Biosorption of Uranyl Ions from Aqueous Solution by Parachlorella sp. AA1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Preparation of U(VI) Solutions
2.3. Determination of Growth Conditions
2.4. U(VI) Biosorption Experiments
2.5. X-ray Absorption near Edge Structure Analysis
3. Results and Discussion
3.1. AA1 Strain Properties
3.2. Effects of Initial pH and U(VI) Concentration
3.3. U(VI) Biosorption Experiments
3.4. Detection of Biosorption of U(VI) Using XANES Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gok, C.; Aytas, S. Biosorption of uranium(VI) from aqueous solution using calcium alginate beads. J. Hazard. Mater. 2009, 168, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Veglio, F.; Beolchini, F. Removal of metals by biosorption: A review. Hydrometallurgy 1997, 44, 301–316. [Google Scholar] [CrossRef]
- Markich, S.J. Uranium speciation and bioavailability in aquatic systems: An overview. Sci. World J. 2002, 2, 707–729. [Google Scholar] [CrossRef] [Green Version]
- Fortin, C.; Dutel, L.; Garnier-Laplace, J. Uranium complexation and uptake by a green alga in relation to chemical speciation; the importance of the free uranyl ion. Environ. Toxicol. Chem. 2004, 23, 974–981. [Google Scholar] [CrossRef]
- Kalinowski, B.E.; Oskarsson, A.; Albinsson, Y.; Arlinger, J.; Ödegaard-Jensen, A.; Andlid, T.; Pedersen, K. Microbial leaching of uranium and other trace elements from shale mine tailings at Ranstad. Geoderma 2004, 122, 177–194. [Google Scholar] [CrossRef]
- Ma, M.; Wang, R.; Xu, L.; Xu, M.; Liu, S. Emerging health risks and underlying toxicological mechanisms of uranium contamination: Lessons from the past two decades. Environ. Int. 2020, 145, 106107. [Google Scholar] [CrossRef]
