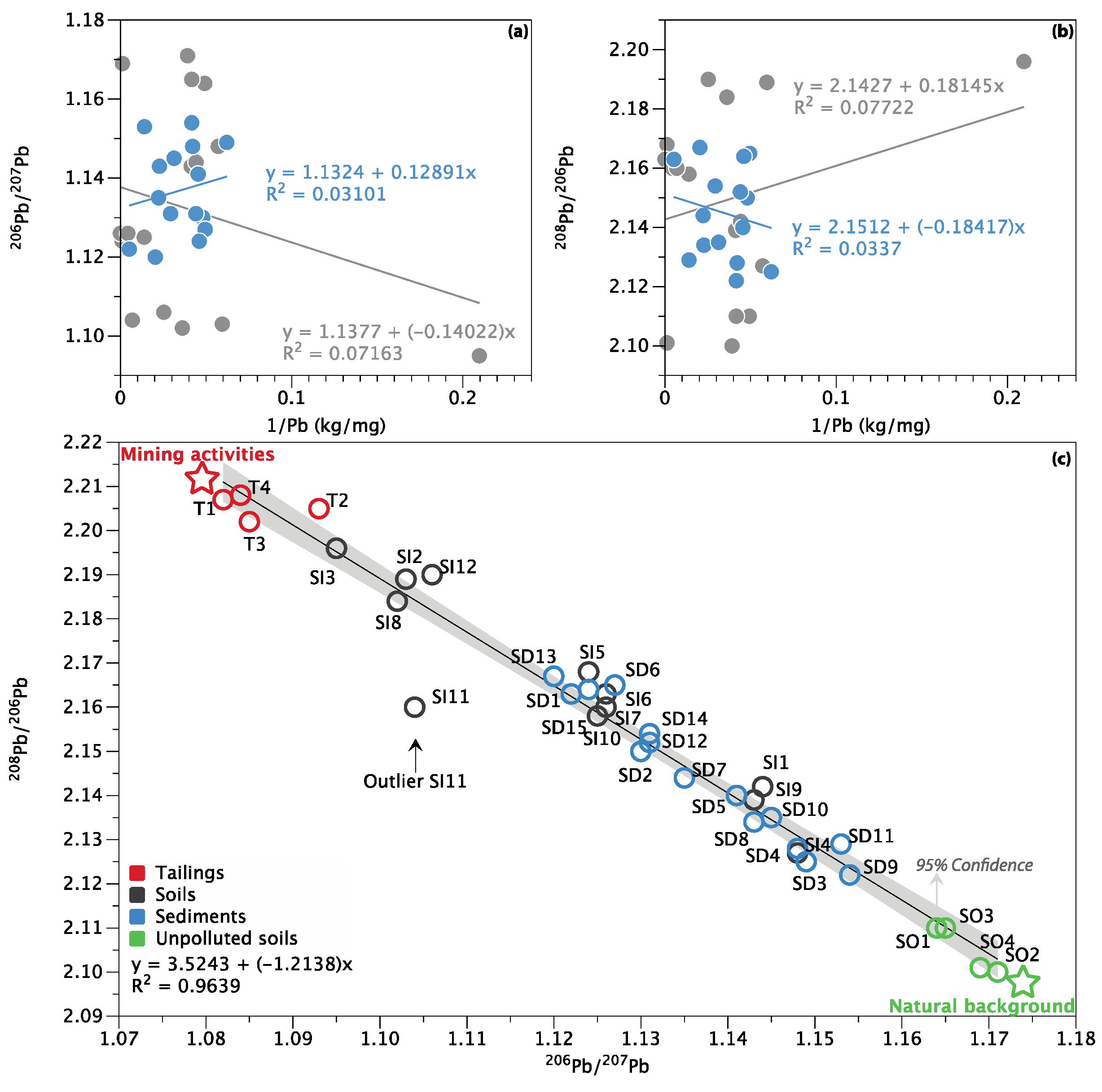
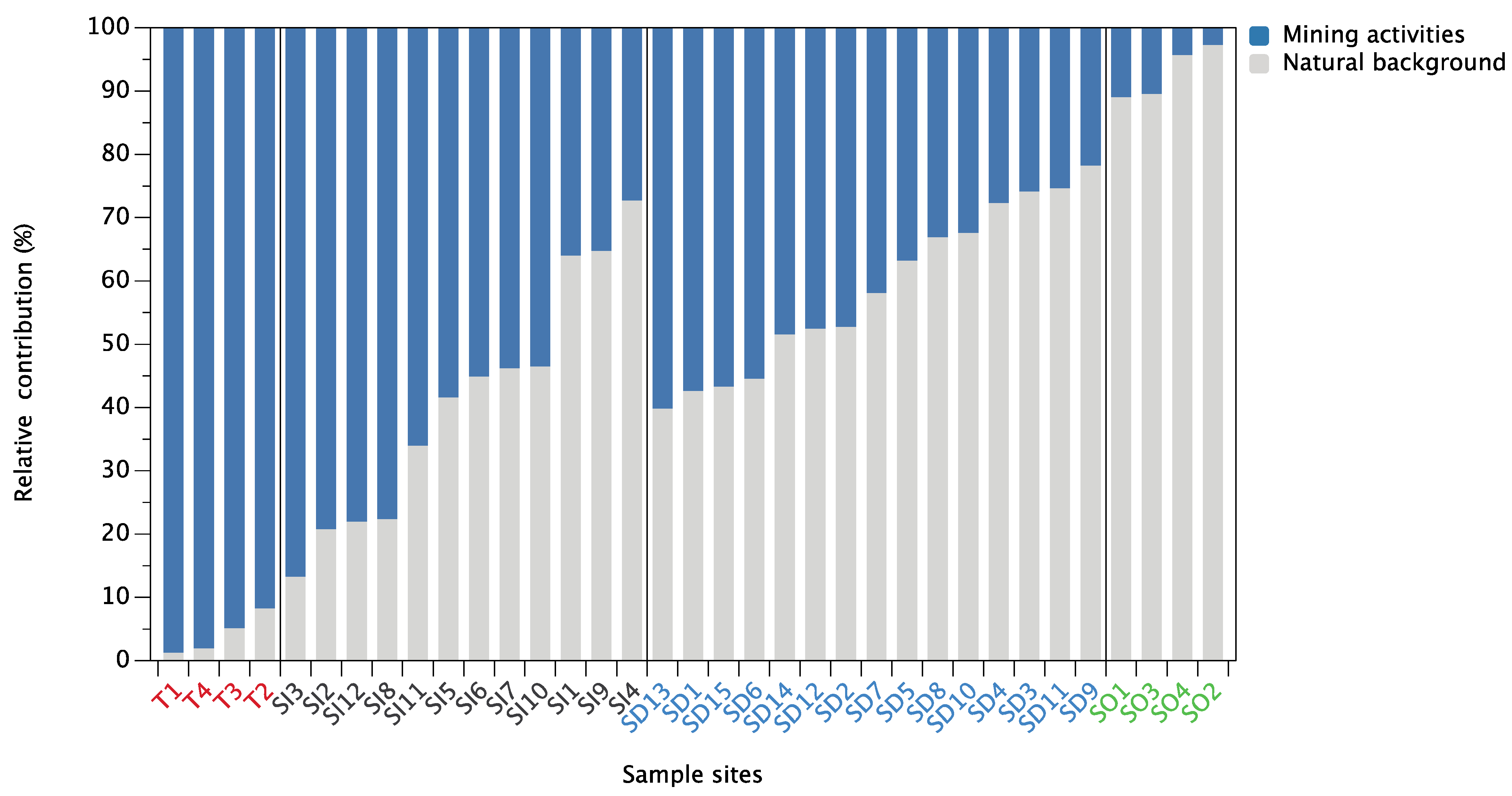
3.4. Pb Isotope Ratios and Source Apportionment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Murguía, D.I.; Bringezu, S.; Schaldach, R. Global direct pressures on biodiversity by large-scale metal mining: Spatial distribution and implications for conservation. J. Environ. Manag. 2016, 180, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef]
- Purves, D. Trace-Element Contamination of the Environment; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Agudelo-Echavarría, D.M.; Olid, C.; Molina, F.; Vallejo-Toro, P.P.; Garcia-Orellana, J. Historical reconstruction of small-scale gold mining activities in tropical wetland sediments in Bajo Cauca-Antioquia, Colombia. Chemosphere 2020, 254, 126733. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Hudson-Edwards, K.A.; Blake, R.; Zhao, F.; Yuan, Z.; Gao, W. Characterization of mining-related aromatic contaminants in active and abandoned metal(loid) tailings ponds. Environ. Sci. Technol. 2020, 54, 15097–15107. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, T.C.; Whitby, L.M. Heavy-metal pollution in the sudbury mining and smelting region of canada, I. Soil and vegetation contamination by nickel, copper, and other metals. Environ. Conserv. 1974, 1, 123–132. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Lopez-Soler, A.; Plana, F. Levels and chemistry of atmospheric particulates induced by a spill of heavy metal mining wastes in the Doñana area, Southwest Spain. Atmos. Environ. 2000, 34, 239–253. [Google Scholar] [CrossRef]
- Resongles, E.; Casiot, C.; Freydier, R.; Dezileau, L.; Viers, J.; Elbaz-Poulichet, F. Persisting impact of historical mining activity to metal (Pb, Zn, Cd, Tl, Hg) and metalloid (As, Sb) enrichment in sediments of the Gardon River, Southern France. Sci. Total Environ. 2014, 481, 509–521. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Chen, Y.; Wang, L.; Long, Z.; Hughes, S.S.; Ni, S.; Cheng, X.; Wang, J.; Li, T.; et al. Tracing Pb and possible correlated Cd contamination in soils by using lead isotopic compositions. J. Hazard. Mater. 2020, 385, 121528. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Liu, J.; Bi, X.; Ning, Y.; Yang, S.; Yang, X. Apportionment of sources of heavy metals to agricultural soils using isotope fingerprints and multi-variate statistical analyses. Environ. Pollut. 2019, 249, 208–216. [Google Scholar] [CrossRef]
