Dietary Exposure of the Taiwan Population to Mercury Content in Various Seafood Assessed by a Total Diet Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Constructing a Representative Diet List
2.2. Planning the Sampling Strategy
2.3. Analysis of THg Concentrations in Seafood
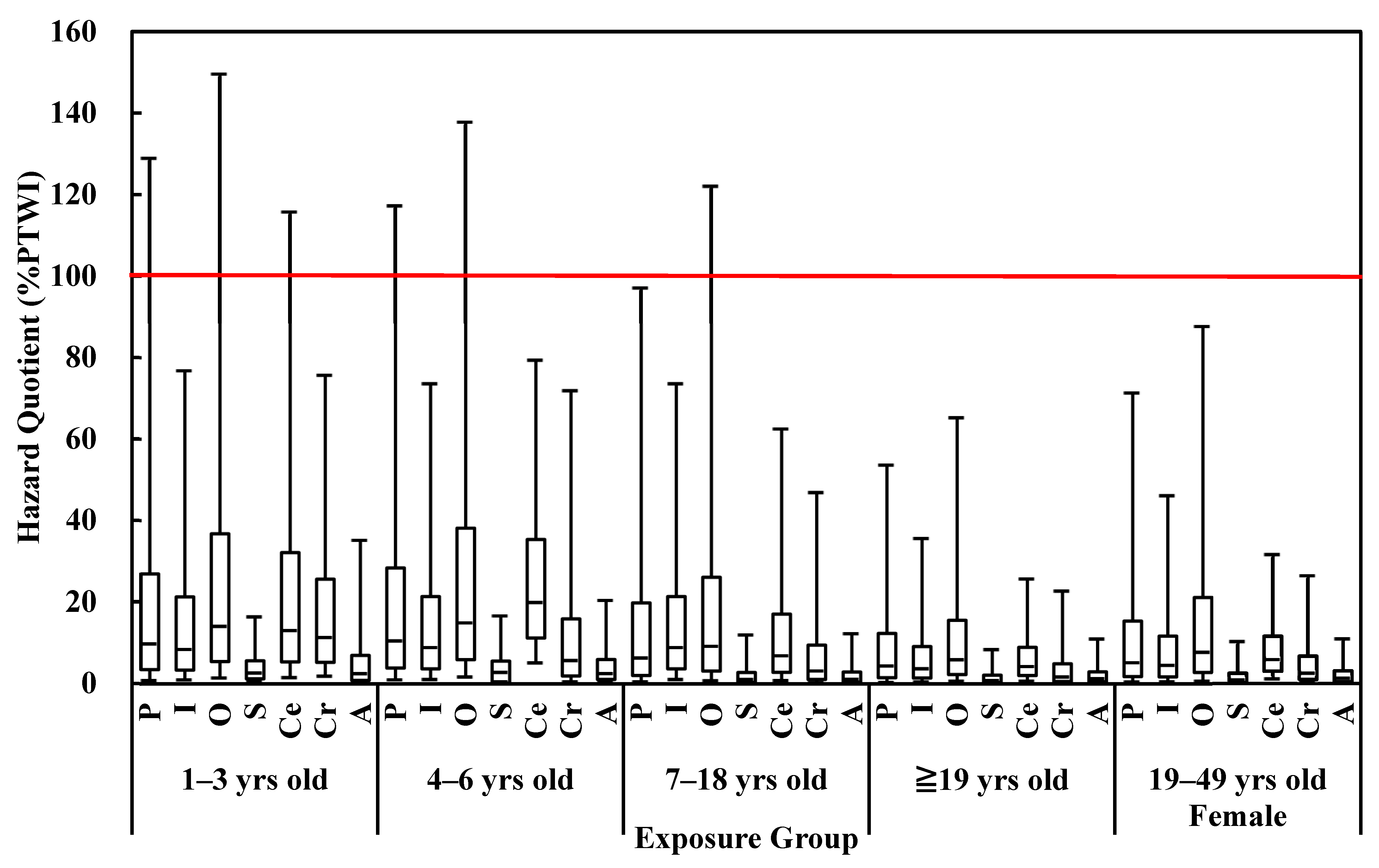
2.4. Seafood Safety Risk Assessment
2.5. Risk-Based Consumption Advisories
2.6. Uncertainty Analyses
3. Results and Discussion
3.1. THg and MeHg Concentration in Seafood
3.2. Human Health Risk Assessment
3.3. Risk and Nutrition-Based Recommended Weekly Consumption Advisory
3.4. Comparison of Raw and Cooked Food
3.5. Correlation between Seafood Weight and THg Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Category | Common Name | Hg Concentration (mg/kg) | Converted MeHg Concentration (mg/kg) | EPA+DHA Concentration (mg/g) |
|---|---|---|---|---|
| Pelagic Fish | Bastard Albacore | 0.48 | 0.45 | 2.300 [39] |
| 0.400 [5] | ||||
| Bigeye Tuna | 0.13 | 0.12 | - | |
| Blue Marlin | 0.14 | 0.73 | 12.000 [42] | |
| Pacific Sailfish | 0.03 | 0.03 | - | |
| Pointed Nose Shark | 0.25 | 0.25 | - | |
| Requiem Shark | 0.08 | 0.06 | 2.200 [40] | |
| Snake Mackerel | 0.32 | 0.29 | - | |
| Swordfish | 0.71 | 0.70 | 5.800 [40] | |
| 3.200 [5] | ||||
| 6.930 [44] | ||||
| Tribon Blou | 1.68 | 1.22 | - | |
| Yellow-fin Tuna | 0.16 | 0.15 | 1.000–1.200 [40] | |
| Inshore Fish | Anglerfish | 0.11 | 0.10 | 0.700 [44] |
| Barracuda | 0.05 | 0.05 | - | |
| Black Sea Bream | 0.08 | 0.08 | - | |
| Bullet Tuna | 0.10 | 0.09 | - | |
| Catfish | 0.00 | 0.00 | 2.800 [40] | |
| 1.900 [44] | ||||
| Cod | 0.78 | 0.76 | 2.400 [40] | |
| 8.300 [5] | ||||
| 3.000 [45] | ||||
| 1.860 [44] | ||||
| 2.620 [8] | ||||
| Cutlassfish | 0.06 | 0.06 | - | |
| Eel | 0.08 | 0.08 | - | |
| Freshwater Eel | 0.06 | 0.06 | - | |
| Golden Threadfin Bream | 0.08 | 0.08 | 8.200 [39] | |
| Haliubut | 0.13 | 0.13 | - | |
| Pike Conger | 0.14 | 0.11 | - | |
| Pompano | 0.02 | 0.02 | 2.000 [42] | |
| Red Seabream | 0.17 | 0.17 | - | |
| Righteye Flounder | 0.03 | 0.03 | 1.500 [40] | |
| Scat | 0.04 | 0.04 | - | |
| Silverfish | 0.16 | 0.16 | 4.100 [5] | |
| Skipjack Tuna | 0.13 | 0.12 | 2.560–3.280 [40] | |
| Sole | 0.00 | 0.00 | 1.920 [44] | |
| Stingray | 0.13 | 0.13 | 0.690 [44] | |
| Sturgeon | 0.00 | 0.00 | - | |
| Yellow Seabream | 0.06 | 0.06 | - | |
| Other Fish | Barramundi | 0.01 | 0.01 | 4.900 [40] |
| 4.900 [45] | ||||
| Blue Mackerel | 0.08 | 0.07 | 2.752 [46] | |
| Butterfish | 0.01 | 0.01 | - | |
| Cobia | 0.13 | 0.13 | 6.000 [42] | |
