Soil Biological Responses under Different Vegetation Types in Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physico-Chemical Analyses
2.2. Biological Analyses
2.3. Integrative Biological Response Index (IBR)
2.4. Statistical Analyses
3. Results
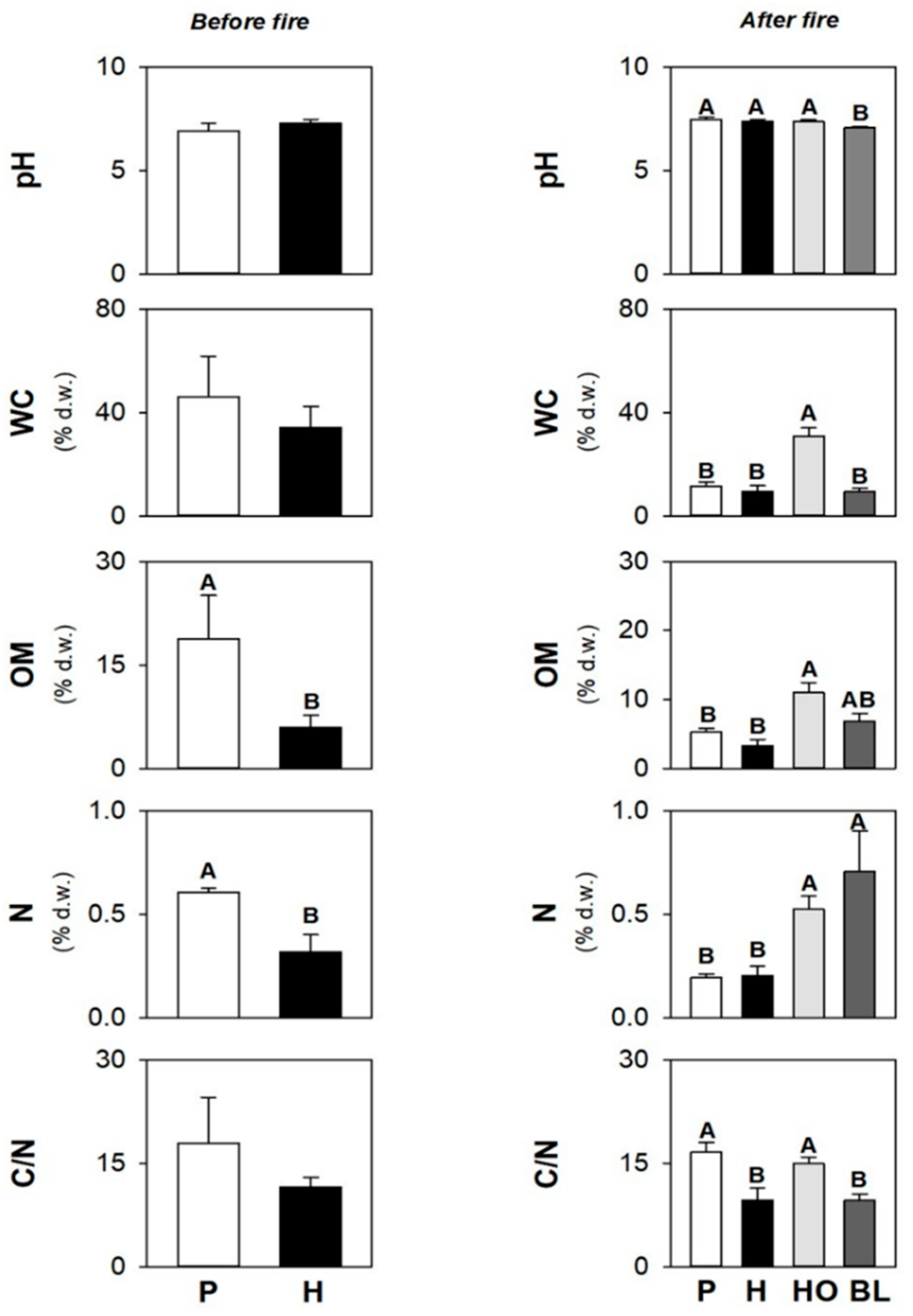
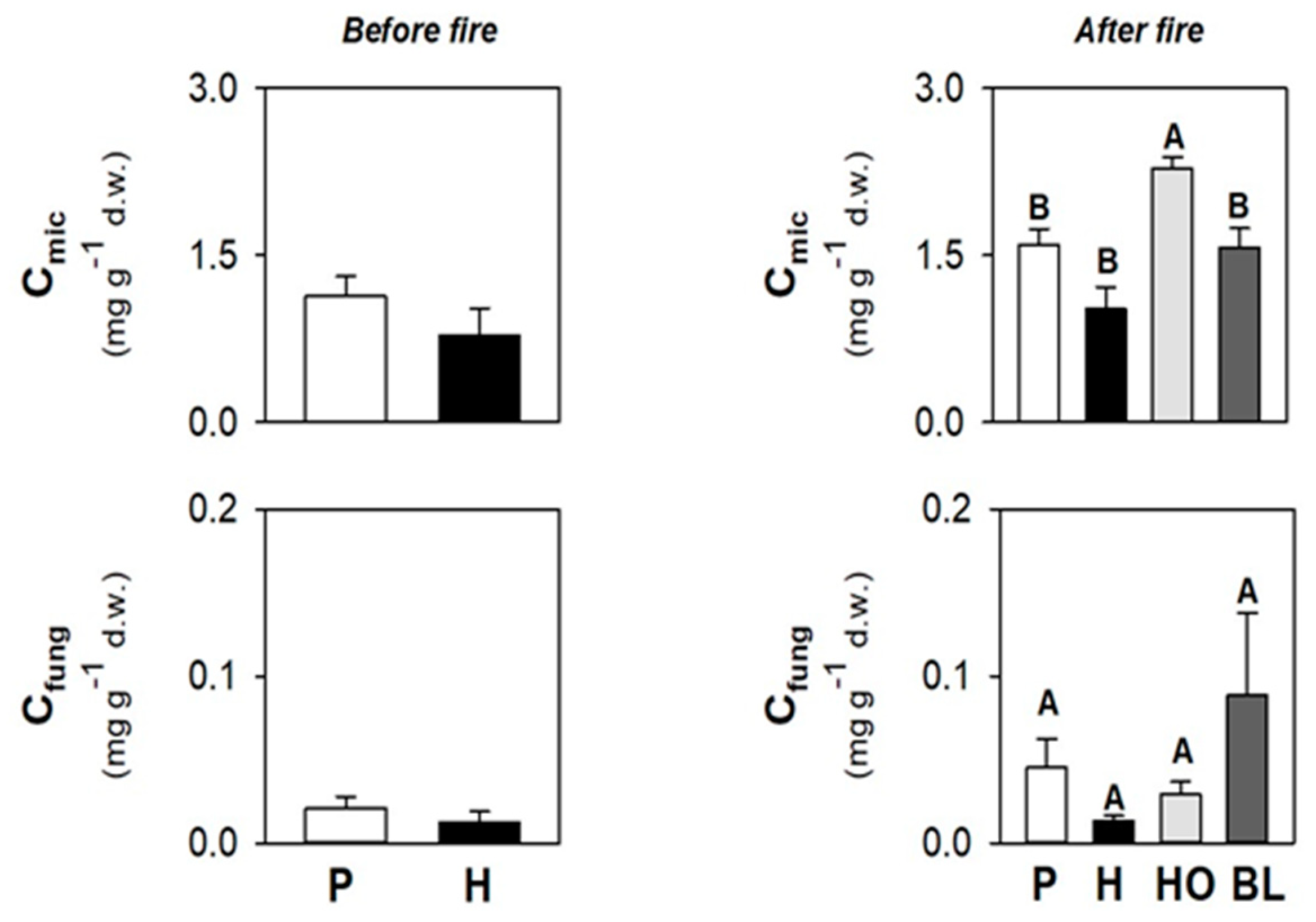
3.1. Soil Physico-Chemical and Biological Properties before the Fire
3.2. Correlations between Biotic and Abiotic Properties in P and H Soils Collected before the Fire
3.3. Soil Physico-Chemical and Biological Properties after the Fire
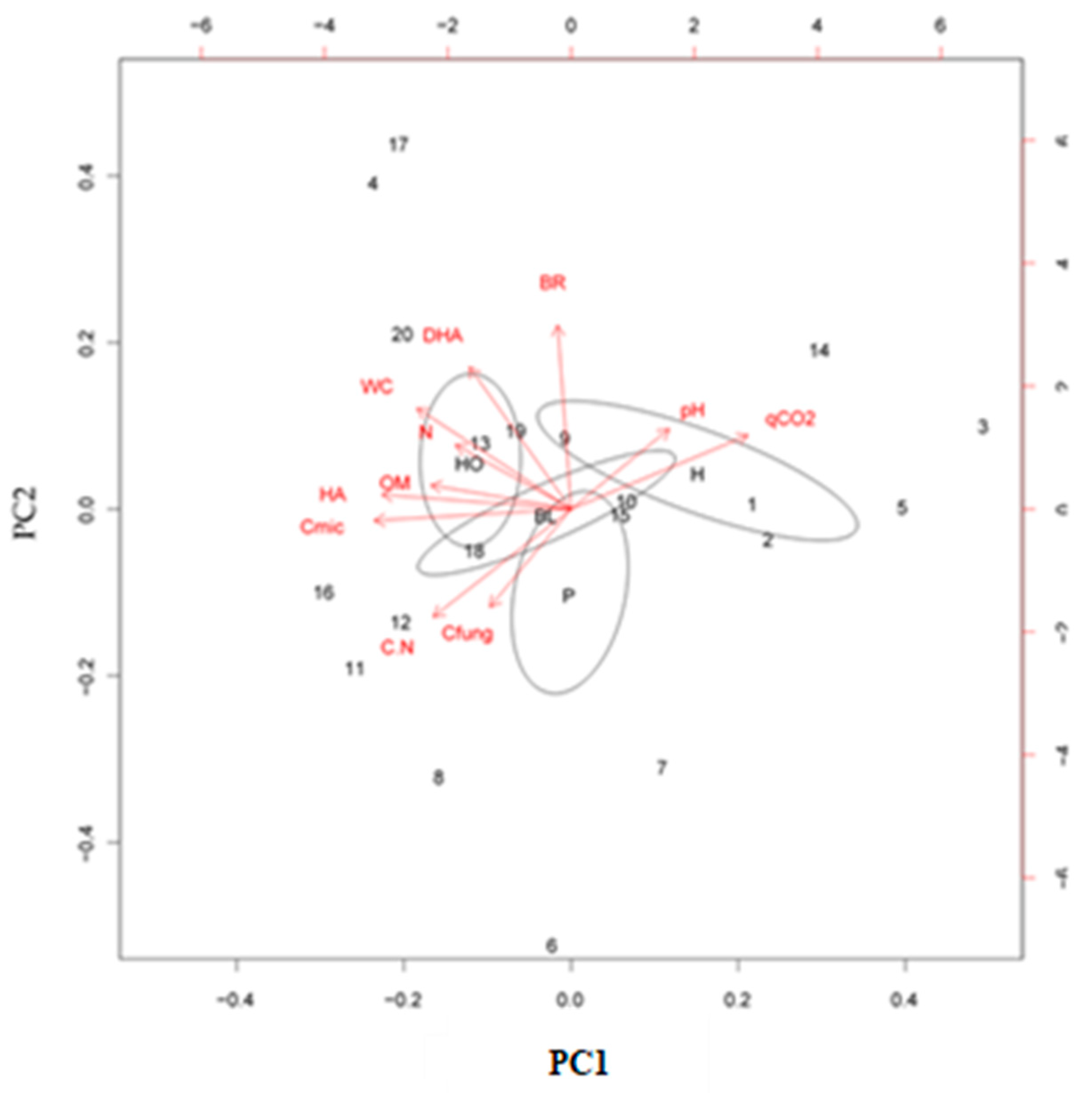
3.4. PCA on Dataset after the Fire
3.5. Correlations between Biotic and Abiotic Characteristics in P, H, OH, and BL Soils Collected after the Fire
3.6. Differences in Soil Properties under Herb and Pine Covers before and after the Fire
3.7. Integrative Biological Response Index (IBR) before and after the Fire
4. Discussion
4.1. Differences in Soil Properties between Pines and Herbs before and after Fire Occurrence
4.2. Impacts of Fire and Vegetation Type on Soil Properties after Fire Occurrence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marinari, S.; Moscatelli, M.C.; Marabottini, R.; Moretti, P.; Vingiani, S. Enzyme activities as affected by mineral properties in buried volcanic soils of southern Italy. Geoderma 2019, 362, 114123. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; D’ascoli, R.; Virzo De Santo, A. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 2004, 436, 1719–1729. [Google Scholar] [CrossRef]
- Steinauer, K.; Tilman, D.; Wragg, P.D.; Cesarz, S.; Cowles, J.M.; Pritsch, K.; Reich, P.B.; Weisser, W.W.; Eisenhauer, N. Plant diversity effects on soil microbial functions and enzymes are stronger than warming in a grassland experiment. Ecology 2015, 96, 99–112. [Google Scholar] [CrossRef]
- Mitchell, S.R.; Harmon, M.E.; O’Connell, K.E.B. Carbon debt and carbon sequestration parity in forest bioenergy production. Glob. Change Biol. Bioenergy 2012, 4, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Lonzano, Y.M.; Hortal, S.; Armas, C.; Pugnaire, F.I. Interactions among soil, plants, and microorganisms drive secondary succession in a dry environment. Soil Biol. Biochem. 2014, 78, 298–306. [Google Scholar] [CrossRef]
