The Potential Use of Nephelium lappaceum Seed as Coagulant–Coagulant Aid in the Treatment of Semi-Aerobic Landfill Leachate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Characterisation of Leachate
2.2. Extraction and Characterisation of Nephelium lappaceum Seed
2.3. Coagulation–Flocculation Experiment
2.4. Determination of Optimum pH and Coagulant Dosage
3. Results and Discussion
3.1. Landfill Leachate Compositions
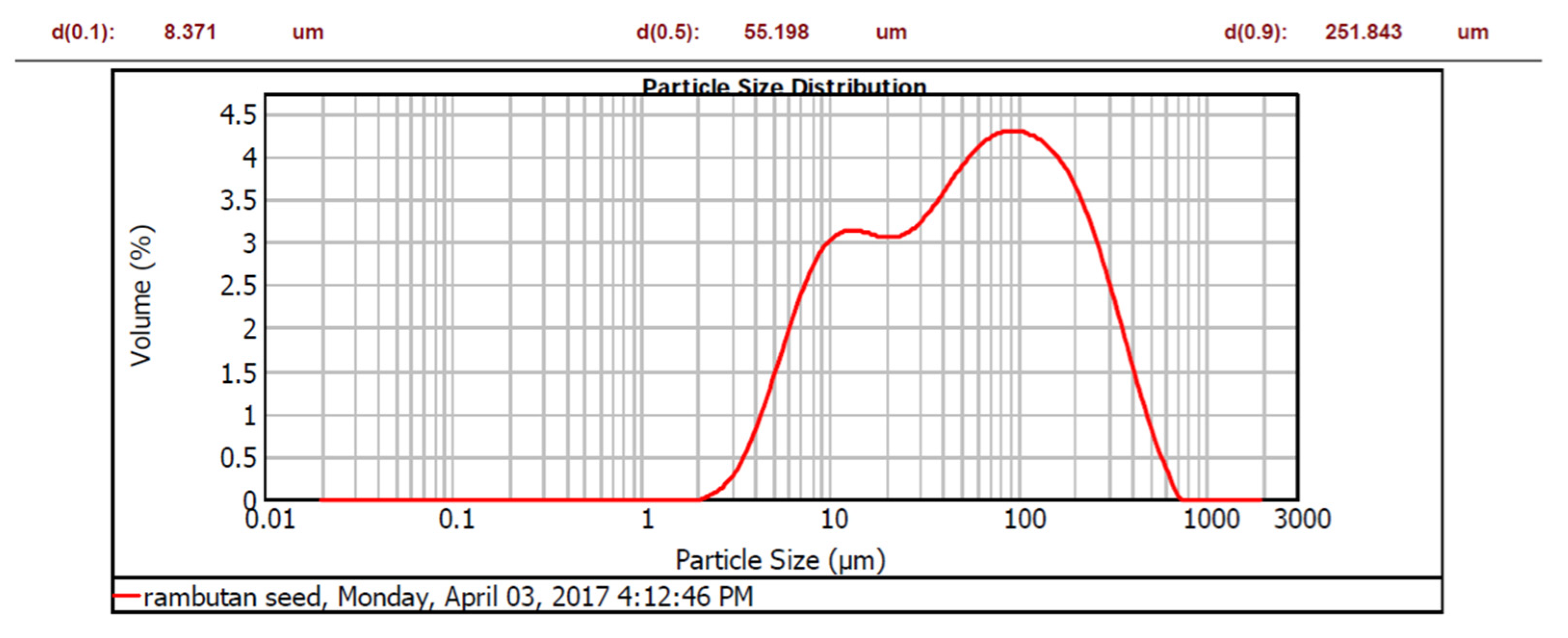
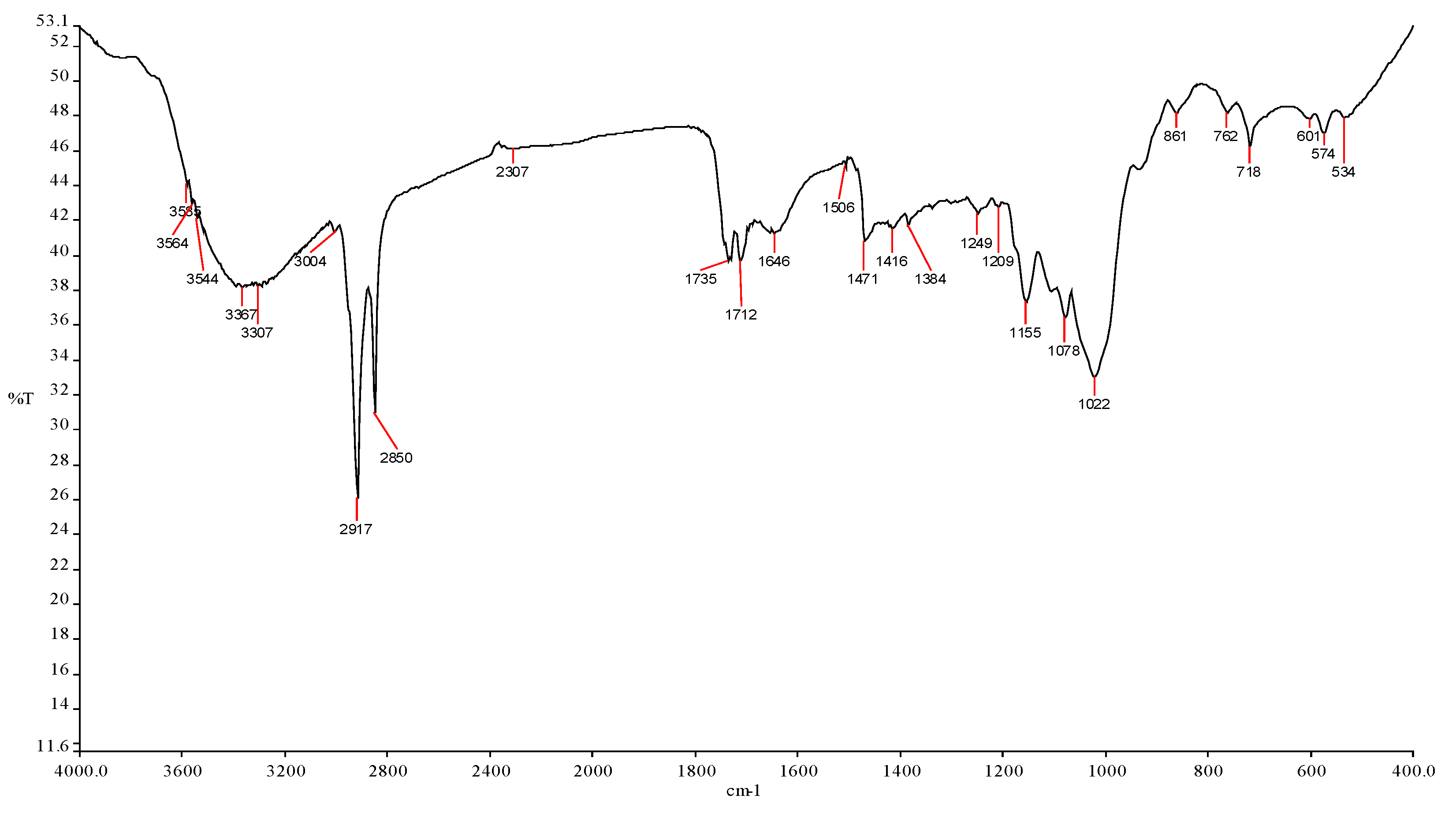
3.2. Characteristic of Nephelium lappaceum Seeds
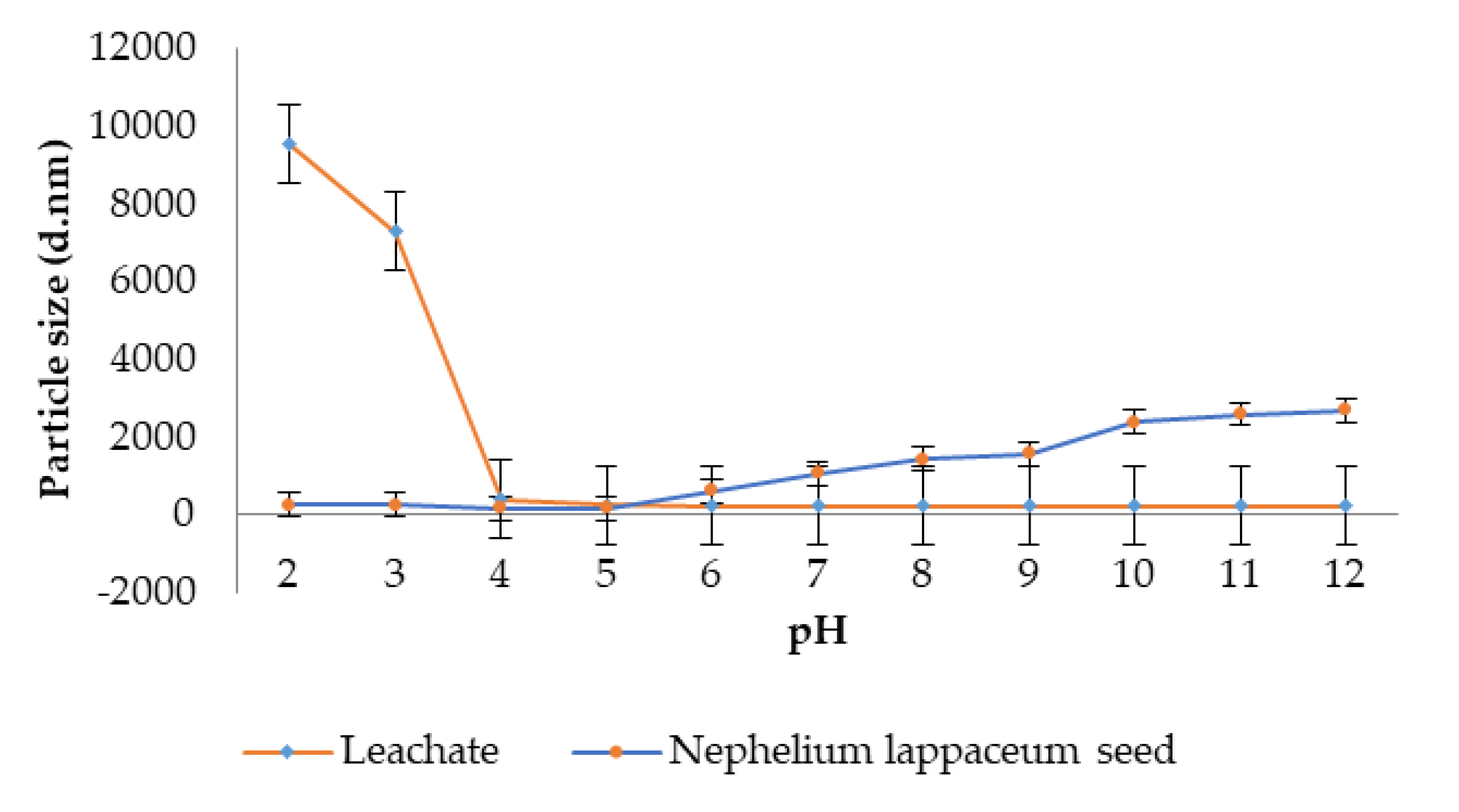
3.3. Zeta Potential and Particle Size
3.4. Optimum Operating Conditions of Nephelium lappaceum Seed as a Sole Coagulant
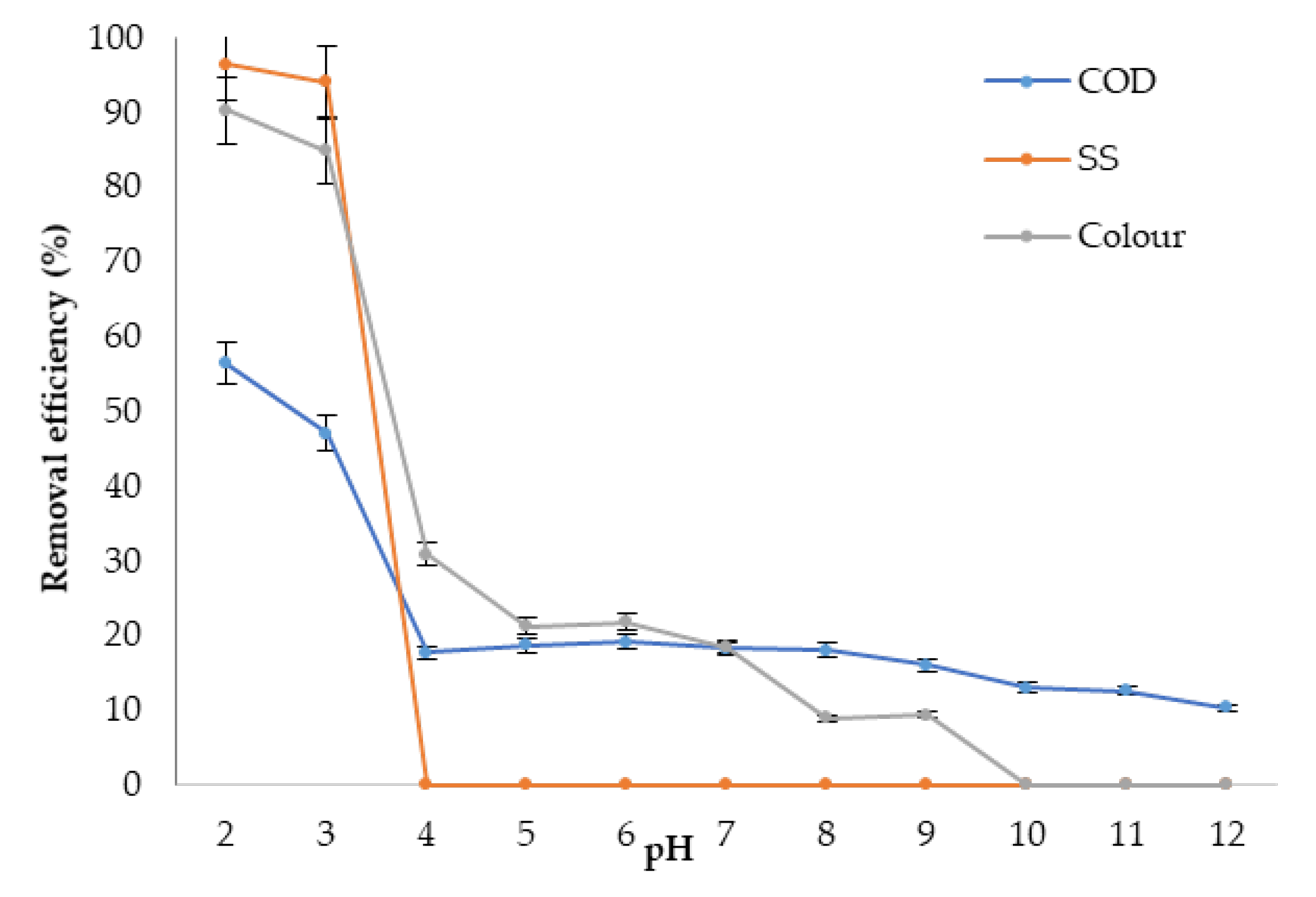
3.4.1. Optimum pH
3.4.2. Optimum Dosage
3.5. Optimum Operating Conditions of SnCl4 a Sole Coagulant
3.5.1. Optimum pH
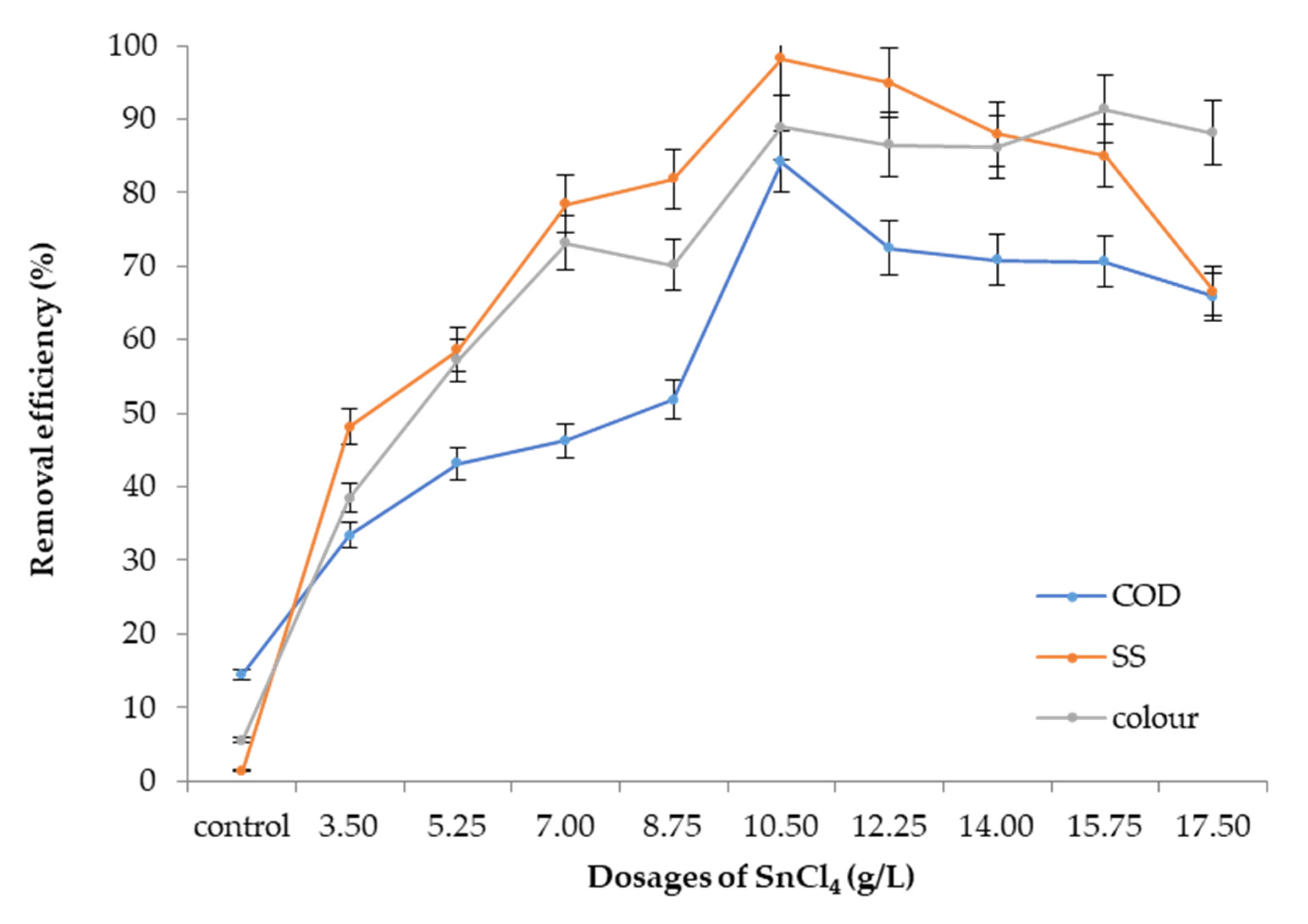
3.5.2. Optimum Dosage
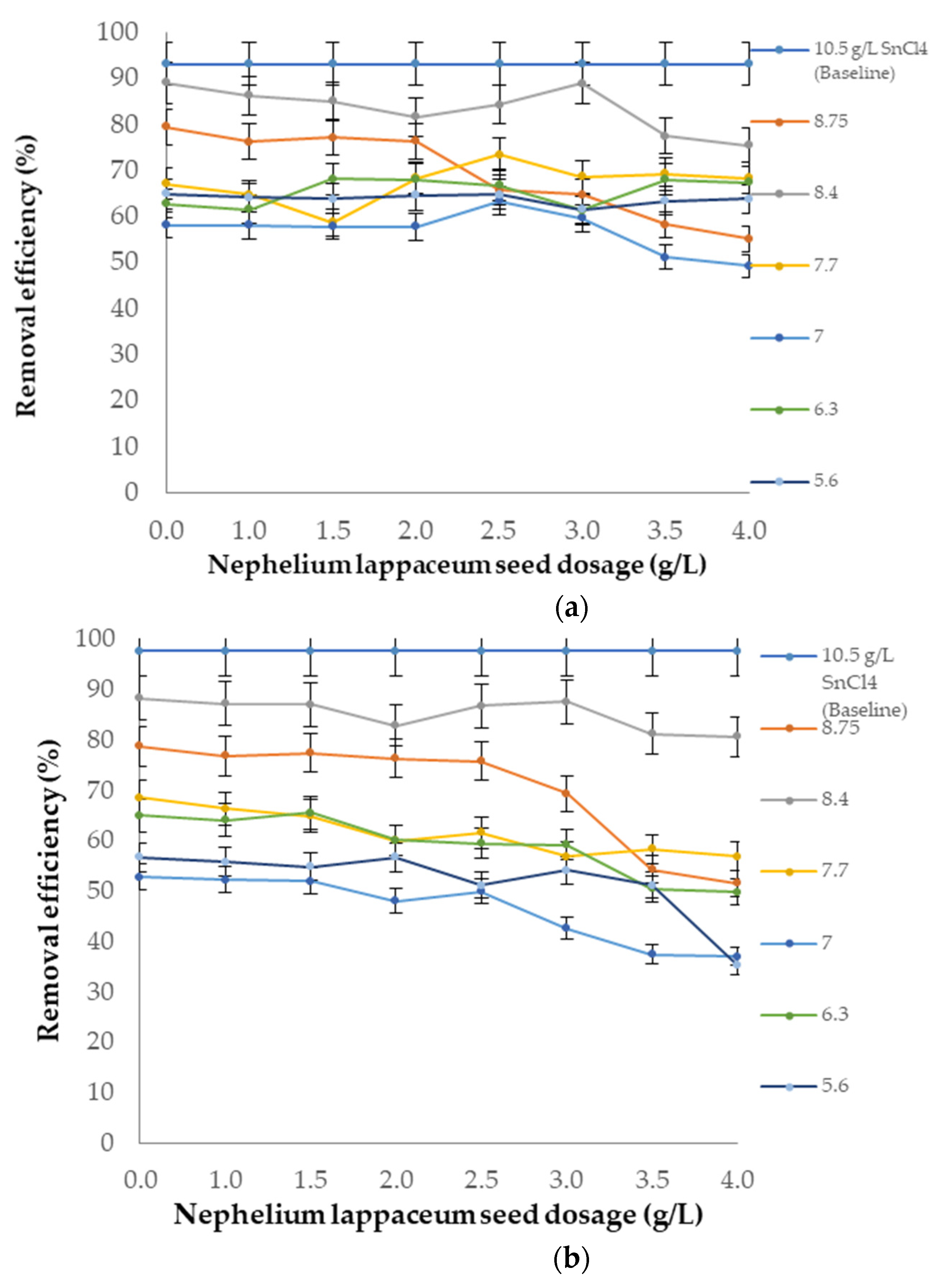
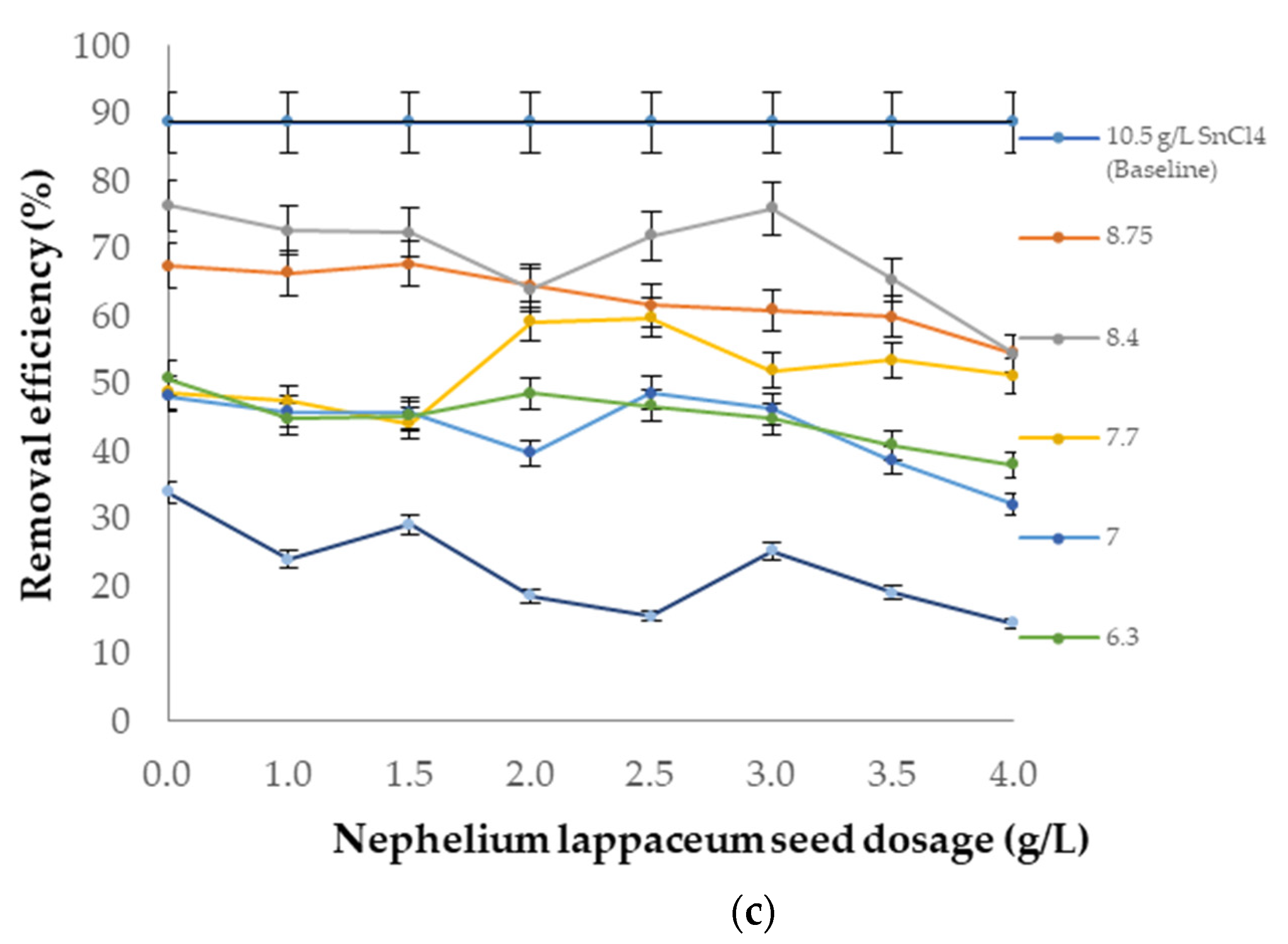
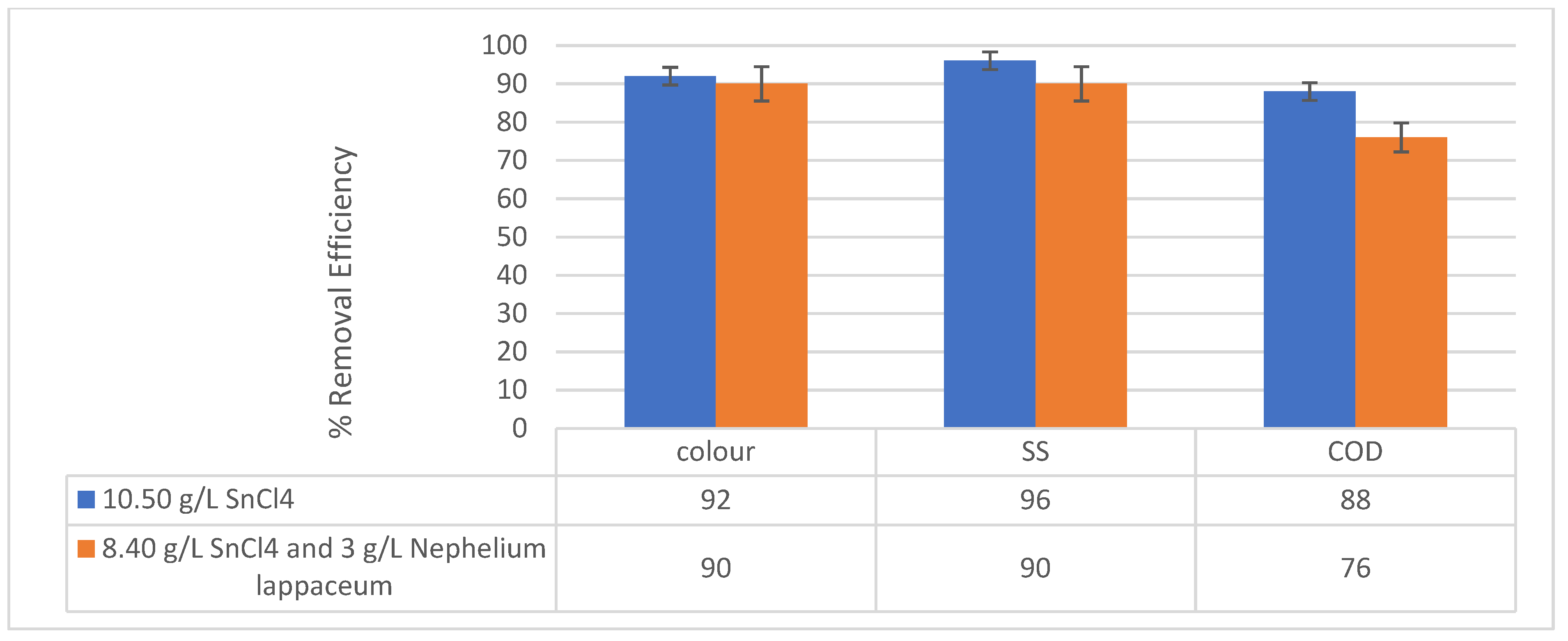
3.6. Performance of SnCl4 as a Coagulant and Nephelium lappaceum Seeds as a Flocculant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, H.A.; Alias, S.; Assari, F.; Adlan, M.N. The use of alum, ferric chloride and ferrous sulphate as coagulants in removing suspended solids, colour and COD from semi-aerobic landfill leachate at controlled pH. Waste Manag. Res. 2007, 25, 556–565. [Google Scholar] [CrossRef]
- Yusoff, M.S.; Aziz, H.A.; Alazaiza, M.Y.; Rui, L.M. Potential use of oil palm trunk starch as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. Water Qual. Res. J. 2019, 54, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Zainal, S.F.F.S.; Aziz, H.A.; Omar, F.M.; Alazaiza, M.Y. Influence of Jatropha curcas seeds as a natural flocculant on reducing Tin (IV) tetrachloride in the treatment of concentrated stabilised landfill leachate. Chemosphere 2021, 285, 131484. [Google Scholar] [CrossRef]
- Aziz, H.A.; Yusoff, M.S.; Adlan, M.N.; Adnan, N.H.; Alias, S. Physico-chemical removal of iron from semi-aerobic landfill leachate by limestone filter. Waste Manag. 2004, 24, 353–358. [Google Scholar] [CrossRef]