| Common Dolphinfish | 0.02 | 0.02 | - | |
| Giant Grouper | 0.07 | 0.07 | - | |
| Greater Amberjack | 0.00 | 0.00 | 13.200 [5] | |
| Japanese Butterfish | 0.01 | 0.01 | - | |
| Lizardfish | 0.10 | 0.10 | - | |
| Milkfish | 0.00 | 0.00 | 3.867 [42] | |
| 2.000 [5] | ||||
| Moonfish | 0.06 | 0.06 | - | |
| Oceanic Anchovy | 0.00 | 0.00 | - | |
| Orange-Spotted Grouper | 0.06 | 0.06 | 3.500 [39] | |
| 4.000 [45] | ||||
| Round Scad | 0.07 | 0.07 | - | |
| Salmon Trout | 0.00 | 0.00 | 15.900 [40] | |
| 8.200 [39] | ||||
| 21.600 [5] | ||||
| 25.000 [45] | ||||
| 15.360 [44] | ||||
| 4.242 [8] | ||||
| Saury | 0.06 | 0.04 | - | |
| Sea Cucumber | 0.02 | 0.02 | - | |
| Shishamo | 0.00 | 0.00 | - | |
| Shrimp Scad | 0.33 | 0.31 | ||
| Silver Stripe Round Herring | 0.02 | 0.02 | - | |
| Sweet Fish | 0.04 | 0.04 | 7.000 [42] | |
| Tilapia | 0 | 0 | 1.600 [42] | |
| 1.400 [5] | ||||
| Tilefish | 0.12 | 0.12 | 5.100 [39] |
Appendix B
| Seafood Category | Daily Intake of DHA and EPA a,b,c,d (mg/day) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1–3 Years Old | 4–6 Years Old | 19–49-Year-Old Women | ||||||
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 1 | Scenario 2 | Scenario 3 | |
| Pelagic Fish | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 38.9 | 38.9 | 38.9 |
| Inshore Fish | 3.7 | 7.5 | 15.0 | 7.5 | 15.0 | 74.7 | 89.7 | 104.6 |
| Other Farmed Fish | 512.7 | 414.3 | 223.2 | 892.3 | 739.4 | 968.2 | 597.3 | 214.4 |
References
- Wang, Y.L.; Fang, M.D.; Chien, L.C.; Lin, C.C.; His, H.C. Distribution of mercury and methylmercury in surface water and surface sediment of river, irrigation canal, reservoir, and wetland in Taiwan. Environ. Sci. Pollut. Res. Int. 2019, 26, 17762–17773. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Mercury. Agency for Toxic Substances and Disease Registry. 1999. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=115&tid=24 (accessed on 31 August 2020).
- Subhavana, K.L.; Keerthana, R.T.; Qureshi, A. Mercury in marine, freshwater and aquaculture species from south India and human exposure risk assessment. Expo Health 2020, 12, 897–903. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, Y.M.; Lee, K.E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353. [Google Scholar] [CrossRef] [Green Version]