- De Marco, A.; Panico, S.C.; Memoli, V.; Santorufo, L.; Zarrelli, A.; Barile, R.; Maisto, G. Differences in soil carbon and nitrogen pools between afforested pine forests and natural shrublands in a Mediterranean area. Appl. Soil Ecol. 2022, 170, 104262. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Tigabu, M.; Liping, C.; Fanrui, M.; Aiqin, L.; Xiangqing, M. Changes in soil physico-chemical properties following vegetation restoration mediate bacterial community composition and diversity in Changting, China. Ecol. Eng. 2019, 138, 171–179. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Panico, S.C.; Ceccherini, M.T.; Memoli, V.; Maisto, G.; Pietramellara, G.; Barile, R.; De Marco, A. Effects of different vegetation types on burnt soil properties and microbial communities. Int. J. Wildland Fire 2020, 29, 628–636. [Google Scholar] [CrossRef]
- Brunel, C.; Gros, R.; Lerch, T.; Farnet da Silva, A.M. Changes in soil organic matter and microbial communities after fine and coarse residues inputs from Mediterranean tree species. Appl. Soil Ecol. 2020, 149, 103516. [Google Scholar] [CrossRef]
- Hart, S.C.; DeLuca, T.H.; Newman, G.S.; Derek MacKenzie, M.; Boyle, S.I. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For. Ecol. Manag. 2015, 220, 166–184. [Google Scholar] [CrossRef]
- Caminä, F.A.; Trasar-Cepeda, C.; Gil-Stores, F.; Leiroâ, C. Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Fterich, A.; Mahdhi, M.; Mars, M. The effects of Acacia tortilis subsp. raddiana, soil texture and soil depth on soil microbial and biochemical characteristics in arid zones of Tunisia. Land Degrad. Dev. 2014, 25, 143–152. [Google Scholar] [CrossRef]
- Ferreira, A.C.C.; Leite, L.F.C.; de Araújo, A.S.F.; Eisenhauer, N. Land-use type effects on soil organic carbon and microbial properties in a semi-arid region of northeast Brazil. Land Degrad. Dev. 2014, 27, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Physico-chemical and microbial perturbations of Andalusian pine forest soils following a wildfire. Sci. Total. Environ. 2018, 634, 650–660. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Esposito, F.; Panico, S.C.; Barile, R.; Maisto, G. Seasonality, altitude and human activities control soil quality in a national park surrounded by an urban area. Geoderma 2019, 337, 1–10. [Google Scholar] [CrossRef]
- Devin, S.; Buffet, P.E.; Châtel, A.; Perrein-Ettajani, H.; Valsami-Jones, E.; Mouneyrac, C. The integrated biomarker response: A suitable tool to evaluate toxicity of metal-based nanoparticles. Nanotoxicology 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guasch, H.; Bonet, B.; Bonnineau, C.; Barral, L. Microbial Biomarkers. In Microbial Ecotoxicology; Cravo-Laureau, C., Cagnon, C., Lauga, B., Duran, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 251–281. [Google Scholar]