- Segundo, I.D.B.; Moreira, F.C.; Silva, T.F.; Webler, A.D.; Boaventura, R.A.; Vilar, V.J. Development of a treatment train for the remediation of a hazardous industrial waste landfill leachate: A big challenge. Sci. Total Environ. 2020, 741, 140165. [Google Scholar] [CrossRef]
- Umar, M.; Aziz, H.A.; Yusoff, M.S. Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag. 2010, 30, 2113–2121. [Google Scholar] [CrossRef]
- Nai, C.; Tang, M.; Liu, Y.; Xu, Y.; Dong, L.; Liu, J.; Huang, Q. Potentially contamination and health risk to shallow groundwater caused by closed industrial solid waste landfills: Site reclamation evaluation strategies. J. Clean. Prod. 2021, 286, 125402. [Google Scholar] [CrossRef]
- Zainal, S.F.F.S.; Abdul Aziz, H.; Mohd Omar, F.; Alazaiza, M.Y. Sludge performance in coagulation-flocculation treatment for suspended solids removal from landfill leachate using Tin (IV) chloride and Jatropha curcas. Inter. J. Environ. Analyt. Chem. 2021, 1–15. [Google Scholar] [CrossRef]
- Wei, Y.; Ye, Y.; Ji, M.; Peng, S.; Qin, F.; Guo, W.; Ngo, H.H. Microbial analysis for the ammonium removal from landfill leachate in an aerobic granular sludge sequencing batch reactor. Bioresour. Technol. 2021, 324, 124639. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Daud, Z.; Adlan, M.N.; Hung, Y.-T. The use of polyaluminium chloride for removing colour, COD and ammonia from semi-aerobic leachate. Inter. J. Environ. Eng. 2009, 1, 20–35. [Google Scholar] [CrossRef]
- Bashir, M.J.; Aziz, H.A.; Yusoff, M.S.; Huqe, A.; Mohajeri, S. Effects of ion exchange resins in different mobile ion forms on semi-aerobic landfill leachate treatment. Water Sci. Technol. 2010, 61, 641–649. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.; Yusoff, M.S.; Umar, M.; Bashir, M.J.; Adlan, M.N. Application of psyllium husk as coagulant and coagulant aid in semi-aerobic landfill leachate treatment. J. Hazard. Mater. 2011, 190, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M.S.; Adam, N.H.; Watalinggam, K.; Aziz, H.A.; Alazaiza, M.Y. Effectiveness of oil palm frond activated carbon for removing COD, color and Fe from landfill leachate. J. Eng. Technol. Sci. 2021, 53, 512–521. [Google Scholar] [CrossRef]
