Assessment of Magnetic Nanomaterials for Municipality Wastewater Treatment Using Biochemical Methane Potential (BMP) Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochemical Methane Potential (BMP) Test
2.2. Water Quality Analysis
3. Results and Discussion
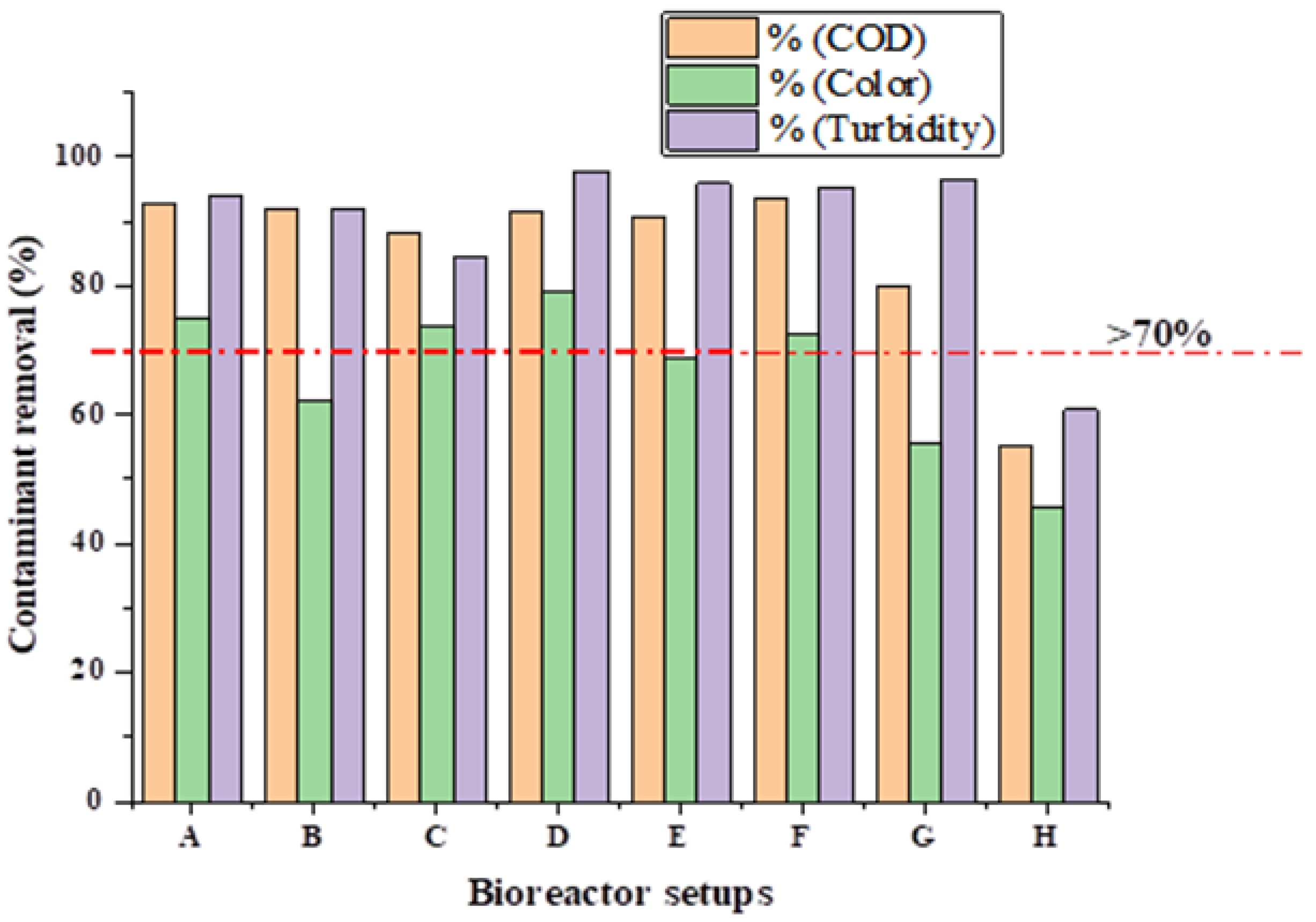
3.1. Effect of MNMs on Contaminants Removal from BS Wastewater
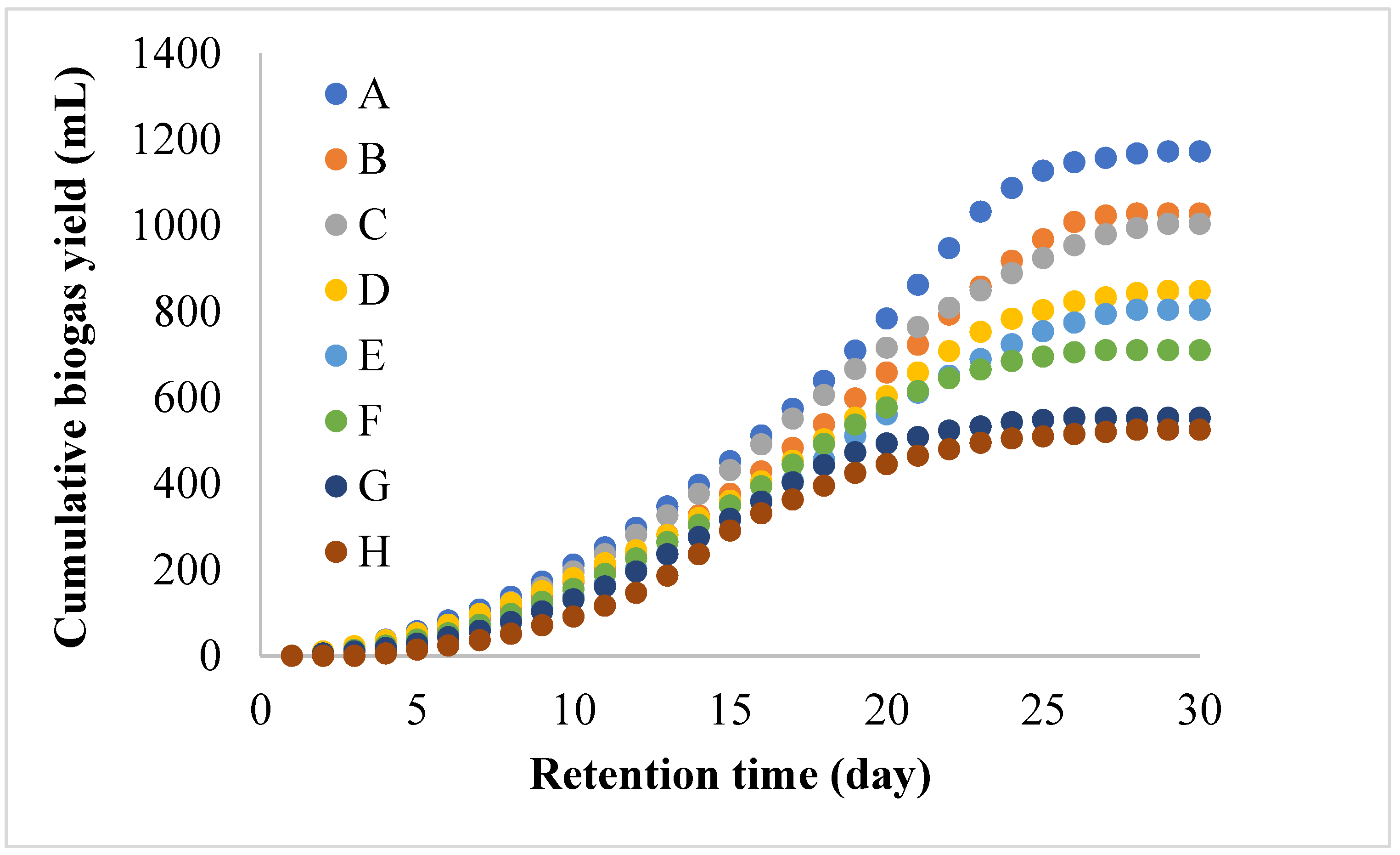
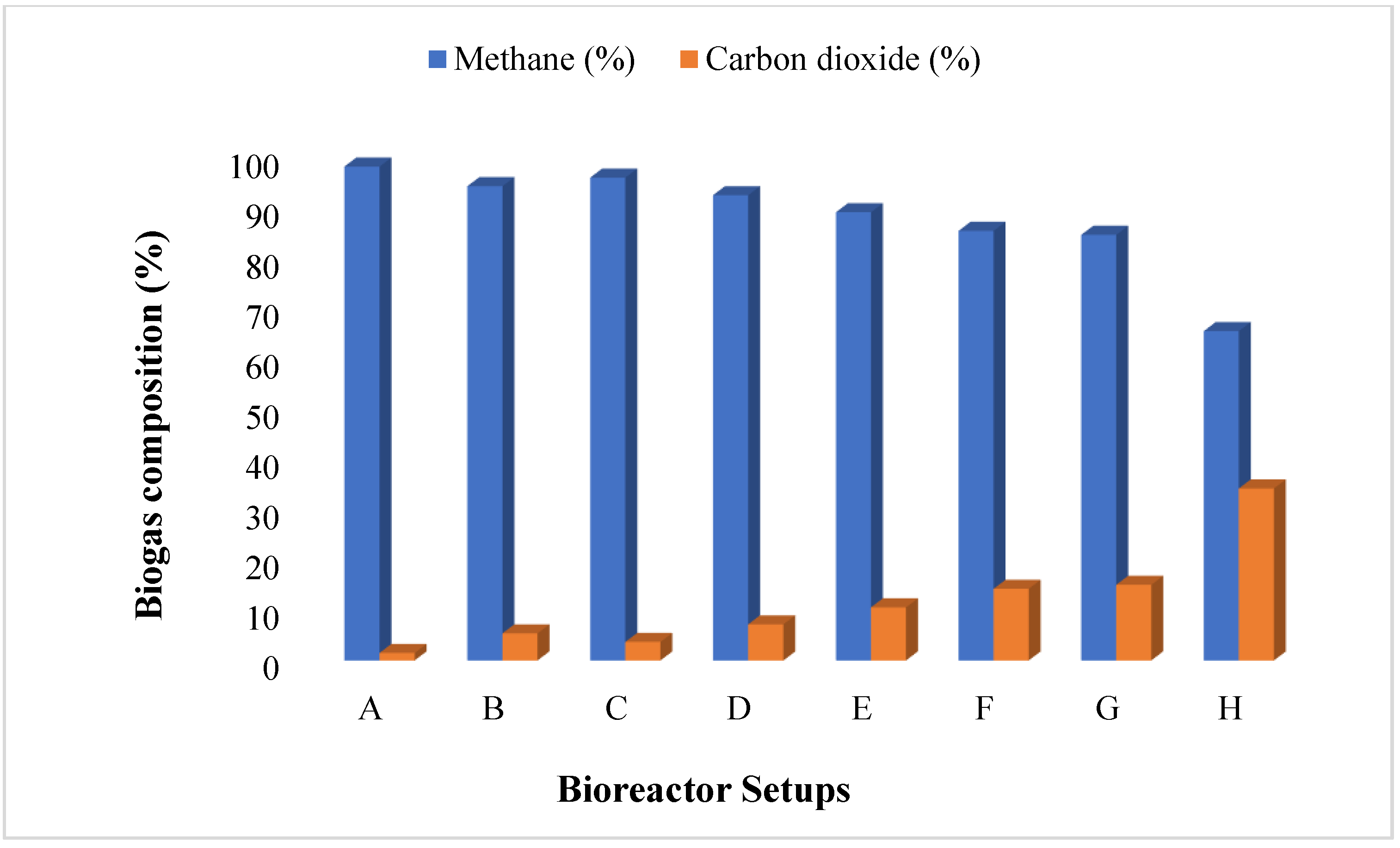
3.2. Biogas and Methane Yield of BMP System for BS Wastewater
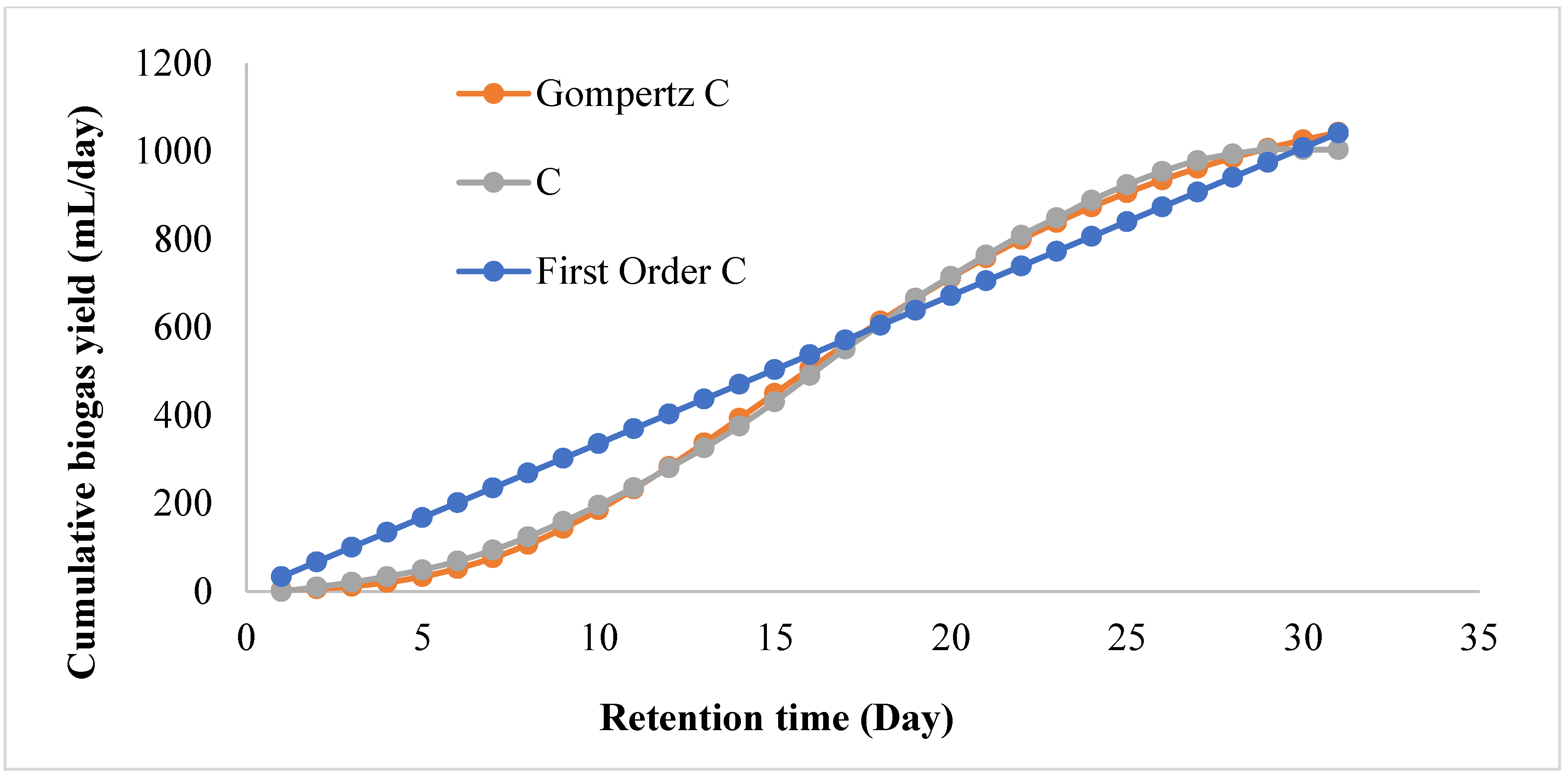
3.3. Kinetic Study of the BMP System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isiaka, A.A.; Olaniran, A.O. Treatment of industrial oily wastewater by advanced technologies: A review. Appl. Water Sci. 2021, 11, 98. [Google Scholar]
- Divyapriya, G.; Singh, S.; Martínez-Huitle, C.A.; Scaria, J.; Karim, A.V.; Nidheesh, P. Treatment of real wastewater by photoelectrochemical methods: An overview. Chemosphere 2021, 276, 130188. [Google Scholar] [CrossRef]
- Ángeles, R.; Vega-Quiel, M.J.; Batista, A.; Fernández-Ramos, O.; Lebrero, R.; Muñoz, R. Influence of biogas supply regime on photosynthetic biogas upgrading performance in an enclosed algal-bacterial photobioreactor. Algal Res. 2021, 57, 102350. [Google Scholar] [CrossRef]