- Hsi, H.C.; Hsu, Y.W.; Chang, T.C.; Chein, L.C. Methylmercury concentration in fish and risk-benefit assessment of fish intake among pregnant versus infertile women in Taiwan. PLoS ONE 2016, 11, e0155704. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, U.; Palviainen, M.; Eronen, A.; Piirainen, S.; Laurén, A.; Akkanen, J.; Kankaala, P. Spatial variability of mercury and polyunsaturated fatty acids in the European perch (Perca fluviatilis)—Implications for risk-benefit analyses of fish consumption. Environ. Pollut. 2016, 219, 305–314. [Google Scholar] [CrossRef]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, M.J.; Henao, J.J.A.; Reyes, E.S.; Stark, K.D.; Low, G.; Swanson, S.K.; Laird, B.D. Mercury and omega-3 fatty acid profiles in freshwater fish of the Dehcho Region, Northwest Territories: Informing risk benefit assessments. Sci. Total Environ. 2018, 637, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Watzl, B.; Gelencsér, E.; Hoekstra, J.; Kulling, S.; Lydeking-Olsen, E.; Rowland, I.; Schilter, B.; van Klaveren, J.; Chiodini, A. Application of the BRAFO-tiered approach for benefit-risk assessment to case studies on natural foods. Food Chem. Toxicol. 2012, 50, S699–S709. [Google Scholar] [CrossRef]
- Davidson, P.W.; Strain, J.J.; Myers, G.J.; Thurston, S.W.; Bonham, M.P.; Shamlaye, C.F.; Stokes-Riner, A.; Wallace, J.M.W.; Robson, P.J.; Duffy, E.M.; et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology 2008, 29, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.D.; Chen, C.W.; Chen, C.F. Distribution, enrichment, accumulation and potential ecological risks of mercury in the sediments of Kaohsiung Harbor, Taiwan. J. Chem. Ecol. 2013, 29, 693–708. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F. Health risk and significance of mercury in the environment. Environ. Sci. Pollut. Res. Int. 2015, 22, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in marine and oceanic waters—A review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.F.; Ju, Y.R.; Lin, G.T.; Chen, C.W.; Dong, C.D. Methylmercury in industrial harbor sediments in Taiwan: First observations on its occurrence, distribution, and measurement. Int. J. Environ. Res. Public Health 2018, 15, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Chen, M.H. Mercury levels of seafood commonly consumed in Taiwan. J. Food Drug Anal. 2006, 14, 373. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.W.; Chen, Z.H.; Huo, W.I.; Huang, R.; Huang, W.X.; Peng, J.W.; Yang, X.F. Benefit–risk assessment of commonly consumed fish species from South China Sea based on methyl mercury and DHA. Environ. Geochem. Health 2019, 41, 2055–2066. [Google Scholar] [CrossRef]
- Pennington, J.A.; Gunderson, E.L. History of the Food and Drug Administration’s total diet study—1961 to 1987. J. Assoc. Off. Anal. Chem. 1987, 70, 772–782. [Google Scholar] [CrossRef] [PubMed]