- Bashir, M.J.; Aziz, H.A.; Yusoff, M.S. New sequential treatment for mature landfill leachate by cationic/anionic and anionic/cationic processes: Optimisation and comparative study. J. Hazard. Mater. 2011, 186, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; AlGburi, H.R.; Alazaiza, M.Y.D.; Noor, A.F.M. Sequential treatment for stabilised landfill leachate by ozonation–adsorption and adsorption–ozonation methods. Inter. J. Environ. Sci. Technol. 2021, 18, 861–870. [Google Scholar]
- Chiang, L.-C.; Chang, J.-E.; Chung, C.-T. Electrochemical oxidation combined with physical–chemical pre-treatment processes for the treatment of refractory landfill leachate. Environ. Eng. Sci. 2001, 18, 369–379. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Coll. Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.-H.; Chan, G.Y. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef]
- Aziz, H.A.; Rahim, N.A.; Ramli, S.F.; Alazaiza, M.Y.; Omar, F.M.; Hung, Y.T. Potential use of Dimocarpus longan seeds as a flocculant in landfill leachate treatment. Water 2018, 10, 1672. [Google Scholar] [CrossRef] [Green Version]
- Zurina, A.Z.; Mohd Fadzli, M.; Ghani, A.; Abdullah, L. Preliminary study of rambutan (Nephelium lappaceum) seed as potential biocoagulant for turbidity removal. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2014; Volume 12, pp. 96–105. [Google Scholar]
- Aziz, H.A.; Ling, T.J.; Haque, A.A.M.; Umar, M.; Adlan, M.N. Leachate treatment by swim-bed bio fringe technology. Desalination 2011, 276, 278–286. [Google Scholar] [CrossRef]
- APAH. Standard Methods for the Examination of Water and Waste Water, 21th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Thanki, A.; Padhiyar, H.; Kathiriya, T.; Shah, D.; Singh, N.K. Applications of Moringa Oleifera for wastewater treatment: Concepts and approaches. In Green Innovation, Sustainable Development, and Circular Economy; CRC Press: Boca Raton, FL, USA, 2020; Volume 11, pp. 129–140. [Google Scholar]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Memb. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Awang, N.A.; Aziz, H.A. Hibiscus rosa-sinensis leaf extract as coagulant aid in leachate treatment. Appl. Water Sci. 2012, 2, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Sackey, L.N.A.; Meizah, K. Assesement of the quality of leachate at Sarbah landfill site at Weija in Accra. J. Environ. Chem. Ecotox. 2015, 7, 56–61. [Google Scholar]