- Hou, D.; O′Connor, D.; Nathanail, P.; Tian, L.; Ma, Y. Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: A critical review. Environ. Pollut. 2017, 231, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Mostert, M.; Ayoko, G.A.; Kokot, S. Application of chemometrics to analysis of soil pollutants. TrAC Trends Anal. Chem. 2010, 29, 430–445. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xia, X.; Lin, C.; Chen, X.; Zhou, C. Levels of arsenic and heavy metals in the rural soils of Beijing and their changes over the last two decades (1985–2008). J. Hazard. Mater. 2010, 179, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y. Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: A review. Environ. Pollut. 2010, 158, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.-Y.; Li, Z.-G.; Wang, S.-X.; Zhang, L.; Xu, R.; Liu, J.-L.; Yang, H.-M.; Guo, M.-Z. Lead isotopic compositions of selected coals, Pb/Zn ores and fuels in China and the application for source tracing. Environ. Sci. Technol. 2017, 51, 13502–13508. [Google Scholar] [CrossRef]
- Deng, W.; Liu, W.; Wen, Y.; Li, X. A new inverse distance model to calculate the percentage contribution of various Pb sources. Environ. Res. 2020, 185, 109475. [Google Scholar] [CrossRef]
- Komárek, M.; Ettler, V.; Chrastný, V.; Mihaljevič, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar] [CrossRef]
- Hamilton, E.I. Book Review: Principles of isotope geology. Earth-Sci. Rev. 1978, 14, 190–191. [Google Scholar] [CrossRef]
- ABollhöfer, A.; Rosman, K.J.R. Isotopic source signatures for atmospheric lead: The Southern Hemisphere. Geochim. Cosmochim. Acta 2000, 64, 3251–3262. [Google Scholar] [CrossRef]
- Xu, D.; Wang, R.; Wang, W.; Ge, Q.; Zhang, W.; Chen, L.; Chu, F. Tracing the source of Pb using stable Pb isotope ratios in sediments of eastern Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2019, 141, 127–136. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chakraborty, P.; Hathorne, E.; Sarkar, A.; Linsy, P.; Frank, M.; Nath, B.N. Evidence for increasing anthropogenic Pb concentrations in Indian shelf sediments during the last century. Sci. Total Environ. 2021, 760, 143833. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Wang, J.; Xiao, T.; Yin, M.; Belshaw, N.S.; Lippold, H.; Kong, L.; Xiao, E.; Bao, Z.; et al. Provenance of uranium in a sediment core from a natural reservoir, South China: Application of Pb stable isotope analysis. Chemosphere 2018, 193, 1172–1180. [Google Scholar] [CrossRef]
- Wang, Z.; Dwyer, G.S.; Coleman, D.S.; Vengosh, A. Lead isotopes as a new tracer for detecting coal fly ash in the environment. Environ. Sci. Technol. Lett. 2019, 6, 714–719. [Google Scholar] [CrossRef]
- Xiong, L.; Zhao, X.; Wei, J.; Jin, X.; Fu, L.; Lin, Z. Linking mesozoic lode gold deposits to metal-fertilized lower continental crust in the North China Craton: Evidence from Pb isotope systematics. Chem. Geol. 2020, 533, 119440. [Google Scholar] [CrossRef]