- Adeogun, A.I.; Bhagawati, P.; Shivayogimath, C. Pollutants removals and energy consumption in electrochemical cell for pulping processes wastewater treatment: Artificial neural network, response surface methodology and kinetic studies. J. Environ. Manag. 2021, 281, 111897. [Google Scholar] [CrossRef]
- Stafford, W.; Cohen, B.; Pather-Elias, S.; Von Blottnitz, H.; Van Hille, R.; Harrison, S.T.; Burton, S.G. Technologies for recovery of energy from wastewaters: Applicability and potential in South Africa. J. Energy S. Afr. 2013, 24, 15–26. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Opoku Amankwa, M.; Armah, E.K.; Rathilal, S. Fate of covid-19 occurrences in wastewater systems: Emerging detection and treatment technologies—a review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Ponce-Robles, L.; Masdemont-Hernández, B.; Munuera-Pérez, T.; Pagán-Muñoz, A.; Lara-Guillén, A.J.; García-García, A.J.; Pedrero-Salcedo, F.; Nortes-Tortosa, P.A.; Alarcón-Cabañero, J.J. WWTP effluent quality improvement for agricultural reuse using an autonomous prototype. Water 2020, 12, 2240. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Removal of antibiotics during the anaerobic digestion of slaughterhouse wastewater. Planning 2020, 15, 335–343. [Google Scholar] [CrossRef]
- Nethengwe, N.S.; Uhunamure, S.E.; Tinarwo, D. Potentials of biogas as a source of renewable energy: A case study of South Africa. Int. J. Renew. Energy Res. 2018, 8, 1112–1123. [Google Scholar]