- Maqbool, F.; Bhatti, Z.; Malik, A.; Pervez, A.; Mahmood, Q. Effect of landfill leachate on the stream water quality. Inter. J. Environ. Res. 2011, 5, 491–500. [Google Scholar]
- Aziz, H.; Yii, Y.; Syed Zainal, S.; Ramli, S.; Akinbile, C. Effects of using Tamarindus indica seeds as a natural coagulant aid in landfill leachate treatment. Global NEST J. 2018, 20, 373–380. [Google Scholar]
- Aziz, S.Q.; Aziz, H.A.; Yusoff, M.S.; Bashir, M.J.; Umar, M. Leachate scharacterisation in semi-aerobic and anaerobic sanitary landfills: A comparative study. J. Environ. Manag. 2010, 91, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-S.; Abbas, A.A.; Chen, Y.-P.; Liu, Z.-P.; Fang, F.; Chen, P. Treatment of landfill leachate using a combined stripping, Fenton, SBR, and coagulation process. J. Hazard. Mater. 2010, 178, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, S.N.F.; Abdul Aziz, H.; Amr, A.; Salem, S. Performance of ozone/ZrCl4 oxidation in sstabilised landfill leachate treatment. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2015; pp. 501–506. [Google Scholar]
- Ramli, S.F.; Aziz, H.A.; Omar, F.M.; Yusoff, M.S.; Halim, H.; Kamaruddin, M.A.; Ariffin, K.S.; Hung, Y.T. Reduction of COD and highly coloured mature landfill leachate by tin tetrachloride with rubber seed and polyacrylamide. Water 2021, 13, 3062. [Google Scholar] [CrossRef]
- Arenas, M.G.H.; Angel, D.N.; Damian, M.T.M.; Ortiz, D.T.; Díaz, C.N.; Martinez, N.B. Characterization of rambutan (Nephelium lappaceum) fruits from outstanding mexican selections. Rev. Bras. Frutic. 2010, 32, 1098–1104. [Google Scholar] [CrossRef] [Green Version]
- Ebeling, J.M.; Rishel, K.L.; Sibrell, P.L. Screening and evaluation of polymers as flocculation aids for the treatment of aquacultural effluents. Aquacultur. Eng. 2005, 33, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.S.; Tausif, M.; Mohsin, M.; Ahmad, S.W.; Zia-ul-Haq, M. Potato starch as a coagulant for dye removal from textile wastewater. Water Air Soil Poll. 2015, 226, 244. [Google Scholar] [CrossRef]
