Laser Treatment Increases the Antimicrobial Efficacy of Cyanobacterial Extracts against Staphylococcusaureus (SA) and Methicillin-resistantStaphylococcus aureus (MRSA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Purification, and Morphological Identification of Cyanobacterial Samples
2.2. Pathogenic Bacterial Samples Used in Antimicrobial Bioassay
2.3. Initial Screening of Cyanobacterial Extracts and Antimicrobial Bioassays against Pathogenic Bacteria
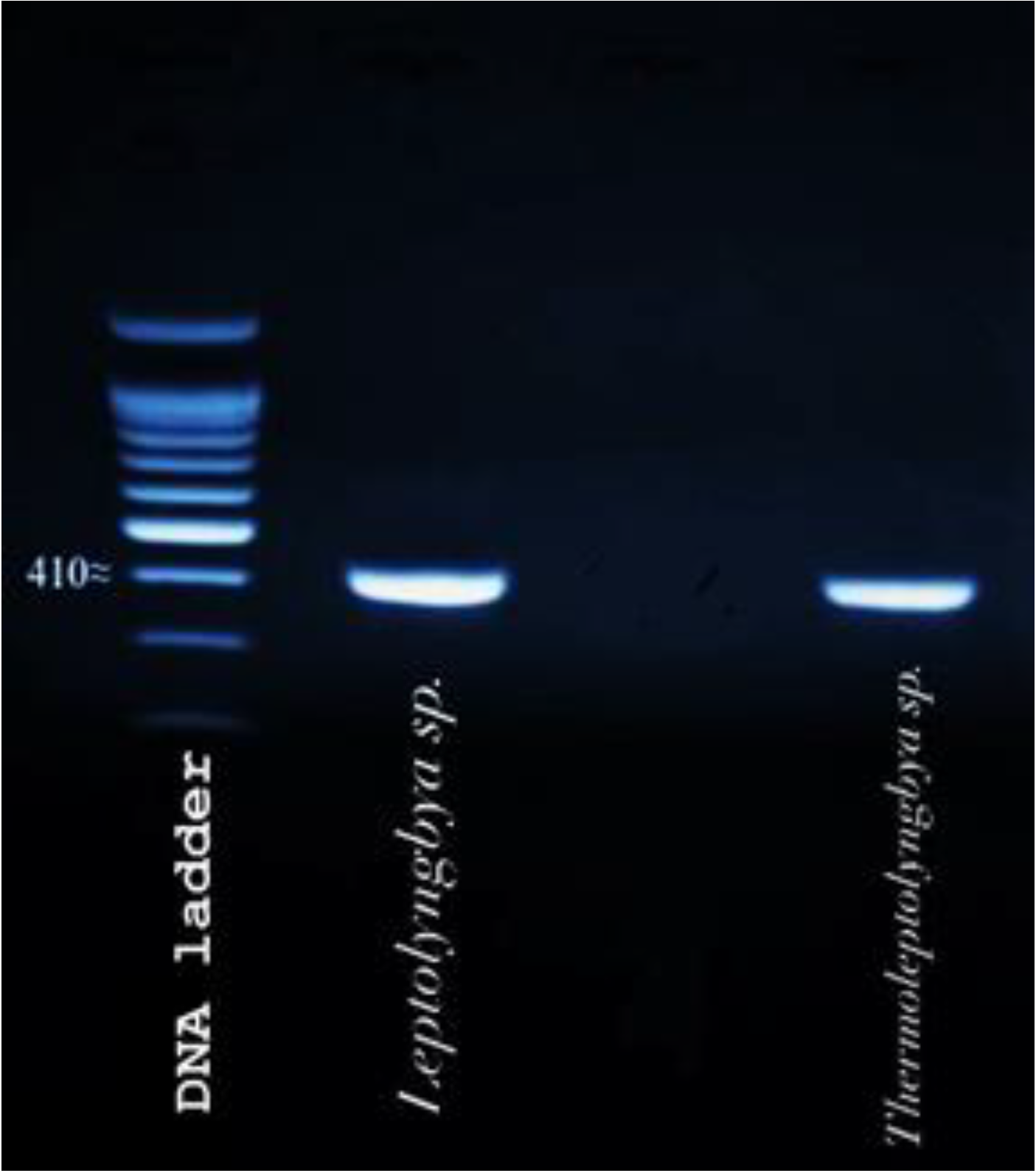
2.4. Molecular Identification of Cyanobacterial Strains
2.4.1. DNA Extract of Cyanobacterial Strains
2.4.2. PCR Amplification of Cyanobacterial Genomic DNA
- Initial denaturation at 94 °C for 2 min.
- Amplification cycles (35×). Each cycle consists of:
- Denaturation at 94 °C for 20 s;
- Annealing at 55 °C for 30 s;
- Extension at 72 °C for 10 s;
- Final extension step at 72 °C for 10 min [47].