- Chen, X.; Ji, H.; Yang, W.; Zhu, B.; Ding, H. Speciation and distribution of mercury in soils around gold mines located upstream of Miyun Reservoir, Beijing, China. J. Geochem. Explor. 2016, 163, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, Y.; You, S.; Wu, Y.; Zheng, F. A framework for assessing the effects of afforestation and South-to-North Water Transfer on nitrogen and phosphorus uptake by plants in a critical riparian zone. Sci. Total Environ. 2019, 651, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Andersen, T.; Burch, M.; Jia, Z.; An, W.; Yu, J.; Yang, M. Succession and interaction of surface and subsurface cyanobacterial blooms in oligotrophic/mesotrophic reservoirs: A case study in Miyun Reservoir. Sci. Total Environ. 2019, 649, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, B.; Lu, J.; Zhou, Y.; Xu, D.; Gao, L.; Sun, K. Pollution characteristics and source identification of trace metals in riparian soils of Miyun Reservoir, China. Ecotoxicol. Environ. Saf. 2017, 144, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ji, H.; Chen, Y.; Qiao, M.; Tang, L. Assessment and sources of heavy metals in surface sediments of Miyun Reservoir, Beijing. Environ. Monit. Assess. 2013, 185, 6049–6062. [Google Scholar] [CrossRef]
- Li, Q.; Ji, H.; Qin, F.; Tang, L.; Guo, X.; Feng, J. Sources and the distribution of heavy metals in the particle size of soil polluted by gold mining upstream of Miyun Reservoir, Beijing: Implications for assessing the potential risks. Environ. Monit. Assess. 2014, 186, 6605–6626. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhu, Y.; Ji, H. Distribution, speciation, and risk assessment of selected metals in the gold and iron mine soils of the catchment area of Miyun Reservoir, Beijing, China. Environ. Monit. Assess. 2013, 185, 8525–8545. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gao, B.; Zhou, Y.; Xu, D.; Sun, K. Predicting remobilization characteristics of cobalt in riparian soils in the Miyun Reservoir prior to water retention. Ecol. Indic. 2017, 80, 196–203. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, B. Defining and modeling the soil geochemical background of heavy metals from the Hengshi River watershed (southern China): Integrating EDA, stochastic simulation and magnetic parameters. J. Hazard. Mater. 2010, 180, 542–551. [Google Scholar] [CrossRef]
- Kinimo, K.C.; Yao, K.M.; Marcotte, S.; Kouassi, N.L.B.; Trokourey, A. Distribution trends and ecological risks of arsenic and trace metals in wetland sediments around gold mining activities in central-southern and southeastern Côte d′Ivoire. J. Geochem. Explor. 2018, 190, 265–280. [Google Scholar] [CrossRef]