- Solidum, J. Potential nutritional and medicinal sources from fruit peels in Manila, Philippines. Inter. J. Biosci. Biochem. Bioinform. 2012, 2, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.A.; Mohamad Sobri, N.I. Extraction and application of starch-based coagulants from sago trunk for semi-aerobic landfill leachate treatment. J. Environ. Sci. Poll. Res. 2015, 22, 16943–16950. [Google Scholar] [CrossRef]
- Mohd-Asharuddin, S.; Othman, N.; Mohd Zin, N.; Tajarudin, H.A. A chemical and morphological study of cassava peel: A potential waste as coagulant aid. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 103. [Google Scholar]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468, 195–201. [Google Scholar] [CrossRef]
- Ab Ghani, Z.; Yusoff, M.S.; Zaman, N.Q.; Zamri, M.F.M.A.; Andas, J. Optimization of preparation conditions for activated carbon from banana pseudo-stem using response surface methodology on removal of color and COD from landfill leachate. Waste Manag. 2017, 62, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Gao, B.; Xu, C.; Fu, Y.; Liu, X. Effects of pH on coagulation behavior and floc properties in Yellow River water treatment using ferric based coagulants. Chin. Sci. Bulletin. 2010, 55, 1382–1387. [Google Scholar] [CrossRef]
- Kruszewski, S.; Cyrankiewicz, M. Aggregated silver sols as SERS substrates. Acta Phys. Pol. Ser. A Gen. Phys. 2012, 121, A68. [Google Scholar] [CrossRef]
- Borja, K.; Mercado, J.; Combatt, E. Methods of mechanical dispersion for determining granulometric fractions in soils using four dispersant solutions. Agron. Colomb. 2015, 33, 253–260. [Google Scholar] [CrossRef]
- Bruice, P.Y. Organic Chemistry: Pearson New International Edition; Pearson Education Limited: New York, NY, USA, 2014. [Google Scholar]
- Mishra, A.; Bajpai, M. The flocculation performance of Tamarindus mucilage in relation to removal of vat and direct dyes. Bioresour. Technol. 2006, 97, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Muaz, M.; Yusof, M.S.; Aziz, H.A. The study of flocculant characteristics for landfill leachate treatment using starch based flocculant from Durio zibethinus seed. Adv. Environ. Biol. 2014, 8, 129–135. [Google Scholar]