- Manikandan, N.; Prasath, C.S.; Prakash, S. Biosorption of uranium and thorium by Marine micro algae. Indian J. Geo Mar. Sci. 2011, 40, 121–124. [Google Scholar]
- Newsome, L.; Morris, K.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Petrie, L.; North, N.N.; Dollhopf, S.L.; Balkwill, D.L.; Kostka, J.E. Enumeration and characterization of iron(III)-reducing microbial communities from acidic subsurface sediments contaminated with uranium(VI). Appl. Environ. Microbiol. 2003, 69, 7467–7479. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kelly, S.D.; Kemner, K.M.; Banfield, J.F. Nanometre-size products of uranium bioreduction. Nature 2002, 419, 134. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; García-Balboa, C.; López-Rodas, V.; Fernández Díaz, M.; Costas, E. Evidence of microalgal isotopic fractionation through enrichment of depleted uranium. Sci. Rep. 2019, 9, 1973. [Google Scholar] [CrossRef]
- Kalin, M.; Wheeler, W.N.; Meinrath, G. The removal of uranium from mining waste water using algal/microbial biomass. J. Environ. Radioact. 2005, 78, 151–177. [Google Scholar] [CrossRef]
- Manoyan, J.; Gabrielyan, L.; Kozel, N.; Trchounian, A. Regulation of biohydrogen production by protonophores in novel green microalgae Parachlorella kessleri. J. Photochem. Photobiol. B Biol. 2019, 199, 111597. [Google Scholar] [CrossRef]
- Bold, H.C.; Wynne, M.J. Introduction to the Alga; Prentice-Hall Press: Upper Saddle River, NJ, USA, 1985. [Google Scholar]
- Gao, Y.; Feng, J.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Physiological changes of Parachlorella Kessleri TY02 in lipid accumulation under nitrogen stress. Int. J. Environ. Res. Public Health 2019, 16, 1188. [Google Scholar] [CrossRef] [Green Version]