- WHO; FAO; EFSA. Towards a Harmonised Total Diet Study Approach: A Guidance Document: Joint Guidance of EFSA, FAO and WHO. 2011. Available online: https://apps.who.int/iris/handle/10665/75330 (accessed on 31 August 2021).
- Bradley, M.A.; Barst, B.D.; Basu, N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Greenfield, B.K.; Dang, F.; Gong, Y.; Bu, W.; Zhong, H. Mechanistic understanding of low methylmercury bioaccessibility from crayfish (Procambarus clarkii) muscle tissue. Sci. Total Environ. 2017, 603, 453–460. [Google Scholar] [CrossRef]
- Gong, Y.; Nunes, L.M.; Greenfield, B.K.; Qin, Z.; Yang, Q.; Huang, L.; Bu, W.; Zhong, H. Bioaccessibility-corrected risk assessment of urban dietary methylmercury exposure via fish and rice consumption in China. Sci. Total Environ. 2018, 630, 222–230. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Contaminants in the Food Chain (CONTAM) Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar] [CrossRef]
- Peng, X.; Liu, F.; Wang, W.X. Organ-specific accumulation, transportation, and elimination of methylmercury and inorganic mercury in a low Hg accumulating fish. Environ. Toxicol. Chem. 2016, 35, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Toxicological Effects of Methylmercury; National Academies Press: Washington, DC, USA, 2000.
- You, S.H.; Wang, S.L.; Pan, W.H.; Chan, W.C.; Fan, A.M.; Lin, P. Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children. Int. J. Hyg. Environ. Health 2018, 221, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.P.; Wang, S.W.; Kao, Y.H.; Chen, J.S. Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ. Geochem. Health 2016, 38, 1271–1281. [Google Scholar] [CrossRef]
- Liang, C.P.; Jang, C.S.; Chen, J.S.; Wang, S.W.; Lee, J.J.; Liu, C.W. Probabilistic health risk assessment for ingestion of seafood farmed in arsenic contaminated groundwater in Taiwan. Environ. Geochem. Health 2013, 35, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Buchanan, S.; Anderson, H.A.; Xiao, Z.; Persky, V.; Turyk, M.E. Association of methylmercury intake from seafood consumption and blood mercury level among the Asian and Non-Asian populations in the United States. Environ. Res. 2018, 160, 212–222. [Google Scholar] [CrossRef]
- WHO. Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives. In Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives. 2004. Available online: https://apps.who.int/iris/handle/10665/42849 (accessed on 31 August 2021).