- Yong, C.; Aziz, H. Utilisation of Tamarindus Indica Seed as Natural Coagulant/Flocculant in Landfill Leachate Treatment. Master’s Thesis, Universiti Sains Malaysia, Nibong Tebal, Malaysia, 2016, (unpublished). [Google Scholar]
- Coffman, N. Recovering Titanium Dioxide (TiO2) after Its Useto Treat Leachate for Reuse on Future Leachate Flows; Florida Atlantic University: Boca Raton, FL, USA, 2015. [Google Scholar]
- Badrus, Z. Potential of natural flocculant in coagulation-flocculation wastewater treatment process. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; p. 05006. [Google Scholar]
- Baghvand, A.; Zand, A.D.; Mehrdadi, N.; Karbassi, A. Optimising coagulation process for low to high turbidity waters using aluminum and iron salts. Am. J. Environ. Sci. 2010, 6, 442–448. [Google Scholar] [CrossRef]
- Osei, J.A. Utilization of Agricultural Food Waste Products for Bioethanol Generation, Kiambu County, Kenya. Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2021. [Google Scholar]
- Kalyanasundaram, K. Photochemical applications of solar energy: Photocatalysis and photodecomposition of. Photochemistry 2013, 41, 182. [Google Scholar]




| Parameter | Min | Max | Average * | Permissible Limit |
|---|---|---|---|---|
| Temperature (°C) | 26.48 | 32.07 | 29.47 | 40 |
| pH | 8.04 | 8.90 | 8.59 | 6.0–9.0 |
| Total Dissolved Solids (g/L) | 8.532 | 9.950 | 9.440 | |
| Dissolved Oxygen (mg/L) | 2.14 | 4.38 | 3.17 | |
| BOD5 (mg/L) | 160 | 333 | 241 | 20 |
| COD (mg/L) | 2533 | 4215 | 3440 | 400 |
| Ratio BOD5/COD | 0.057 | 0.080 | 0.070 | |
| Suspended Solids (mg/L) | 350 | 604 | 469 | 50 |
| Colour (PtCo) | 13750 | 18733 | 16829 | 100 |
| NH3-N | 1040 | 1357 | 1227 | 5 |
| Zeta Potential (mV) | −22.4 | −20.7 | −21.5 |
| Wavelength (cm−1) | Wavenumber Range | Intensity | Bond | Group Vibration | Functional Group |
|---|---|---|---|---|---|
| 3585 | 3700–3584 | Medium | O–H | Stretching | Alcohol |
| 3564 | 3700–3500 | Medium | N–H | Stretching | Amide |
| 3544 | 3550–3200 | Strong | O–H | Stretching | Alcohol |