- Jordaan, G.P. Evaluating the Sustainable Potential of Biogas Generation in South Africa. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- McCabe, B.K.; Schmidt, T. Integrated Biogas Systems: Local Applications of Anaerobic Digestion towards Integrated Sustainable Solutions; IEA Bioenergy: Paris, France, 2018. [Google Scholar]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Hülsemann, B.; Zhou, L.; Merkle, W.; Hassa, J.; Müller, J.; Oechsner, H. Biomethane potential test: Influence of inoculum and the digestion system. Appl. Sci. 2020, 10, 2589. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Application of nanoparticles for biogas production: Current status and perspectives. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–13. [Google Scholar] [CrossRef]
- Abdelsalam, E.M.; Samer, M. Biostimulation of anaerobic digestion using nanomaterials for increasing biogas production. Rev. Environ. Sci. Bio/Technol. 2019, 18, 525–541. [Google Scholar] [CrossRef]
- Kweinor Tetteh, E.; Rathilal, S. Biogas production from wastewater treatment-evaluating anaerobic and biomagnetic systems. Water-Energy Nexus 2021, 4, 165–173. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ajay, C.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Abou Kana, M.T.; Radi, M.; Elsabee, M.Z. Wastewater treatment with chitosan nano-particles. Int. J. Nanotechnol. Appl. 2013, 3, 39–50. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.; Surampalli, R. Engineered nanoparticles in wastewater and wastewater sludge–Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef]