- Santorufo, L.; Memoli, V.; Panico, S.C.; Santini, G.; Barile, R.; Di Natale, G.; Trifuoggi, M.; De Marco, A.; Maisto, G. Early post-fire changes in properties of Andosols within a Mediterranean area. Geoderma 2021, 394, 115016. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Parelho, C.; Rodrigues, A.S.; Barreto, M.C.; Ferreira, N.G.C.; Garcia, P. Assessing microbial activities in metal contaminated agricultural volcanic soils—An integrative approach. Ecotoxicol. Environ. Saf. 2016, 129, 242–249. [Google Scholar] [CrossRef]
- Si, W.; He, X.; Li, A.; Liu, L.; Li, J.; Gong, D.; Liu, J.; Liu, J.; Shen, W.; Zhang, X. Application of an integrated biomarker response index to assess ground water contamination in the vicinity of a rare earth mine tailings site. Environ. Sci. Pollut. Res. 2016, 23, 17345–17356. [Google Scholar] [CrossRef] [PubMed]
- Di Gennaro, A. I Sistemi di Terre Della Campania; Assessorato Regionale alla Ricerca Scientifica: Firenze, Italy, 2002. [Google Scholar]
- De Nicola, F.; Maisto, G.; Alfani, A. Assessment of nutritional status and trace element contamination of holm oak woodlands through analyses of leaves and surrounding soils. Sci. Total Environ. 2003, 311, 191–203. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Santorufo, L.; De Marco, A.; Barile, R.; Maisto, G. Total and fraction content of elements in volcanic soil: Natural or anthropogenic derivation. Sci. Total Environ. 2018, 625, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, A.; Esposito, F.; Berg, B.; Giordano, M.; Virzo De Santo, A. Soil C and N sequestration in organic and mineral layers of two coeval forest stands implanted on pyroclastic material (Mount Vesuvius, South Italy). Geoderma 2013, 209–210, 128–135. [Google Scholar] [CrossRef]
- Vega, J.A.; Fontùrbel, T.; Merino, A.; Fernàndez, C.; Ferreiro, A.; Jiménez, E. Testing the suitability of visual indicators of soil burn severity to reflect changes in soil chemical and microbial properties in pine stands and shrublands. Plant Soil 2013, 369, 73–91. [Google Scholar] [CrossRef]
- Saulino, L.; Rita, A.; Migliozzi, A.; Maffei, C.; Allevato, E.; Garonna, A.P.; Saracino, A. Detecting burn severity across Mediterranean forest types by coupling medium-spatial resolution satellite imagery and field data. Remote Sens. 2020, 12, 741. [Google Scholar] [CrossRef] [Green Version]
- USDA-NRCS. Soil Survey Manual; Issue 18 of Agricultural Handbook; Government Printing Office: Washington, DC, USA, 2017.