- Agah, H.; Leermakers, M.; Elskens, M.; Fatemi, S.M.R.; Baeyens, W. Total mercury and methyl mercury concentrations in fish from the Persian Gulf and the Caspian Sea. Water Air Soil Pollut. 2007, 181, 95–105. [Google Scholar] [CrossRef]
- Baeyens, W.; Leermakers, M.; Papina, T.; Saprykin, A.; Brion, N.; Noyen, J.; Gieter, M.D.; Elskens, M.; Goeyens, L. Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt Estuary fish. Arch. Environ. Contam. Toxicol. 2003, 45, 498–508. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, L.; Zhao, W.; Liu, X.; Sakamoto, M.; Wang, W. Mercury levels in fisherman and their household members in Zhoushan, China: Impact of public health. Sci. Total Environ. 2009, 407, 2625–2630. [Google Scholar] [CrossRef]
- Clémens, S.; Monperrus, M.; Donard, O.F.X.; Amouroux, D.; Guerin, T. Mercury speciation analysis in seafood by species-specific isotope dilution: Method validation and occurrence data. Anal. Bioanal. Chem. 2011, 401, 2699. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, D.S.; Casey, V.; Dabeka, R.W.; McKenzie, A. Methylmercury levels in predatory fish species marketed in Canada. Food Addit. Contam. A 2004, 21, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hight, S.C.; Cheng, J. Determination of methylmercury and estimation of total mercury in seafood using high performance liquid chromatography (HPLC) and inductively coupled plasma-mass spectrometry (ICP-MS): Method development and validation. Anal. Chim. Acta 2006, 567, 160–172. [Google Scholar] [CrossRef]
- Kuballa, T.; Moellers, M.; Schoeberl, K.; Lachenmeier, D.W. Survey of methylmercury in fish and seafood from the southwestern German market. Eur. Food Res. Technol. 2011, 232, 737–742. [Google Scholar] [CrossRef]
- Miklavčič, A.; Cuderman, P.; Mazej, D.; Tratnik, J.S.; Krsnik, M.; Planinšek, P.; Osredkar, J.; Horvat, M. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ. Res. 2011, 111, 1201–1207. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; D’Addabbo, R.; Palermo, C.; Marcotrigiano, G.O. Survey of total mercury and methylmercury levels in edible fish from the Adriatic Sea. Food Addit. Contam. 2003, 20, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.Y.; Wong, W.W.K.; Chung, S.W.C.; Tran, C.H.; Chan, B.T.P.; Ho, Y.Y.; Xiao, Y. Quantitative risk–benefit analysis of fish consumption for women of child-bearing age in Hong Kong. Food Addit. Contam. A 2014, 31, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Mahaffey, K.R.; Sunderland, E.M.; Chan, H.M.; Choi, A.L.; Grandjean, P.; Marien, K.; Oken, E.; Sakamoto, M.; Schoeny, R.; Weihe, P.; et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr. Rev. 2011, 69, 493–508. [Google Scholar] [CrossRef]
- GEMS/Food-EURO. Second Workshop on Reliable Evaluation of Low-Level Contamination of Food: Report on a Workshop in the Frame of GEMS. 1995. Available online: http://toolbox.foodcomp.info/References/LOD/GEMS-Food-EURO%20%20-%20%20Reliable%20Evaluation%20of%20Low-Level%20Contamination%20of%20Food.pdf (accessed on 31 August 2021).
- Del Gobbo, L.C.; Archbold, J.A.; Vanderlinden, L.D.; Eckley, C.S.; Diamond, M.L.; Robson, M. Risks and benefits of fish consumption for childbearing women. Can. J. Diet Pract. Res. 2010, 71, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO; WHO. Fats and fatty acids in human nutrition. Proceedings of the Joint FAO/WHO Expert Consultation. November 10-24, 2008, Geneva, Switzerland. Ann. Nutr. Metab. 2009, 55, 308. Available online: https://apps.who.int/nutrition/publications/nutrientrequirements/anm_fatsandfattyacids_human_nut/en/index.html (accessed on 31 August 2021).