- Esakkimuthu, T.; Sivakumar, D.; Akila, S. Application of nanoparticles in wastewater treatment. Pollut. Res 2014, 33, 567–571. [Google Scholar]
- Lombi, E.; Donner, E.; Tavakkoli, E.; Turney, T.W.; Naidu, R.; Miller, B.W.; Scheckel, K.G. Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol. 2012, 46, 9089–9096. [Google Scholar] [CrossRef] [PubMed]
- Inozemtseva, O.A.; German, S.V.; Navolokin, N.A.; Bucharskaya, A.B.; Maslyakova, G.N.; Gorin, D.A. Encapsulated magnetite nanoparticles: Preparation and application as multifunctional tool for drug delivery systems. In Nanotechnology and Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–192. [Google Scholar]
- Majidi, S.; Zeinali Sehrig, F.; Samiei, M.; Milani, M.; Abbasi, E.; Dadashzadeh, K.; Akbarzadeh, A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Tetteh, E.K.; Amo-Duodu, G.; Rathilal, S. Synergistic Effects of Magnetic Nanomaterials on Post-Digestate for Biogas Production. Molecules 2021, 26, 6434. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Kweinor Tetteh, E.; Rathilal, S.; Chollom, M.N. Synthesis and characterization of magnetic nanoparticles: Biocatalytic effects on wastewater treatment. Mater. Today Proc. 2022, 62, S79–S84. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Armah, E.K.; Adedeji, J.; Chollom, M.N.; Chetty, M. Effect of Engineered Biomaterials and Magnetite on Wastewater Treatment: Biogas and Kinetic Evaluation. Polymers 2021, 13, 4323. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Rathilal, S.; Chollom, M.N.; Kweinor Tetteh, E. Application of metallic nanoparticles for biogas enhancement using the biomethane potential test. Sci. Afr. 2021, 12, e00728. [Google Scholar] [CrossRef]
- Enayati Ahangar, L.; Movassaghi, K.; Emadi, M.; Yaghoobi, F. Photocatalytic application of TiO2/SiO2-based magnetic nanocomposite (Fe3O4@ SiO2/TiO2) for reusing of textile wastewater. Nanochemistry Res. 2016, 1, 33–39. [Google Scholar]
- Esfandiari, N.; Kashefi, M.; Afsharnezhad, S.; Mirjalili, M. Insight into enhanced visible light photocatalytic activity of Fe3O4–SiO2–TiO2 core-multishell nanoparticles on the elimination of Escherichia coli. Mater. Chem. Phys. 2020, 244, 122633. [Google Scholar] [CrossRef]
- Yang, C.; Wang, P.; Li, J.; Wang, Q.; Xu, P.; You, S.; Zheng, Q.; Zhang, G. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe (III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417, 129340. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Stoller, M.; Ciambelli, P.; Chianese, A. Photocatalytic removal of phenol by ferromagnetic N-TiO2/SiO2/Fe3O4 nanoparticles in presence of visible light irradiation. Chem. Eng. Trans. 2016, 47, 235–240. [Google Scholar]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpää, M. Magnetic field application and its potential in water and wastewater treatment systems. Sep. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Lillo-Rodenas, M.A.; Román-Martínez, M.C. Cu/TiO2 photocatalysts for the conversion of acetic acid into biogas and hydrogen. Catal. Today 2017, 287, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Alvarado-Morales, M.; Tsapekos, P.; Awais, M.; Gulfraz, M.; Angelidaki, I. TiO2/UV based photocatalytic pretreatment of wheat straw for biogas production. Anaerobe 2017, 46, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Lin, H.-M. Photo reduction of CO2 to methanol via TiO2 photocatalyst. Int. J. Photoenergy 2005, 7, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Ren, G.; Chen, P.; Yu, J.; Liu, J.; Ye, J.; Zhou, S. Recyclable magnetite-enhanced electromethanogenesis for biomethane production from wastewater. Water Res. 2019, 166, 115095. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-G.; Li, X.-J.; Zhang, J.; Zhang, L.-G.; Cheng, B. Effect of microscale ZVI/magnetite on methane production and bioavailability of heavy metals during anaerobic digestion of diluted pig manure. Environ. Sci. Pollut. Res. 2017, 24, 12328–12337. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Wan, H.; Chen, Y.; Wang, X.; Yang, G.; Ren, G. Anaerobic co-digestion of animal manure and wheat straw for optimized biogas production by the addition of magnetite and zeolite. Energy Convers. Manag. 2015, 97, 132–139. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, G.; Yu, H.-Q. Kinetic modeling of batch hydrogen production process by mixed anaerobic cultures. Bioresour. Technol. 2006, 97, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