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. A physiological method for the quantitative measurements of microbial biomass in soil. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Baldantoni, D.; De Nicola, F.; Maisto, G. Short- and long-term effects of a single application of two organic amendments. Ecosphere 2017, 8, e02009. [Google Scholar] [CrossRef] [Green Version]
- Sundman, V.; Sivela, S. A comment on the membrane filter technique for the estimation of length of fungal hyphae in soil. Soil Biol. Biochem. 1978, 10, 399–401. [Google Scholar] [CrossRef]
- Olson, F.C.W. Quantitative estimates of filamentous algae. Trans. Am. Microsc. Soc. 1950, 69, 272–279. [Google Scholar] [CrossRef]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystem; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Froment, A. Soil respiration in a mixed oak forest. Oikos 1972, 23, 273–277. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of the soil. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Panico, S.C.; De Marco, A.; Barile, R.; Maisto, G. Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total Environ. 2018, 636, 1099–1108. [Google Scholar] [CrossRef]
- Hódar, J.A.; Lázaro-González, A.; Zamora, R. Beneath the mistletoe: Parasitized trees host a more diverse herbs vegetation and are more visited by rabbits. Ann. For. Sci. 2018, 75, 77. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.; Davey, M.; De Marco, A.; Emmett, B.; Faituri, M.; Hobbie, S.E.; Johansson, M.-B.; Liu, C.; McClaugherty, C.; Norell, L.; et al. Factors influencing limit values for pine needle litter decomposition: A synthesis for boreal and temperate pine forest systems. Biogeochemistry 2010, 100, 57–73. [Google Scholar] [CrossRef]
- De Santo, A.V.; Fierro, A.R.; Berg, B.; De Marco, A. Heavy metals and litter decomposition in coniferous forests. In Developments in Soil Science; Violante, A., Gianfreda, L., Bollag, J.M., Huang, P.M., Eds.; Elsevier: London, UK, 2002; Volume 28, pp. 63–78. [Google Scholar]
- Shedayi, A.A.; Xu, M.; Naseer, I.; Khan, B. Altitudinal gradients of soil and vegetation carbon and nitrogen in a high altitude nature reserve of Karakoram ranges. SpringerPlus 2010, 5, 320. [Google Scholar] [CrossRef] [Green Version]