- Krienitz, L.; Hegewald, E.H.; Hepperle, D.; Huss, V.A.R.; Rohr, T.; Wolf, M. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 2004, 43, 529–542. [Google Scholar] [CrossRef]
- Kuncham, K.; Nair, S.; Durani, S.; Bose, R. Efficient removal of uranium(VI) from aqueous medium using ceria nanocrystals: An adsorption behavioural study. J. Radioanal. Nucl. Chem. 2017, 313, 101–112. [Google Scholar] [CrossRef]
- Warchoł, J.; Matłok, M.; Misaelides, P.; Noli, F.; Zamboulis, D.; Godelitsas, A. Interaction of UaqVI with CHA-type zeolitic materials. Microporous Mesoporous Mater. 2012, 153, 63–69. [Google Scholar] [CrossRef]
- Bampaiti, A.; Yusan, S.; Aytas, S.; Pavlidou, E.; Noli, F. Investigation of uranium biosorption from aqueous solutions by Dictyopteris polypodioides brown algae. J. Radioanal. Nucl. Chem. 2016, 307, 1335–1343. [Google Scholar] [CrossRef]
- Bozkurt, S.S.; Molu, Z.B.; Cavas, L.; Merdivan, M. Biosorption of uranium(VI) and thorium(IV) onto Ulva gigantean (Kützing) bliding: Discussion of adsorption isotherms, kinetics and thermodynamic. J. Radioanal. Nucl. Chem. 2011, 288, 867–874. [Google Scholar] [CrossRef]
- Cecal, A.; Humelnicu, D.; Rudic, V.; Cepoi, L.; Ganju, D.; Cojocari, A. Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour. Technol. 2012, 118, 19–23. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhainsa, K.C.; D’Souza, S.F. Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresour. Technol. 2010, 101, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Study of polyethyleneimine- and amidoxime-functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium (VI) ion. Environ. Sci. Pollut. Res. 2015, 22, 17998–18010. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.V.; Melo, J.S.; Chaugule, B.B.; D’Souza, S.F. Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J. Hazard. Mater. 2008, 158, 628–635. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, L. Removal of uranium(VI) from aqueous solution using citric acid modified pine sawdust: Batch and column studies. J. Radioanal. Nucl. Chem. 2012, 292, 585–595. [Google Scholar] [CrossRef]
- Konstantinou, M.; Pashalidis, I. Adsorption of hexavalent uranium on biomass by-product. J. Radioanal. Nucl. Chem. 2007, 273, 549–552. [Google Scholar] [CrossRef]
- Yi, Z.; Lian, B. Adsorption of U(VI) by Bacillus mucilaginosus. J. Radioanal. Nucl. Chem. 2012, 293, 321–329. [Google Scholar] [CrossRef]
- Prodromou, M.; Pashalidis, I. Uranium adsorption by nontreated and chemically modified cactus fibres in aqueous solutions. J. Radioanal. Nucl. Chem. 2013, 298, 1587–1595. [Google Scholar] [CrossRef]
- Nilchi, A.; Dehaghan, T.S.; Garmarodi, S.R. Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 2013, 321, 67–71. [Google Scholar] [CrossRef]
- Akkaya, R. Removal of radioactive elements from aqueous solutions by adsorption onto polyacrylamide-expanded perlite: Equilibrium, kinetic, and thermodynamic study. Desalination 2013, 321, 3–8. [Google Scholar] [CrossRef]
- Yusan, S.; Erenturk, S.A. Adsorption equilibrium and kinetics of U(VI) on beta type of akaganeite. Desalination 2010, 263, 233–239. [Google Scholar] [CrossRef]
- Sarri, S.; Misaelides, P.; Papanikolaou, M.; Zamboulis, D. Uranium removal from acidic aqueous solutions by Saccharomyces cerevisiae, Debaryomyces hansenii, Kluyveromyces marxianus and Candida colliculosa. J. Radioanal. Nucl. Chem. 2009, 279, 709–711. [Google Scholar] [CrossRef]