- Afonso, C.; Bernardo, I.; Bandarra, N.M.; Martins, L.L.; Cardoso, L. The implications of following dietary advice regarding fish consumption frequency and meal size for the benefit (EPA+ DHA and Se) versus risk (MeHg) assessment. Int. J. Food Sci. Nutr. 2019, 70, 623–637. [Google Scholar] [CrossRef]
- Cantoral, A.; Batis, C.; Basu, N. National estimation of seafood consumption in Mexico: Implications for exposure to methylmercury and polyunsaturated fatty acids. Chemosphere 2017, 174, 289–296. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; González-Barriga, V.; Valenzuela, R.; López-Arana, S.; Remero, J.; Valenzuela, A. Profile and distribution of fatty acids in edible parts of commonly consumed marine fishes in Chile. Food Chem. 2019, 274, 123–129. [Google Scholar] [CrossRef] [PubMed]
- German Federal Risk Assessment Agency (BfR). Opinion No. 030/2009 BfR Recommends the Setting of Maximum Levels for the Fortification of Foods with Omega-3 Fatty Acids. Available online: http://www.bfr.bund.de/cm/349/bfr_recommends_the_setting_of_maximum_levels_for_the_fortification_of_foods_with_omega_3_fatty_acids.pdf (accessed on 31 August 2021).
- US Food and Drug Administration (USFDA). Title 21—Food and Drugs Chapter I: Food and Drug Administration Department of Health and Human Services Subchapter b—Food for Human Consumption (continued) Part 184—Direct Food Substances Affirmed as Generally Recognized as Safe. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1472 (accessed on 31 August 2021).
- Devesa, V.; Martinez, A.; Suner, M.A.; Benito, V.; Velez, D.; Montoro, R. Kinetic study of transformations of arsenic species during heat treatment. J. Agric. Food Chem. 2001, 49, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Perello, G.; Marti-Cid, R.; Llobet, J.M.; Domingo, J.L. Effects of various cooking processes on the concentrations of arsenic, cadmium, mercury, and lead in foods. J. Agric. Food Chem. 2008, 56, 11262–11269. [Google Scholar] [CrossRef]
- Burger, J.; Gaines, K.F.; Boring, C.S.; Snodgrass, J.; Stephens, W.L., Jr.; Gochfeld, M. Effects of cooking on radiocesium in fish from the Savannah River: Exposure differences for the public. Arch. Environ. Contam. Toxicol. 2004, 46, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalogeropoulos, N.; Karavoltsos, S.; Sakellari, A.; Avramidou, S.; Dassenakis, M.; Scoullos, M. Heavy metals in raw, fried and grilled Mediterranean finfish and shellfish. Food Chem. Toxicol. 2012, 50, 3702–3708. [Google Scholar] [CrossRef]
- Diaconescu, C.; Urdes, L.; Diaconescu, S.; Popa, D. Effects of cooking methods on the heavy metal concentrations in the fish meat originating from different areas of Danube River. J. Anim. Sci. 2012, 55, 265–267. [Google Scholar]
- Atta, M.B.; El-Sebaie, L.A.; Noaman, M.A.; Kassab, H.E. The effect of cooking on the content of heavy metals in fish (Tilapia nilotica). Food Chem. 1997, 58, 1–4. [Google Scholar] [CrossRef]



| Seafood Category | Common Name (Sample Size) |
|---|---|
| Pelagic Fish (n = 17) | Bastard Albacore (1), Bigeye Tunny (1), Blue Marlin (2), Marlin (1), Pacific Sailfish (1), Pointed Nose Shark (1), Requiem Shark (1), Snake Mackerel (2), Swordfish (4), Tribon Blou (2), and Yellow-Fin Tuna (1). |