- Mukai, H.; Hirose, A.; Motai, S.; Kikuchi, R.; Tanoi, K.; Nakanishi, T.M.; Yaita, T.; Kogure, T. Cesium adsorption/desorption behavior of clay minerals considering actual contamination conditions in Fukushima. Sci. Rep. 2016, 6, 21543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Moll, J.; Kellner, H.; Leonhardt, S.; Stengel, E.; Dahl, A.; Bässler, C.; Buscot, F.; Hofrichter, M.; Hoppe, B. Bacteria inhabiting deadwood of 13 tree species areheterogeneously distributed between sapwood andheartwood. Environ. Microbiol. 2018, 20, 3744–3756. [Google Scholar] [CrossRef] [Green Version]
- Zeraatpishe, M.; Khormali, F. Carbon stock and mineral factors controlling soil organic carbon in a climatic gradient, Golestan province. J. Soil Sci. Plant Nutr. 2012, 12, 637–654. [Google Scholar] [CrossRef]
- Aislabie, J.; Deslippe, J.R. Soil microbes and their contribution to soil services. J.R. In Ecosystem Services in New Zealand-Conditions and Trends; Dymond, Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- Wang, Q.; He, T.; Wang, S.; Liu, L. Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric. For. Meteorol. 2013, 178–179, 152–160. [Google Scholar] [CrossRef]
- Pausas, J.G.; Llovet, J.; Rodrigo, A.; Vallejo, R. Are wildfires a disaster in the Mediterranean basin?—A review. Int. J. Wildland Fire 2008, 17, 713–723. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Reyes, O.; Calvo, L. Do fire regime attributes affect soil biochemical properties in the same way under different environmental conditions? Forests 2020, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Memoli, V.; Panico, S.C.; Santorufo, L.; Barile, R.; Di Natale, G.; Di Nunzio, A.; Toscanesi, M.; Trifuoggi, M.; De Marco, A.; Maisto, G. Do wildfires cause changes in soil quality in short term? Int. J. Environ. Res. Public Health 2020, 17, 5343. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; De Marco, A.; D’Ascoli, R.; Castaldi, S.; Gentile, A.; De Santo, A.V. Impact of fire on fungal abundance and microbial efficiency in C assimilation and mineralization in a Mediterranean maquis soil. Biol. Fert. Soils 2007, 44, 377–381. [Google Scholar] [CrossRef]
- Tepley, A.J.; Thomann, E.; Veblen, T.T.; Perry, G.L.W.; Holz, A.; Paritsis, J.; Kitzberger, T.; Anderson-Teixeira, A.J. Influences of fire–vegetation feedbacks and post-fire recovery rates on forest landscape vulnerability to altered fire regimes. J. Ecol. 2018, 106, 1925–1940. [Google Scholar] [CrossRef] [Green Version]