- Sherman, L.S.; Blum, J.D.; Dvonch, J.T.; Gratz, L.E.; Landis, M.S. The use of Pb, Sr, and Hg isotopes in Great Lakes precipitation as a tool for pollution source attribution. Sci. Total Environ. 2015, 502, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bohdalkova, L.; Novák, M.; Stepanova, M.; Fottová, D.; Chrastný, V.; Mikova, J.; Kuběna, A.A. The fate of atmospherically derived Pb in Central European catchments: Insights from spatial and temporal pollution gradients and Pb isotope ratios. Environ. Sci. Technol. 2014, 48, 4336–4343. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth′s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Zhao, Y.; Zhang, P. Heavy metal concentrations in roadside soils and correlation with urban traffic in Beijing, China. J. Hazard. Mater. 2010, 181, 640–646. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Muller, G. The heavy metal pollution of the sediments of Neckars and its tributary: A stocktaking. Chem. Zeit 1981, 105, 157–164. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Smith, K.S.; Huyck, H.L.; Plumlee, G.; Logsdon, M.; Filipek, L. An overview of the abundance, relative mobility, bioavailability, and human toxicity of metals. Environ. Geochem. Miner. Depos. 1997, 6, 29–70. [Google Scholar] [CrossRef]
- Bird, G.; Brewer, P.A.; Macklin, M.G.; Nikolova, M.; Kotsev, T.; Mollov, M.; Swain, C. Quantifying sediment-associated metal dispersal using Pb isotopes: Application of binary and multi-variate mixing models at the catchment-scale. Environ. Pollut. 2010, 158, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Gao, B.; Peng, W.; Gao, L.; Wan, X.; Li, Y. Application of DGT/DIFS and geochemical baseline to assess Cd release risk in reservoir riparian soils, China. Sci. Total Environ. 2019, 646, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Han, L.; Peng, W.; Gao, B.; Xu, D.; Wan, X. Identification of anthropogenic inputs of trace metals in lake sediments using geochemical baseline and Pb isotopic composition. Ecotoxicol. Environ. Saf. 2018, 164, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, L.; Li, L.; Liang, T.; Zhang, Y.; Ma, C.; Xing, B. Multivariate geostatistical analysis and source identification of heavy metals in the sediment of Poyang Lake in China. Sci. Total Environ. 2018, 621, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Mclennan, S.M. The continental crust: Its composition and evolution. J. Geol. 1985, 94, 57–72. [Google Scholar]
- Li, T.; Shi, Y.; Li, X.; Zhang, H.; Pi, K.; Gerson, A.R.; Liu, D. Leaching behaviors and speciation of cadmium from river sediment dewatered using contrasting conditioning. Environ. Pollut. 2020, 263, 114427. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. NIH 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Li, Y.; Li, B.; Shen, Z.; Stenstrom, M.K. Metal enrichment and lead isotope analysis for source apportionment in the urban dust and rural surface soil. Environ. Pollut. 2016, 216, 764–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef] [PubMed]
- Shajib MT, I.; Hansen HC, B.; Liang, T. Metals in surface specific urban runoff in Beijing. Environ. Pollut. 2019, 248, 584–598. [Google Scholar] [CrossRef]