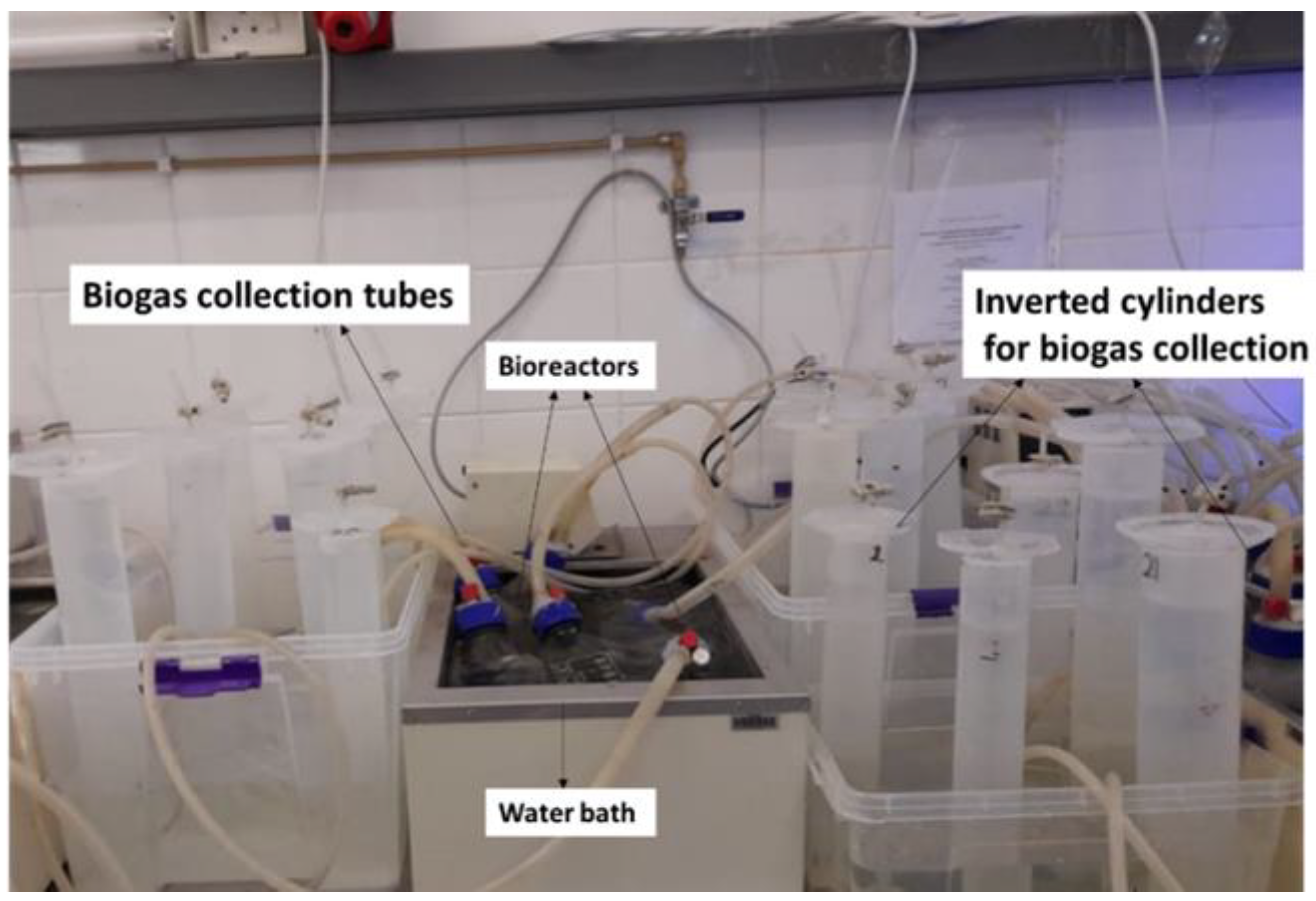
| Wastewater | |
|---|---|
| Parameters | Biofiltration System (BS) |
| Chemical oxygen demand (COD) (mg/L) | 2380 ± 32 |
| Colour (465 nm, Pt.Co) | 570 ± 7.6 |
| Turbidity (NTU) | 73.2 ± 12.5 |
| pH | 7.42 ± 3.6 |
| Activated sludge | |
| Total Solids (TS) (mg TS/L) | 304.5 ± 23.6 |
| Volatile Solid (VS) (mg VS/L) | 229.5 ± 2.65 |
| VS/TS (%) | 75.37 ± 3.5 |
| Setup | MNPs Loading (g) | Symbol (s) | Wastewater (L) | Sludge (L) |
|---|---|---|---|---|
| A | 1.5 Fe3O4 | mF | 0.5 | 0.3 |
| B | 1.5 NiFe2O4 | NmF | 0.5 | 0.3 |
| C | 1.5 CuFe2O4 | CmF | 0.5 | 0.3 |
| D | 1.5 TiO2Fe2O4 | TmF | 0.5 | 0.3 |
| E | 1.5 ChitosanTiO2Fe2O4 | ChTmF | 0.5 | 0.3 |
| F | 1.5 CuTiO2Fe2O4 | CTmF | 0.5 | 0.3 |
| G | 1.5 ALTiO2Fe2O4 | ATmF | 0.5 | 0.3 |
| H | No MNPs (Control) | n/a | 0.5 | 0.3 |
| Setup | COD Removal (%) | Colour Removal (%) | Turbidity Removal (%) |
|---|---|---|---|
| A | 92.59 | 74.86 | 94.13 |
| B | 91.90 | 61.98 | 91.94 |
| C | 88.07 | 73.68 | 84.56 |
| D | 91.60 | 78.95 | 97.81 |
| E | 90.76 | 68.77 | 95.90 |
| F | 93.70 | 72.63 | 95.36 |
| G | 79.83 | 55.61 | 96.45 |
| H | 54.96 | 45.61 | 60.79 |
| Modified Gompertz Model | First-Order Model | ||||||
|---|---|---|---|---|---|---|---|