- Oppio, A.; Corsi, S.; Mattia, S.; Tosini, A. Exploring the relationship among local conflicts and territorial vulnerability: The case study of Lombardy Region. Land Use Policy 2015, 43, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Klimek, B.; Niklińska, M.; Jaźwa, M.; Tarasek, A.; Tekielak, I.; Musielokc, L. Covariation of soil bacteria functional diversity and vegetation diversity along an altitudinal climatic gradient in the Western Carpathians. Pedobiologia 2015, 58, 105–112. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D.D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Li, C.; Zhao, L.; Feng, H.; Yue, G.; Ren, Z.; Cheng, G. The soil carbon/nitrogen ratio and moisture affect microbial community structures in alkaline permafrost-affected soils with different vegetation types on the Tibetan plateau. Res. Microbiol. 2014, 165, 128–139. [Google Scholar] [CrossRef]
- Li, W.; Niu, S.; Liu, X.; Wang, J. Short-term response of the soil bacterial community to differing wildfire severity in Pinus tabulaeformis stands. Sci. Rep. 2019, 9, 1148. [Google Scholar] [CrossRef] [Green Version]
- Halofsky, J.E.; Peterson, D.L.; Harvey, B.J. Changing wildfire, changing forests: The effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol. 2020, 16, 4. [Google Scholar] [CrossRef] [Green Version]
- Ondrasek, G.; Begić, H.B.; Zovko, M.; Filipović, L.; Meriño-Gergichevich, C.; Savić, R.; Rengel, Z. Review—Biogeochemistry of soil organic matter in agroecosystems & environmental implications. Sci. Total Environ. 2019, 658, 1559–1573. [Google Scholar]
- Liang, H.; Xue, Y.; Li, Z.; Wang, S.; Wu, X.; Gao, G.; Liu, G.; Fu, B. Soil moisture decline following the plantation of Robinia pseudoacacia forests: Evidence from the Loess Plateau. For. Ecol. Manag. 2018, 412, 62–69. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Cuesta, B.; Benayas, J.M.R.; Gallardo, A.; Villar-Salvador, P.; González-Espinosa, M. Soil chemical properties in abandoned Mediterranean cropland after succession and oak reforestation. Acta Oecologica 2012, 38, 58–65. [Google Scholar] [CrossRef]
- Rahmonov, O. The chemical composition of plant litter of black locust (Robinia pseudoacacia L.) and its ecological role in sandy ecosystems. Acta Ecol. Sin. 2009, 29, 237–243. [Google Scholar] [CrossRef]
- Teutscherova, N.; Lojka, B.; Houška, J.; Masaguer, A.; Benito, M.; Vazquez, E. Application of holm oak biochar alters dynamics of enzymatic and microbial activity in two contrasting Mediterranean soils. Eur. J. Soil Biol. 2018, 88, 15–26. [Google Scholar] [CrossRef]
- Januszek, K.; Błońska, E.; Długa, J.; Socha, J. Dehydrogenase activity of forest soils depends on the assay used. Int. Agrophys. 2015, 29, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.; Roß, C.L.; Hoffmann, M.; Muskolus, A.; Ellmer, F.; Kautz, T. The chemical Composition of biogas digestates determines their effect on soil microbial activity. Agriculture 2020, 10, 244. [Google Scholar] [CrossRef]