- Shi, G.; Chen, Z.; Xu, S.; Zhang, J.; Wang, L.; Bi, C.; Teng, J. Potentially toxic metal contamination of urban soils and roadside dust in Shanghai, China. Environ. Pollut. 2008, 156, 251–260. [Google Scholar] [CrossRef]
- Cheng, X.; Danek, T.; Drozdova, J.; Huang, Q.; Qi, W.; Zou, L.; Yang, S.; Zhao, X.; Xiang, Y. Soil heavy metal pollution and risk assessment associated with the Zn-Pb mining region in Yunnan, Southwest China. Environ. Monit. Assess. 2018, 190, 194. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mackay, A.K.; Hudson-Edwards, K.A.; Holz, E. Soil Cd, Cu, Pb and Zn contaminants around Mount Isa city, Queensland, Australia: Potential sources and risks to human health. Appl. Geochem. 2010, 25, 841–855. [Google Scholar] [CrossRef]
- Veiga, M.M.; Maxson, P.A.; Hylander, L.D. Origin and consumption of mercury in small-scale gold mining. J. Clean. Prod. 2006, 14, 436–447. [Google Scholar] [CrossRef]
- Bing-Quan, Z.; Yu-Wei, C.; Xiang-Yang, C. Application of Pb isotopic mapping to environment evaluation in China. Chem. Speciat. Bioavailab. 2002, 14, 49–56. [Google Scholar] [CrossRef]
- Renberg, I.; Brännvall, M.-L.; Bindler, R.; Emteryd, O. Stable lead isotopes and lake sediments—A useful combination for the study of atmospheric lead pollution history. Sci. Total Environ. 2002, 292, 45–54. [Google Scholar] [CrossRef]
- Pan, L.; Fang, G.; Wang, Y.; Wang, L.; Su, B.; Li, D.; Xiang, B. Potentially toxic element pollution levels and risk assessment of soils and sediments in the upstream river, miyun reservoir, China. Int. J. Environ. Res. Public Health 2018, 15, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Gao, B.; Wei, X.; Gao, L.; Xu, D.; Sun, K. The characteristic of Pb isotopic compositions in different chemical fractions in sediments from Three Gorges Reservoir, China. Environ. Pollut. 2015, 206, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, O.; Rhoujjati, A.; EL Hachimi, M.L. Contamination, sources and environmental risk assessment of heavy metals in water, sediment and soil around an abandoned Pb mine site in North East Morocco. Environ. Earth Sci. 2021, 80, 96. [Google Scholar] [CrossRef]

| Element | Cu | Pb | Zn | Cr | Ni | Cd | As | Hg |
|---|---|---|---|---|---|---|---|---|
| mg/kg | ||||||||
| Soils (n = 36) | ||||||||
| Max | 1580 | 27400 | 792 | 195 | 62.3 | 4.10 | 59.1 | 1.140 |
| Min | 13.0 | 4.77 | 18.7 | 44.3 | 18.2 | 0.0970 | 2.07 | 0.004 |
| Mean | 199 | 2470 | 181 | 117 | 40.4 | 0.791 | 8.52 | 0.168 |
| Meadium | 71.4 | 55.7 | 93.2 | 105 | 38.4 | 0.315 | 3.92 | 0.026 |
| SD | 438 | 785 | 211 | 52.7 | 13.8 | 1.12 | 16.0 | 0.329 |
| CV% | 220 | 31.8 | 116 | 45.2 | 34.1 | 141 | 188 | 197 |
| Unpolluted Soils (n = 12) | ||||||||
| Max | 29.0 | 25.5 | 71.4 | 80.1 | 33.9 | 0.150 | 7.45 | 0.000019 |