| 3367 | 3400–3300 | Medium | N–H | Stretching | Aliphatic primary amine |
| 3307 | 3300–2500 | Strong | O–H | Stretching | Carboxylic acid |
| 3004 | 3000–2800 | Strong | N–H | Stretching | Amine salt |
| 3100–3000 | Medium | C–H | Stretching | Alkene | |
| 2917 | 3000–2800 | Strong | N–H | Stretching | Amine salt |
| 2850 | 3000–2840 | Medium | C–H | Stretching | Alkane |
| 2307 | 2280–2440 | Medium | P–H | Stretching | Phosphine |
| 1735 | 1750–1735 | Strong | C=O | Stretching | δ–lactone |
| 1712 | 1725–1705 | Strong | C=O | Stretching | Aliphatic ketone |
| 1720–1706 | Strong | C=O | Stretching | Carboxylic acid | |
| 1650–1580 | Medium | N–H | Bending | Amine | |
| 1650–1566 | Medium | C=C | Stretching | Cyclic alkene | |
| 1506 | 1550–1500 | Strong | N–O | Stretching | Nitro compound |
| 1471 | 1400–1480 | Strong | C–O | Stretching | Methylene |
| 1416 | 1440–1395 | Medium | O–H | Bending | Carboxylic acid |
| 1420–1330 | Medium | O–H | Bending | Alcohol | |
| 1384 | 1415–1380 | Strong | S=O | Stretching | Sulfate |
| 1410–1380 | Strong | S=O | Stretching | Sulfonyl chloride | |
| 1400–1000 | Strong | C–F | Stretching | Fluoro compound | |
| 1249 | 1342–1266 | Strong | C–N | Stretching | Aromatic amine |
| 1250–1020 | Medium | C–N | Stretching | Amine | |
| 1209 | 1275–1200 | Strong | C–O | Stretching | Alkyl aryl ether |
| 1225–1200 | Strong | C–O | Stretching | Vinyl ether | |
| 1210–1163 | Strong | C–O | Stretching | Ester | |
| 1155 | 1170–1155 | Strong | S=O | Stretching | Sulphonamide |
| 1165–1150 | Strong | S=O | Stretching | Sulfonic acid | |
| 1160–1120 | Strong | S=O | Stretching | Sulfone | |
| 1205–1124 | Strong | C–O | Stretching | Tertiary alcohol | |
| 1078 | 1085–1050 | Strong | C–O | Stretching | Primary alcohol |
| 1022 | 1050–1040 | Strong | CO–O–CO | Stretching | Anhydride |
| 861 | 880 ± 20 | Strong | C–H | Bending | 1,2,4—trisubstituted |
| 880 ± 20 | Strong | C–H | Bending | 1,3—disubstituted | |
| 762 | 850–550 | Strong | C–Cl | Stretching | Halo compound |
| 780 ± 20 | Strong | C–H | Bending | 1,2,3—trisubstituted | |
| 755 ± 20 | Strong | C–H | Bending | 1,2—disubstituted | |
| 750 ± 20 | Strong | C–H | Bending | Monosubstituted benzene derivative | |