| Setup | Measured Yield, (mL/day) | Predicted Yield (mL/day), Y2 | Y1–Y2 (mL/day) | R2 | Predicted Yield (mL/day), Y3 | Y1–Y3 (mL/day) | R2 |
| A | 1172 | 1460 | 288 | 0.9931 | 1872 | 700 | 0.9688 |
| B | 1028 | 1316 | 288 | 0.9943 | 1956 | 928 | 0.9689 |
| C | 1004 | 1174 | 170 | 0.9986 | 1372 | 368 | 0.9786 |
| D | 848 | 986 | 138 | 0.9952 | 3476 | 2658 | 0.9758 |
| E | 804 | 899 | 95 | 0.9960 | 3387 | 2583 | 0.9716 |
| F | 710 | 729 | 19 | 0.9854 | 1162 | 452 | 0.9618 |
| G | 553 | 557 | 4 | 0.9813 | 584 | 31 | 0.9404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Chollom, M.N. Assessment of Magnetic Nanomaterials for Municipality Wastewater Treatment Using Biochemical Methane Potential (BMP) Tests. Int. J. Environ. Res. Public Health 2022, 19, 9805. https://doi.org/10.3390/ijerph19169805
Amo-Duodu G, Tetteh EK, Rathilal S, Chollom MN. Assessment of Magnetic Nanomaterials for Municipality Wastewater Treatment Using Biochemical Methane Potential (BMP) Tests. International Journal of Environmental Research and Public Health. 2022; 19(16):9805. https://doi.org/10.3390/ijerph19169805
Chicago/Turabian StyleAmo-Duodu, Gloria, Emmanuel Kweinor Tetteh, Sudesh Rathilal, and Martha Noro Chollom. 2022. "Assessment of Magnetic Nanomaterials for Municipality Wastewater Treatment Using Biochemical Methane Potential (BMP) Tests" International Journal of Environmental Research and Public Health 19, no. 16: 9805. https://doi.org/10.3390/ijerph19169805