- The amplicons were purified and sequenced (Macrogen, South Korea), and the sequences were checked in Genbank for similarity check (Blastn). The sequences were deposited in GenBank to await accession number designation.
2.5. Laser Treatments Using Different Exposure Time Intervals and Distances
2.6. Measurement the Phycobiliprotein Pigments Quantity of Cyanobacterial Extracts Spectrophotometrically before and after Exposing to Laser
2.7. Statistical Analysis
3. Results
3.1. Molecular Identification of Cyanobacterial Isolate Strains
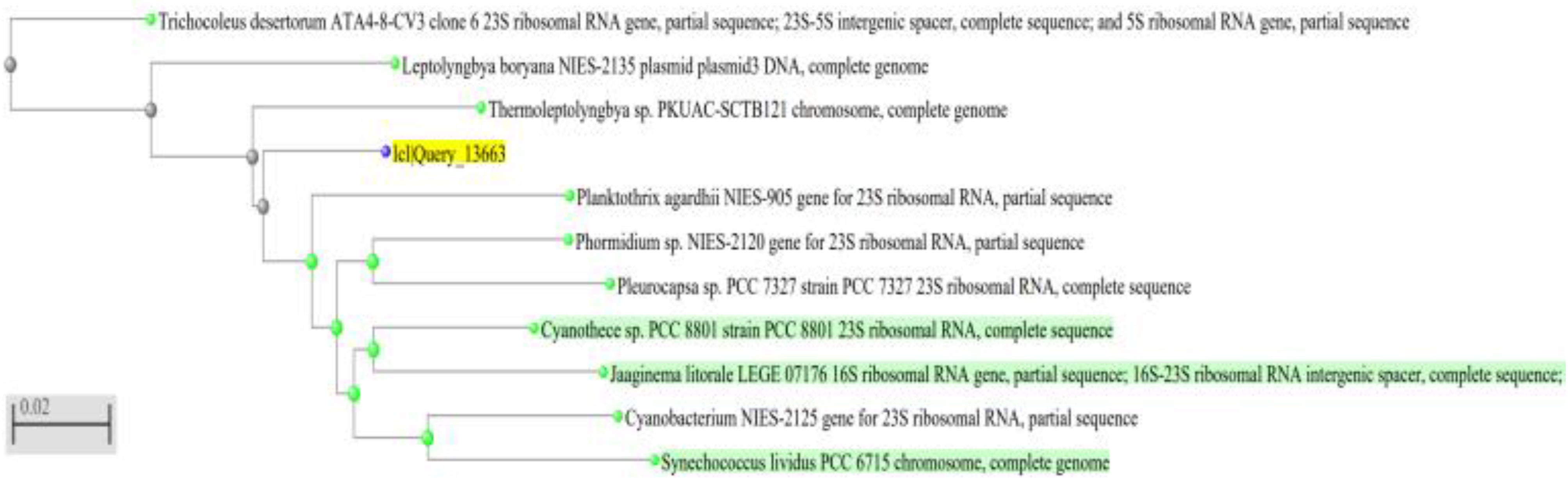
3.2. Phylogenetic Inference
- The filamentous unbranched nonheterocystous strain that was isolated from the thermal sulfur water spring belonged to Thermoleptolyngbya sp., which exhibited 92.92% sequence homology.
- The filamentous unbranched nonheterocystous strain that was isolated from the Hassawi rice field belonged to Leptolyngbya sp., which exhibited 94.58% sequence homology.
3.3. Molecular Identification of Bacteria
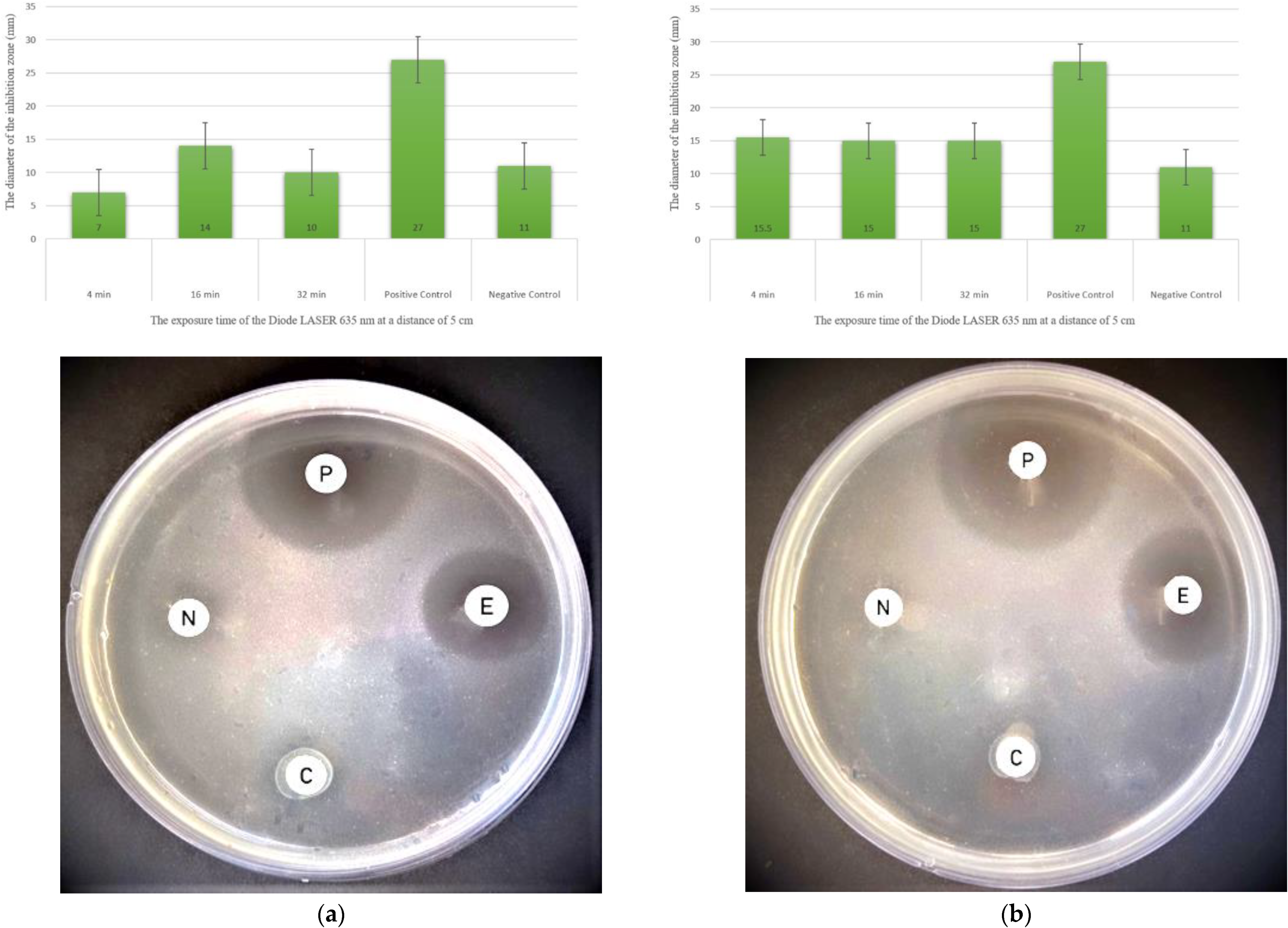
3.4. Antimicrobial Activity of Cyanobacterial Extracts against Pathogenic Bacteria
- Thermoleptolyngbya sp. extracts directly exposed to laser against Staphylococcus aureus, with an inhibition zone of 22 mm at 10 cm and 32 min.
- Thermoleptolyngbya sp. extracts directly exposed to the laser against Methicillin-resistant Staphylococcus aureus, with an inhibition zone of 19 mm at 5 cm and 16 min.
3.5. Measurement the Phycobiliprotein Pigments Quantification of Cyanobacteria by Spectrophotometric