| 718 | 730–665 | Strong | C=C | Bending | Alkene |
| 601 | 850–550 | Strong | C–C | Stretching | Halo compound |
| 690–515 | Strong | C–Br | Stretching | Halo compound | |
| 574 | 850–550 | Strong | C–Cl | Stretching | Halo compound |
| 534 | 850–550 | Strong | C–Cl | Stretching | Halo compound |
| 600–500 | Strong | C–I | Stretching | Halo compound |
| Natural Coagulant | Optimum pH | Pollutant Removal Rate (%) | Reference | ||
|---|---|---|---|---|---|
| SS | Colour | COD | |||
| Commercial sago starch (CSS) | 4 | 29.5 | 15.1 | 28.0 | [37] |
| Chitosan | 4 | - | 14.7 | - | [32] |
| Durian seed starch | 6 | - | 34.0 | - | [45] |
| Tamarindus indica seed (TiS) | 4 | - | 30.2 | 7.5 | [46] |
| Longan seed | 4 | - | 10.3 | 5.2 | [19] |
| Nephelium lappaceum seed | 6 | - | 21.8 | 19.2 | Current Study |
| Natural Coagulant. | Optimum Dosage (g/L) | Pollutant Removal Rate (%) | Source | ||
|---|---|---|---|---|---|
| SS | Colour | COD | |||
| Commercial sago starch | 6 | 29.5 | 15.1 | 28 | [37] |
| Chitosan | 0.06 | - | 14.7 | - | [32] |
| Durian seed starch | 4 | - | 34 | - | [45] |
| Tamarindus indica seed | 5.00 | - | 41.90 | 5.90 | [46] |
| Longan seed | 2.00 | 29.5 | 15.10 | 28.00 | [19] |
| Nephelium lappaceum seed | 2.00 | - | 19.48 | 35.62 | Current Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, H.A.; Rahmat, N.S.; Alazaiza, M.Y.D. The Potential Use of Nephelium lappaceum Seed as Coagulant–Coagulant Aid in the Treatment of Semi-Aerobic Landfill Leachate. Int. J. Environ. Res. Public Health 2022, 19, 420. https://doi.org/10.3390/ijerph19010420
Aziz HA, Rahmat NS, Alazaiza MYD. The Potential Use of Nephelium lappaceum Seed as Coagulant–Coagulant Aid in the Treatment of Semi-Aerobic Landfill Leachate. International Journal of Environmental Research and Public Health. 2022; 19(1):420. https://doi.org/10.3390/ijerph19010420
Chicago/Turabian StyleAziz, Hamidi Abdul, Nur Syahirah Rahmat, and Motasem Y. D. Alazaiza. 2022. "The Potential Use of Nephelium lappaceum Seed as Coagulant–Coagulant Aid in the Treatment of Semi-Aerobic Landfill Leachate" International Journal of Environmental Research and Public Health 19, no. 1: 420. https://doi.org/10.3390/ijerph19010420