- Chungu, D.; Ngándwe, P.; Mubanga, H.; Chilesh, F. Fire alters the availability of soil nutrients and accelerates growth of Eucalyptus grandis in Zambia. J. For. Res. 2019, 31, 1637–1645. [Google Scholar] [CrossRef] [Green Version]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Change Biol. 2019, 26, 669–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sites | Sampling Campaign | Coordinates | Plant Cover | |
|---|---|---|---|---|
| 2016 (BF) | 2018 (AF) | |||
| H_1 | X | X | 40°81′31″ 14°43′66″ | |
| H_2 H_3 H_4 | X X X | X X X | 40°81′81″ 14°43′50″ 40°82′17″ 14°43′57″ 40°82′30″ 14°39′96″ | Mosses, lichens, Centranthus ruber L., Helichrysum italicum R., Artemisia campestris L., Rumex scutatus L. |
| H_5 | X | X | 40°83′07″ 14°25′28″ | |
| P_1 | X | X | 40°83′10″ 14°25′02″ | |
| P_2 P_3 P_4 | X X X | X X X | 40° 82′ 41″ 14°39′18″ 40°80′19″ 14°26′13″ 40°79′71″ 14°43′87″ | Pinus pinea A., (Pine) |
| P_5 | X | X | 40°80′19″ 14°43′85″ | |
| BL_1 | X | 40°81′20″ 14°44′07″ | ||
| BL_2 BL_3 BL_4 | X X X | 40°80′88″ 14°43′92″ 40°82′86″ 14°43′04″ 40°82′36″ 14°43′53″ | Robinia pseudoacacia L. (Black locust) | |
| BL_5 | X | 40°82′13″ 14°43′62″ | ||
| HO_1 | X | 40°80′72″ 14°43′46″ | ||
| HO_2 HO_3 | X X | 40°80′88″ 14°43′92″ 40°81′03″ 14°40′86″ | Quercus ilex L. (Holm oak) | |
| HO_4 | X | 40°81′67″ 14°40′86″ | ||
| HO_5 | X | 40°81′99″ 14°39′96″ | ||
| Pines | Cmic | Cfung | BR | HA | DHA | qCO2 |
|---|---|---|---|---|---|---|
| pH | 0.281 | −0.194 | −0.130 | −0.021 | −0.130 | 0.0216 |
| WC | −0.615 | 0.832 | −0.874 | 0.930 | 0.837 | 0.685 |
| OM | −0.490 | 0.748 | −0.965 | 0.832 | 0.754 | 0.783 |
| N | 0.699 | −0.818 | 0.734 | −0.860 | −0.908 | −0.469 |
| C/N | 0.399 | −0.329 | 0.315 | −0.245 | −0.373 | −0.168 |
| Herbaceous | ||||||
| pH | −0.151 | 0.347 | −0.440 | −0.217 | −0.324 | 0.178 |
| WC | −0.308 | 0.400 | −0.018 | 0.898 | 0.867 | 0.345 |
| OM | 0.427 | −0.427 | 0.209 | −0.867 | 0.783 | −0.255 |
| N | −0.006 | −0.100 | −0.109 | −0.249 | −0.301 | −0.300 |
| C/N | 0.357 | −0.255 | 0.264 | −0.740 | −0.657 | 0.027 |
| Pines | Cmic | Cfung | BR | HA | DHA | qCO2 |
|---|---|---|---|---|---|---|
| pH | 0.546 | 0.496 | 0.442 | 0.260 | 0.439 | −0.025 |
| WC | −0.003 | −0.359 | −0.260 | −0.012 | −0.207 | −0.082 |
| OM | 0.010 | −0.137 | 0.009 | 0.012 | 0.214 | 0.039 |
| N | 0.472 | 0.346 | 0.567 | 0.254 | 0.418 | 0.343 |