| Inshore Fish (n = 35) | Anglerfish (2), Barracuda (3), Bullet Mackerel (1), Catfish (2), Cod (1), Cutlassfish (1), Eel (1), Freshwater Eel (2), Halibut (3), Pike Congers (2), Pompano (1), Red Seabream (2), Righteye Flounder (2), Scat (2), Silverfish (3), Skipjack (2), Stingray (3), Sturgeon (1), and Yellow Seabream (1). |
| Other Farmed Fish (n = 47) | Barramundi (3), Blue Mackerel (3), Butterfish (2), Cobia (1), Common Dolphinfish (1), Giant Grouper (2), Golden Threadfin Bream (2), Greater Amberjack (1), Japanese Butterfish (3), Japanese Horse Mackerel (1), Lizardfish (1), Milkfish (4), Moonfish (1), Oceanic Anchovy (1), Orange-Spotted Grouper (4), Round Scad (1), Salmon Trout (1), Saury (2), Sea Cucumber (1), Shishamo (2), Shrimp Scad (2), Silver-Stripe Round Herring (1), Sweet Fish (1), Tilapia (4), and Tilefish (1). |
| Shellfish (n = 9) | Babylonia (3), Clam (1), Freshwater Clam (1), Locos (1), Mussel (1), Oyster (1), and Variegate Venus (1). |
| Cephalopods (n = 10) | Argentine Shortfin Squid (2), Cuttlefish (1), Jumbo Flying Squid (1), Loligo Squid (1), Octopus (2), and Squid (3). |
| Crustaceans (n = 16) | Big Head Shrimp (3), Blue Crab (1), Crayfish (1), Giant River Prawn (1), Grass Shrimp (1), Lobster (2), Pelagic Crab (3), Sakura Shrimp (2), Serrated Crab (1), and Whiteleg Shrimp (1). |
| Algae (n = 6) | Eucheuma (1), Gracilaria Seaweed (1), Purple Laver (2), and Sea Tangle (2). |
| Seafood Category | Consumption Rates (g/Week) for Various Age Groups | ||||
|---|---|---|---|---|---|
| 1–3 Years Old | 4–6 Years Old | 7–18 Years Old | ≥19 Years Old | 19–49-Year-Old Women | |
| Pelagic Fish | LN(9.24, 16.73) a | LN(14.28, 15.68) | LN(19.32, 16.10) | LN(19.95, 16.87) | LN(20.86, 18.34) |
| Inshore Fish | LN(31.29, 16.73) | LN(48.30, 15.68) | LN(65.66, 16.10) | LN(67.55, 16.87) | LN(70.84, 18.34) |
| Other Farmed Fish | LN(123.90, 16.73) | LN(191.24, 15.68) | LN(259.70, 16.10) | LN(267.40, 16.87) | LN(280.42, 18.34) |
| Shellfish | LN(67.13, 16.52) | LN(102.06, 16.03) | LN(83.16, 21.77) | LN(107.45, 22.47) | LN(104.86, 24.36) |
| Cephalopods | LN(167.09, 21.14) | LN(371.00, 11.13) | LN(294.49, 16.24) | LN(277.76, 15.82) | LN(322.49, 14.14) |
| Crustaceans | LN(149.45, 13.09) | LN(102.83, 24.29) | LN(136.43, 20.86) | LN(107.45, 24.57) | LN(139.23, 19.74) |
| Algae | LN(64.05, 30.80) | LN(94.57, 22.12) | LN(90.30, 20.65) | LN(155.82, 22.75) | LN(147.00, 21.35) |
| Species | Scientific Name | % MeHg/THg | Reference |
|---|---|---|---|
| Anglerfish | Anglerfish Lophiomus setigerus | 92.5 | [31] |
| Cephalopods | Argentine Shortfin Squid Illex argentinus | 81.3 | [32] |
| Jumbo Flying Squid Dosidicus gigas | |||
| Cuttlefish Sepia Esculenta | 72.8 | [32] | |
| Octopus Octopus vulgare | 92 | [37] | |
| Cod | Cod Coelorinchus kamoharai | 97.4 | [31] |
| Crustaceans | Big Head Shrimp Macrobrachium rosenbergii | 93 | [33] |
| Grass Shrimp Penaeus monodon | |||
| Whiteleg Shrimp Sergia lucens | |||
| Eel | Freshwater Eel Anguilla luzonensis | 100 | [36] |
| Eel Muraenesox cinereus | 83.6 | [32] | |
| Flatfish | Sole Cynoglossus arel | 77.3 | [32] |
| Hairtail | Cutlassfish Trichiurus nanhaiensis | 99 | [38] |
| Silverfish Trichiurus lepturus | |||
| Herring | Silver-Stripe Round Herring Spratelloides gracilis | 100 | [38] |
| Lizardfish | Lizardfish Saurida tumbil | 100 | [30] |