- Ghasemi, M.; Keshtkar, A.R.; Dabbagh, R.; Safdari, S.L. Biosorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: Breakthrough curves studies and modeling. J. Hazard. Mater. 2011, 189, 141–149. [Google Scholar] [CrossRef]
- Khani, M.H. Uranium biosorption by Padina sp. Algae biomass: Kinetics and thermodynamics. Environ. Sci. Pollut. Res. 2011, 18, 1593–1605. [Google Scholar] [CrossRef]
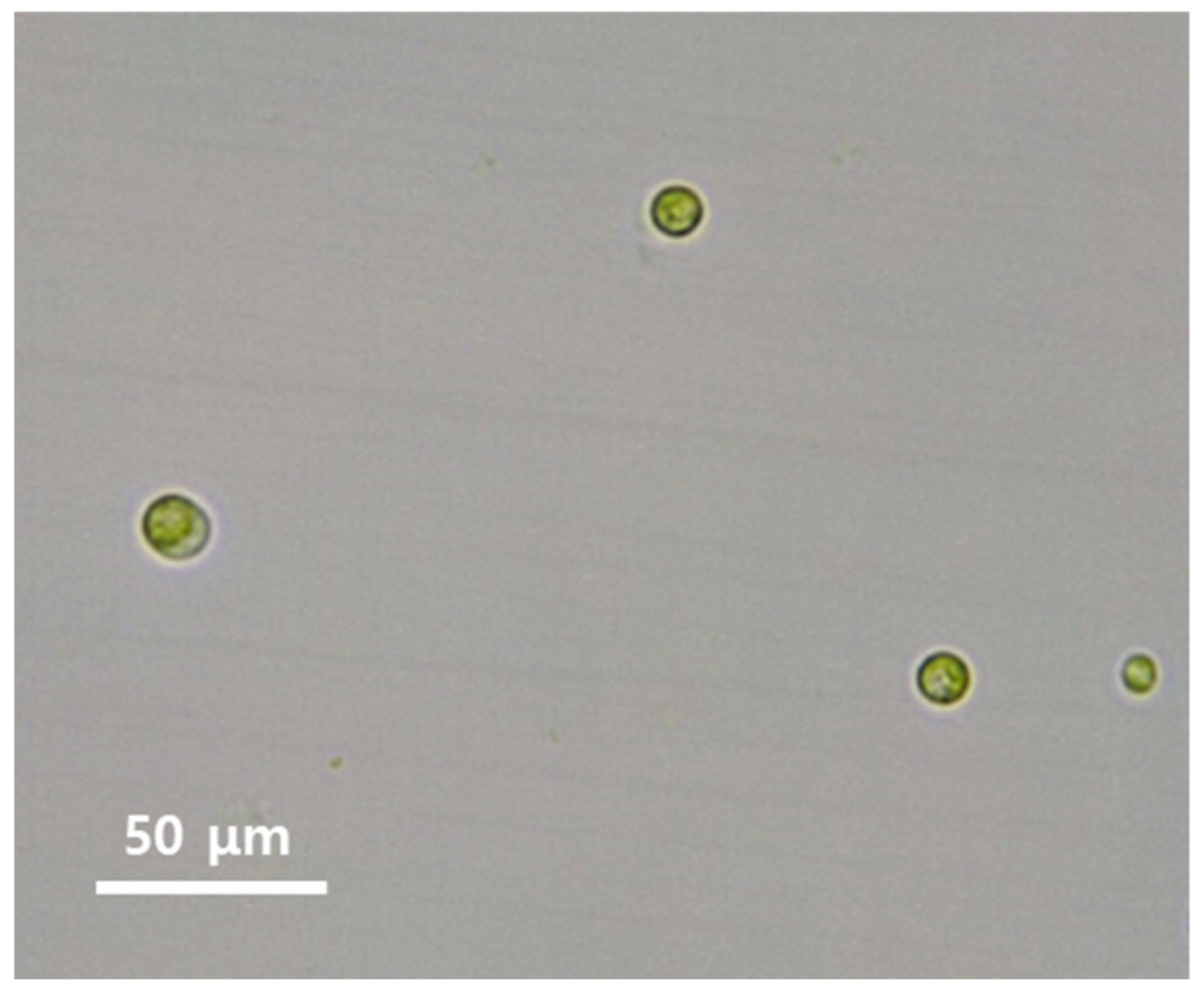

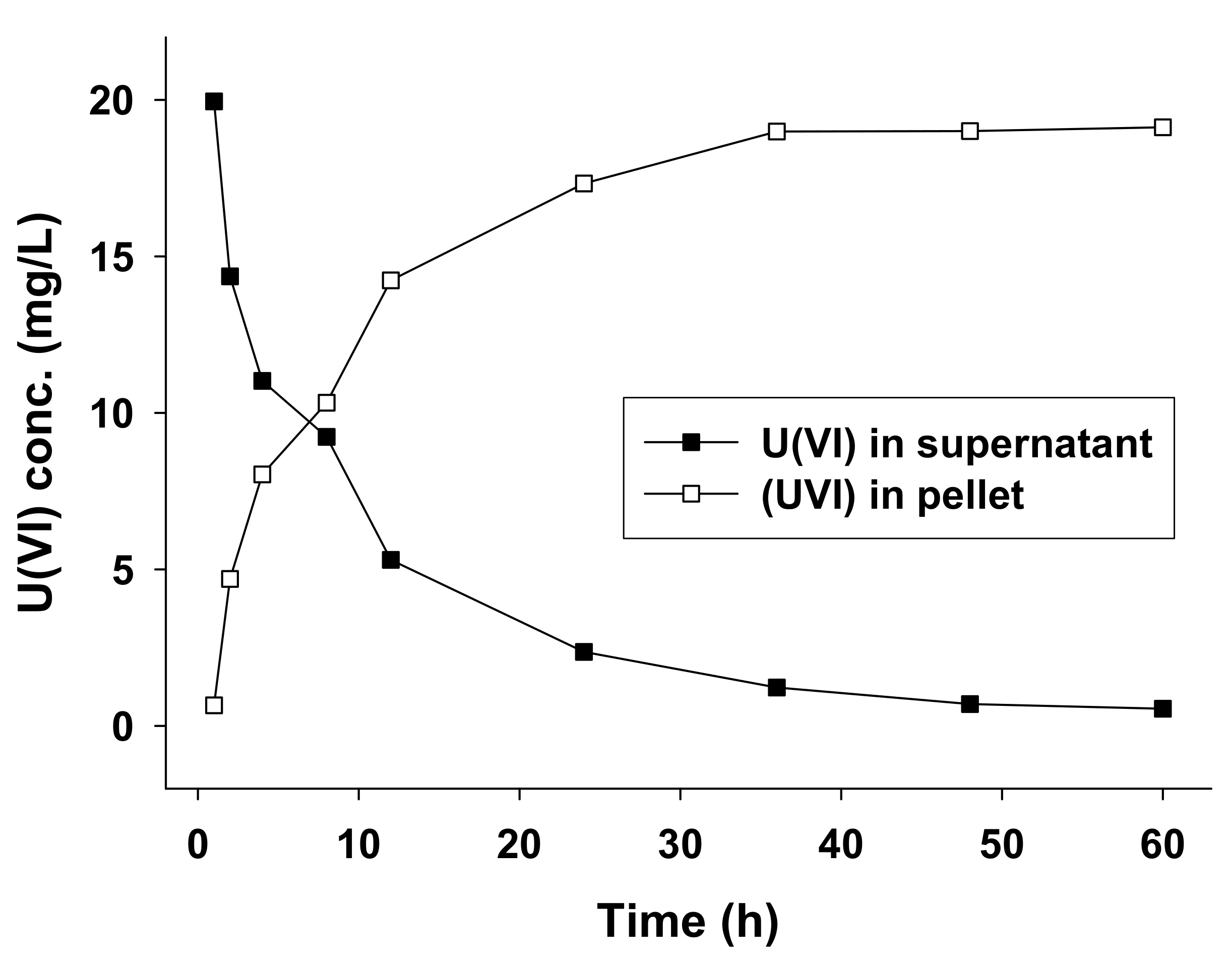
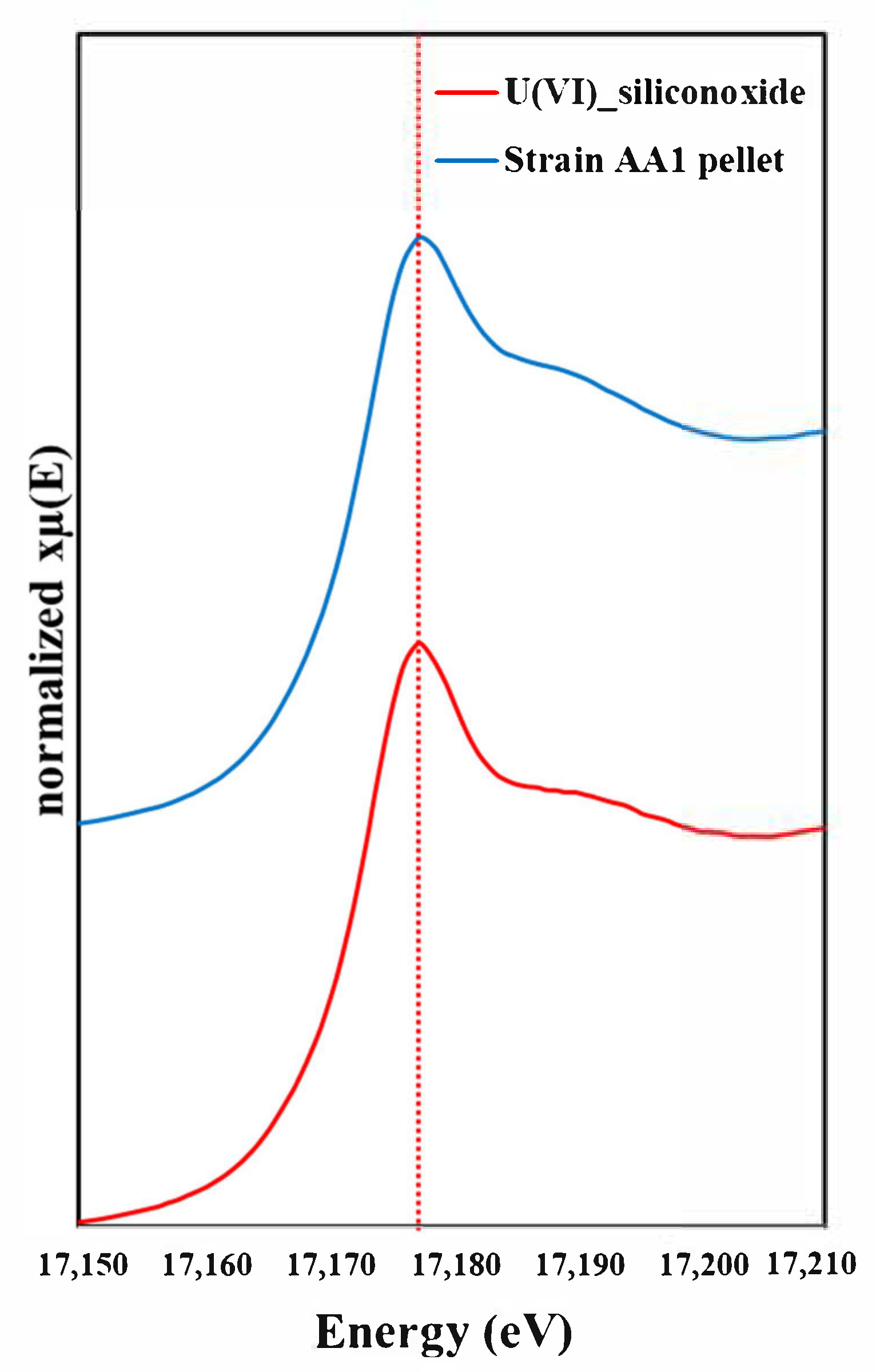
| Initial pH of Medium | Optical Density (680 nm) b | Biosorption Rate (mean ± SD b %) c |
|---|---|---|
| 5.0 | 0.297 ± 0.007 | 62.6 ± 1.99 |
| 6.0 | 0.385 ± 0.010 | 71.5 ± 1.12 |
| 7.0 | 0.501 ± 0.009 | 82.2 ± 0.84 |
| 8.0 | 0.535 ± 0.011 | 90.5 ± 0.75 |
| 9.0 | 0.548 ± 0.015 | 97.2 ± 0.43 |
| Initial U(VI) Concentration (mg/L) | Biosorption Rate (mean ± SD b %) c |
|---|---|
| 0.5 | 95.7 ± 0.59 |
| 1.0 | 96.2 ± 1.03 |
| 5.0 | 96.8 ± 0.44 |
| 10.0 | 96.9 ± 0.81 |
| 20.0 | 97.4 ± 0.34 |
| 30.0 | 91.2 ± 0.16 |
| 50.0 | 89.8 ± 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-Y.; Nam, I.-H.; Yoon, M.-H. Biosorption of Uranyl Ions from Aqueous Solution by Parachlorella sp. AA1. Int. J. Environ. Res. Public Health 2021, 18, 3641. https://doi.org/10.3390/ijerph18073641
Yoon J-Y, Nam I-H, Yoon M-H. Biosorption of Uranyl Ions from Aqueous Solution by Parachlorella sp. AA1. International Journal of Environmental Research and Public Health. 2021; 18(7):3641. https://doi.org/10.3390/ijerph18073641
Chicago/Turabian StyleYoon, Ja-Young, In-Hyun Nam, and Min-Ho Yoon. 2021. "Biosorption of Uranyl Ions from Aqueous Solution by Parachlorella sp. AA1" International Journal of Environmental Research and Public Health 18, no. 7: 3641. https://doi.org/10.3390/ijerph18073641