| C/N | −0.262 | −0.218 | −0.343 | −0.051 | −0.260 | −0.182 |
| Herbaceous | ||||||
| pH | 0.243 | 0.316 | −0.108 | 0.039 | 0.353 | −0.104 |
| WC | 0.308 | 0.398 | 0.226 | 0.183 | 0.110 | 0.128 |
| OM | 0.376 | 0.296 | 0.337 | 0.501 | 0.352 | −0.045 |
| N | 0.769 | 0.731 | 0.056 | 0.803 | 0.571 | −0.519 |
| C/N | 0.353 | 0.111 | 0.600 | 0.486 | 0.689 | −0.040 |
| Holm oak | ||||||
| pH | 0.140 | −0.008 | 0.027 | 0.330 | −0.203 | −0.392 |
| WC | 0.283 | −0.320 | 0.147 | 0.444 | 0.465 | −0.202 |
| OM | 0.138 | −0.266 | 0.326 | 0.206 | 0.531 | 0.131 |
| N | 0.131 | −0.310 | 0.465 | 0.314 | 0.702 | 0.076 |
| C/N | 0.072 | −0.535 | 0.362 | 0.140 | 0.365 | 0.060 |
| Black locust | ||||||
| pH | −0.433 | −0.346 | 0.051 | −0.633 | −0.117 | 0.107 |
| WC | −0.040 | 0.132 | 0.110 | 0.553 | 0.088 | 0.291 |
| OM | 0.232 | −0.186 | −0.055 | 0.277 | 0.171 | 0.090 |
| N | 0.691 | 0.546 | 0.240 | 0.254 | 0.312 | −0.231 |
| C/N | 0.377 | 0.219 | 0.116 | 0.093 | −0.001 | −0.422 |
| Herbaceous (H) | Pines (P) | |||
|---|---|---|---|---|
| BF | AF | BF | AF | |
| pH | 7.26 | 7.37 | 6.92 | 7.46 |
| WC (% d.w.) | 36.3 | 9.30 | 46.2 | 11.7 |
| OM (% d.w.) | 5.20 | 3.30 | 18.7 | 11.7 |
| N (% d.w.) | 0.31 | 0.20 | 0.61 | 0.20 |
| C/N | 11.5 | 9.6 | 17.8 | 16.6 |
| Cfung (mg g−1 d.w.) | 0.13 | 0.13 | 0.02 | 0.05 |
| Cmic (mg g−1 d.w.) | 0.80 | 1.02 | 1.13 | 1.60 |
| BR (mg CO2 g−1 d.w.) | 0.05 | 0.05 | 0.14 | 0.13 |
| qCO2 (mg C-CO2 mg−1 Cmic) | 0.10 | 1.21 | 0.17 | 0.03 |
| HA (mmol FDA min−1 g−1 d.w.) | 0.16 | 0.03 | 0.28 | 0.20 |
| DHA (mmol TPF min−1 g−1 d.w.) | 0.43 | 5.60 | 0.50 | 9.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panico, S.C.; Memoli, V.; Santorufo, L.; Aiello, S.; Barile, R.; De Marco, A.; Maisto, G. Soil Biological Responses under Different Vegetation Types in Mediterranean Area. Int. J. Environ. Res. Public Health 2022, 19, 903. https://doi.org/10.3390/ijerph19020903
Panico SC, Memoli V, Santorufo L, Aiello S, Barile R, De Marco A, Maisto G. Soil Biological Responses under Different Vegetation Types in Mediterranean Area. International Journal of Environmental Research and Public Health. 2022; 19(2):903. https://doi.org/10.3390/ijerph19020903
Chicago/Turabian StylePanico, Speranza Claudia, Valeria Memoli, Lucia Santorufo, Stefania Aiello, Rossella Barile, Anna De Marco, and Giulia Maisto. 2022. "Soil Biological Responses under Different Vegetation Types in Mediterranean Area" International Journal of Environmental Research and Public Health 19, no. 2: 903. https://doi.org/10.3390/ijerph19020903