| Min | 19.4 | 20.3 | 49.7 | 43.8 | 19.5 | 0.110 | 2.49 | 0.000010 |
| Mean | 23.6 | 23.1 | 64.2 | 55.7 | 24.9 | 0.128 | 4.71 | 0.000014 |
| Meadium | 23.0 | 23.3 | 67.9 | 49.5 | 23.2 | 0.125 | 4.46 | 0.000014 |
| SD | 4.79 | 2.21 | 9.83 | 17.0 | 6.87 | 0.0171 | 2.10 | 0.000004 |
| CV% | 20.3 | 9.57 | 15.3 | 30.5 | 27.6 | 13.4 | 44.5 | 28.0 |
| Sediments (n = 45) | ||||||||
| Max | 92.1 | 192 | 325 | 129 | 45.5 | 0.910 | 15.0 | 0.000190 |
| Min | 16.7 | 16.1 | 46.9 | 44.7 | 18.4 | 0.100 | 2.46 | 0.000010 |
| Mean | 36.7 | 42.5 | 113 | 68.8 | 28.9 | 0.336 | 5.10 | 0.000034 |
| Meadium | 29.8 | 24.0 | 100 | 64.2 | 27.1 | 0.200 | 4.29 | 0.000017 |
| SD | 19.3 | 43.9 | 74.3 | 22.6 | 8.51 | 0.262 | 3.15 | 0.000044 |
| CV% | 52.7 | 103 | 66 | 32.8 | 29.4 | 77.8 | 61.7 | 129 |
| BBV a | 22.5 | 23.7 | 71.4 | 80.1 | 33.9 | 0.20 | 7.50 | 0.0700 |
| UCC b | 28.0 | 17.0 | 67.0 | 92.0 | 47.0 | 0.0900 | 4.80 | 0.0500 |
| Type | Site | Contamination Factor (CF) | Pollution Load Index (PLI) | Potential Ecological Risk Index (RI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Zn | Cr | Ni | Cd | As | Hg | Values | Pollution Levels | Values | Pollution Levels | ||
| Soils | SI1 | 5.20 | 35.4 | 3.50 | 2.23 | 1.53 | 5.30 | 0.36 | 0.34 | 2.48 | Moderate | 381 | Considerable |
| SI2 | 1.72 | 0.71 | 1.40 | 2.43 | 1.84 | 1.15 | 0.41 | 0.11 | 0.90 | Unpolluted | 66 | Low | |
| SI3 | 0.58 | 0.20 | 0.26 | 2.35 | 1.04 | 0.60 | 0.58 | 0.06 | 0.44 | Unpolluted | 38 | Low | |
| SI4 | 4.53 | 0.74 | 0.86 | 1.31 | 1.50 | 0.65 | 0.79 | 0.19 | 0.93 | Unpolluted | 65 | Low | |
| SI5 | 4.49 | 37.0 | 3.31 | 1.30 | 1.23 | 4.80 | 0.28 | 0.36 | 2.12 | Moderate | 366 | Considerable | |
| SI6 | 70.3 | 1150 | 11.1 | 1.92 | 1.06 | 20.5 | 0.55 | 3.97 | 9.75 | Very high | 6767 | High | |
| SI7 | 3.61 | 9.79 | 3.92 | 1.30 | 1.15 | 6.05 | 0.40 | 0.53 | 2.01 | Moderate | 265 | Moderate | |
| SI8 | 2.04 | 1.16 | 0.90 | 0.80 | 0.90 | 0.49 | 0.29 | 0.14 | 0.65 | Unpolluted | 40 | Low | |
| SI9 | 0.89 | 1.02 | 0.78 | 0.59 | 0.65 | 0.85 | 0.83 | 0.39 | 0.72 | Unpolluted | 49 | Low | |
| SI10 | 2.27 | 3.03 | 1.21 | 0.55 | 0.54 | 2.00 | 0.50 | 5.46 | 1.38 | Moderate to unpolluted | 98 | Low | |
| SI11 | 7.64 | 6.03 | 2.07 | 1.57 | 1.76 | 4.05 | 7.88 | 16.3 | 4.39 | High | 286 | Considerable | |
| SI12 | 2.73 | 1.67 | 1.08 | 1.13 | 1.11 | 1.05 | 0.79 | 0.93 | 1.21 | Moderate to unpolluted | 70 | Low | |
| Unpolluted soils | SO1 | 1.29 | 0.86 | 0.94 | 1.00 | 1.00 | 0.75 | 0.67 | 0.27 | 0.78 | Unpolluted | 48 | Low |
| SO2 | 0.88 | 1.08 | 1.00 | 0.55 | 0.58 | 0.65 | 0.33 | 0.14 | 0.56 | Unpolluted | 38 | Low | |
| SO3 | 1.16 | 1.01 | 0.96 | 0.68 | 0.79 | 0.60 | 0.99 | 0.21 | 0.73 | Unpolluted | 45 | Low | |
| SO4 | 0.86 | 0.95 | 0.70 | 0.56 | 0.58 | 0.55 | 0.52 | 0.17 | 0.56 | Unpolluted | 36 | Low | |