| Marlin | Blue Marlin Makaira nigricans | 84 | [34] |
| Pacific Sailfish Istiophorus platypterus | |||
| Swordfish Xiphias gladius | 99 | [36] | |
| Oilfish | Snake Mackerel Gempylus serpens | 92 | [36] |
| Pomfret | Butterfish Pampus echinogaster | 75.4 | [32] |
| Pompano | Pompano Parastromateus niger | 94 | [38] |
| Salmon | Salmon Trout Oncorhynchus mykiss | 93 | [37] |
| Saury | Saury Saurida undosquamis | 75 | [36] |
| Shark | Requiem Shark Carcharhinus melanopterus | 73 | [34] |
| Tribon Blou Prionace glauca | |||
| Shellfish | Oyster Crassostrea gigas | 82 | [33] |
| Mussel Perna viridis | 35 | [36] | |
| Snapper | Black Sea Bream Acanthopagrus schlegelii | 100 | [38] |
| Red Seabream Pagrus major | |||
| Yellow Seabream Dentex hypselosomus | |||
| Tilapia | Tilapia Oreochromis niloticus | 95 | [35] |
| Tuna | Bastard Albacore Thunnus alalunga | 93 | [37] |
| Bigeye Tunny Thunnus obesus | |||
| Yellow-Fin Tuna Thunnus albacares |
| Seafood Categories | Number of Species | Uncooked (Raw) | Cooked (Steam) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Samples | ≥LOQ | THg Concentration (mg/kg) | Number of Samples | ≥LOQ | THg Concentration (mg/kg) | ||||
| Mean ± SD | Range | Mean ± SD | Range | ||||||
| Pelagic Fish | 11 | 17 | 17 | 0.61 ± 0.82 | 0.03–3.16 | 17 | 17 | 0.97 ± 1.30 | 0.05–4.59 |
| Inshore Fish | 19 | 35 | 32 | 0.11 ± 0.14 | ND a–0.78 | 35 | 34 | 0.15 ± 0.24 | ND b–1.39 |
| Other Farmed Fish | 25 | 47 | 29 | 0.05 ± 0.07 | ND a–0.35 | 47 | 42 | 0.07 ± 0.11 | ND b–0.47 |
| Shellfish | 7 | 9 | 3 | 0.02 ± 0.02 | ND a–0.06 | 9 | 6 | 0.02 ± 0.02 | ND b–0.05 |
| Cephalopods | 6 | 10 | 5 | 0.02 ± 0.01 | ND a–0.05 | 10 | 10 | 0.04 ± 0.03 | 0.02–0.11 |
| Crustaceans | 10 | 16 | 11 | 0.04 ± 0.04 | ND a–0.14 | 16 | 14 | 0.05 ± 0.06 | ND b–0.23 |
| Algae | 4 | 6 | 1 | 0.01 ± 0.00 | ND a | 6 | 1 | 0.01 ± 0.00 | ND b–0.01 |
| Seafood Category | Weekly Consumption Advisory (g/Week) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1–3 Years Old | 4–6 Years Old | 19–49-Year-Old Women | ||||||
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 1 | Scenario 2 | Scenario 1 | Scenario 2 | Scenario 3 | |
| Pelagic Fish | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 70.0 | 70.0 | 70.0 |
| Inshore Fish | 8.8 | 17.5 | 35.0 | 17.5 | 35.0 | 175.0 | 210.0 | 245.0 |
| Other Farmed Fish | 129.2–453.0 | 125.9–366.1 | 119.3–197.3 | 170.1–788.5 | 163.5–653.3 | 120.5–855.5 | 107.3–527.8 | 94.1–189.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Nan, F.-H.; Ling, M.-P. Dietary Exposure of the Taiwan Population to Mercury Content in Various Seafood Assessed by a Total Diet Study. Int. J. Environ. Res. Public Health 2021, 18, 12227. https://doi.org/10.3390/ijerph182212227
Lin P, Nan F-H, Ling M-P. Dietary Exposure of the Taiwan Population to Mercury Content in Various Seafood Assessed by a Total Diet Study. International Journal of Environmental Research and Public Health. 2021; 18(22):12227. https://doi.org/10.3390/ijerph182212227
Chicago/Turabian StyleLin, Pinpin, Fan-Hua Nan, and Min-Pei Ling. 2021. "Dietary Exposure of the Taiwan Population to Mercury Content in Various Seafood Assessed by a Total Diet Study" International Journal of Environmental Research and Public Health 18, no. 22: 12227. https://doi.org/10.3390/ijerph182212227