| Sediments | SD1 | 4.09 | 8.10 | 1.99 | 1.05 | 0.89 | 2.50 | 0.33 | 0.24 | 1.37 | Moderate to unpolluted | 148 | Low |
| SD2 | 1.77 | 0.88 | 1.61 | 0.82 | 0.75 | 0.75 | 0.539 | 0.23 | 0.78 | Unpolluted | 48 | Low | |
| SD3 | 2.30 | 0.68 | 0.66 | 1.61 | 1.27 | 0.75 | 0.49 | 0.21 | 0.80 | Unpolluted | 53 | Low | |
| SD4 | 2.59 | 1.00 | 0.89 | 1.31 | 1.34 | 1.00 | 0.59 | 0.19 | 0.90 | Unpolluted | 64 | Low | |
| SD5 | 1.22 | 0.93 | 0.83 | 0.62 | 0.66 | 0.90 | 0.57 | 0.33 | 0.71 | Unpolluted | 49 | Low | |
| SD6 | 0.74 | 0.85 | 0.83 | 0.75 | 0.54 | 0.50 | 0.55 | 0.20 | 0.57 | Unpolluted | 34 | Low | |
| SD7 | 1.23 | 1.89 | 1.07 | 0.84 | 0.90 | 2.30 | 0.34 | 0.14 | 0.81 | Unpolluted | 95 | Low | |
| SD8 | 1.32 | 1.85 | 1.62 | 0.69 | 0.73 | 1.55 | 0.53 | 2.64 | 1.20 | Moderate to unpolluted | 75 | Low | |
| SD9 | 1.03 | 1.01 | 1.24 | 0.58 | 0.61 | 0.85 | 0.60 | 0.51 | 0.77 | Unpolluted | 47 | Low | |
| SD10 | 1.70 | 1.35 | 1.57 | 0.75 | 0.90 | 1.95 | 0.78 | 0.89 | 1.16 | Moderate to unpolluted | 89 | Low | |
| SD11 | 1.90 | 3.00 | 4.55 | 0.94 | 1.20 | 4.55 | 2.00 | 0.70 | 1.92 | Moderate to unpolluted | 194 | Moderate | |
| SD12 | 1.04 | 0.96 | 0.74 | 0.56 | 0.63 | 0.50 | 0.65 | 0.14 | 0.57 | Unpolluted | 36 | Low | |
| SD13 | 1.54 | 2.07 | 3.19 | 0.80 | 1.00 | 4.45 | 1.19 | 0.46 | 1.45 | Moderate to unpolluted | 173 | Moderate | |
| SD14 | 1.18 | 1.44 | 1.40 | 0.86 | 0.87 | 1.75 | 0.67 | 0.29 | 0.94 | Unpolluted | 80 | Low | |
| SD15 | 0.81 | 0.92 | 1.44 | 0.73 | 0.62 | 0.90 | 0.38 | 0.19 | 0.65 | Unpolluted | 45 | Low | |
| Element | Soils | Sediments | ||
|---|---|---|---|---|
| F1 | F2 | F1 | F2 | |
| Cu | 0.938 | 0.046 | 0.820 | 0.417 |
| Pb | 0.943 | −0.169 | 0.722 | −0.228 |
| Zn | 0.943 | −0.062 | 0.685 | −0.600 |
| Cr | 0.234 | 0.904 | 0.592 | 0.733 |
| Ni | 0.225 | 0.924 | 0.753 | 0.487 |
| Cd | 0.952 | −0.054 | 0.850 | −0.365 |
| As | 0.222 | 0.223 | 0.102 | −0.468 |
| Hg | 0.706 | −0.343 | 0.325 | −0.643 |
| Eigenvalue | 4.22 | 1.88 | 3.42 | 2.13 |
| Variability (%) | 52.7 | 23.4 | 42.7 | 26.6 |
| Cumulative (%) | 52.7 | 76.2 | 42.7 | 69.3 |
| Type | Sample Site | 208Pb/204Pb | 207Pb/204Pb | 206Pb/204Pb | 206Pb/207Pb | 208Pb/206Pb |
|---|---|---|---|---|---|---|
| Soils | SI1 | 37.92 | 15.48 | 17.70 | 1.144 | 2.142 |
| SI2 | 36.72 | 15.21 | 16.77 | 1.103 | 2.189 | |
| SI3 | 36.77 | 15.30 | 16.75 | 1.095 | 2.196 | |
| SI4 | 37.78 | 15.47 | 17.76 | 1.148 | 2.127 | |
| SI5 | 38.04 | 15.61 | 17.55 | 1.124 | 2.168 | |
| SI6 | 37.91 | 15.56 | 17.52 | 1.126 | 2.163 | |
| SI7 | 37.61 | 15.47 | 17.41 | 1.126 | 2.160 | |
| SI8 | 36.91 | 15.34 | 16.90 | 1.102 | 2.184 | |
| SI9 | 37.81 | 15.47 | 17.68 | 1.143 | 2.139 | |
| SI10 | 37.51 | 15.45 | 17.38 | 1.125 | 2.158 | |
| SI11 | 36.27 | 15.21 | 16.79 | 1.104 | 2.160 | |
| SI12 | 37.15 | 15.33 | 16.96 | 1.106 | 2.190 | |
| mean | 37.37 ± 0.558 | 15.41 ± 0.124 | 17.26 ± 0.382 | 1.121 ± 0.0175 | 2.165 ± 0.0210 | |
| median | 37.56 | 15.46 | 17.40 | 1.125 | 2.162 | |
| Unpolluted soils | SO1 | 38.21 | 15.55 | 18.11 | 1.164 | 2.110 |
| SO2 | 38.21 | 15.54 | 18.19 | 1.171 | 2.100 | |
| SO3 | 38.15 | 15.52 | 18.08 | 1.165 | 2.110 | |
| SO4 | 38.15 | 15.54 | 18.16 | 1.169 | 2.101 | |
| mean | 38.18 ± 0.030 | 15.54 ± 0.011 | 18.14 ± 0.043 | 1.167 ± 0.0029 | 2.105 ± 0.0048 | |
| median | 38.18 | 15.54 | 18.14 | 1.167 | 2.106 | |
| Sediments | SD1 | 37.48 | 15.45 | 17.33 | 1.122 | 2.163 |
| SD2 | 37.47 | 15.42 | 17.42 | 1.130 | 2.150 | |
| SD3 | 37.82 | 15.48 | 17.79 | 1.149 | 2.125 | |
| SD4 | 37.79 | 15.47 | 17.76 | 1.148 | 2.128 | |
| SD5 | 37.80 | 15.48 | 17.66 | 1.141 | 2.140 | |
| SD6 | 37.66 | 15.43 | 17.39 | 1.127 | 2.165 | |
| SD7 | 37.67 | 15.48 | 17.57 | 1.135 | 2.144 | |
| SD8 | 37.76 | 15.48 | 17.69 | 1.143 | 2.134 | |
| SD9 | 37.90 | 15.47 | 17.85 | 1.154 | 2.122 | |
| SD10 | 37.84 | 15.48 | 17.73 | 1.145 | 2.135 | |
| SD11 | 37.99 | 15.49 | 17.85 | 1.153 | 2.129 | |
| SD12 | 37.58 | 15.43 | 17.46 | 1.131 | 2.152 | |
| SD13 | 37.42 | 15.41 | 17.27 | 1.120 | 2.167 | |
| SD14 | 37.57 | 15.42 | 17.44 | 1.131 | 2.154 | |
| SD15 | 37.52 | 15.430 | 17.34 | 1.124 | 2.164 | |
| mean | 37.68 ± 0.168 | 15.45 ± 0.027 | 17.57 ± 0.196 | 1.137 ± 0.0111 | 2.145 ± 0.0152 | |
| median | 37.67 | 15.47 | 17.57 | 1.135 | 2.144 | |
| Tailings | T1 | 36.63 | 15.30 | 16.60 | 1.085 | 2.207 |
| T2 | 36.69 | 15.23 | 16.64 | 1.093 | 2.205 | |
| T3 | 36.53 | 15.30 | 16.60 | 1.085 | 2.202 | |
| T4 | 36.63 | 15.24 | 16.60 | 1.085 | 2.208 | |
| mean | 36.62 ± 0.057 | 15.27 ± 0.033 | 16.61 ± 0.017 | 1.087 ± 0.0035 | 2.205 ± 0.0023 | |
| median | 36.63 | 15.27 | 16.60 | 1.085 | 2.206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Zhang, Y.; Ma, S.; Yan, C.; Geng, H.; Yu, G.; Ji, H.; Wang, F. Potentially Toxic Element Contaminations and Lead Isotopic Fingerprinting in Soils and Sediments from a Historical Gold Mining Site. Int. J. Environ. Res. Public Health 2021, 18, 10925. https://doi.org/10.3390/ijerph182010925
Tang L, Zhang Y, Ma S, Yan C, Geng H, Yu G, Ji H, Wang F. Potentially Toxic Element Contaminations and Lead Isotopic Fingerprinting in Soils and Sediments from a Historical Gold Mining Site. International Journal of Environmental Research and Public Health. 2021; 18(20):10925. https://doi.org/10.3390/ijerph182010925
Chicago/Turabian StyleTang, Lei, Yiyue Zhang, Shuai Ma, Changchun Yan, Huanhuan Geng, Guoqing Yu, Hongbing Ji, and Fei Wang. 2021. "Potentially Toxic Element Contaminations and Lead Isotopic Fingerprinting in Soils and Sediments from a Historical Gold Mining Site" International Journal of Environmental Research and Public Health 18, no. 20: 10925. https://doi.org/10.3390/ijerph182010925