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.P.; Häder, D.-P.; Sinha, R.P. Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res. Rev. 2010, 9, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine Cyanobacteria. Curr. Opin. Biotechnol. 2010, 21, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Francés, E.; Escudero-Oñate, C. Cyanobacteria and microalgae in the production of valuable bioactive compounds. Microalgal Biotechnol. 2018, 6, 104–128. [Google Scholar]
- Mandal, S.; Rath, J. Secondary metabolites of Cyanobacteria and drug development. In Extremophilic Cyanobacteria for Novel Drug Development; Springer: Berlin/Heidelberg, Germany, 2015; pp. 23–43. [Google Scholar]
- Fernandes, P. Antibacterial discovery and development—The failure of success. Nat. Biotechnol. 2006, 24, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive compounds from Cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef]
- Jaki, B.; Zerbe, O.; Heilmann, J.; Sticher, O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (eawag 195). J. Nat. Prod. 2001, 64, 154–158. [Google Scholar] [CrossRef]
- Goud, M.J.P.; Seshikala, D.; Charya, M.A.S. Antibacterial activity and biomolecular composition of certain fresh water microalgae collected from river godavari (India). Int. J. Algae 2007, 9, 350–358. [Google Scholar] [CrossRef]
- Muthulakshmi, M.; Saranya, A.; Sudha, M.; Selvakumar, G. Extraction, partial purification, and antibacterial activity of phycocyanin from Spirulina isolated from fresh water body against various human pathogens. J. Algal Biomass Util. 2012, 3, 7–11. [Google Scholar]
- Santoyo, S.; Herrero, M.; Senorans, F.J.; Cifuentes, A.; Ibáñez, E.; Jaime, L. Functional characterization of pressurized liquid extracts of Spirulina platensis. Eur. Food Res. Technol. 2006, 224, 75. [Google Scholar] [CrossRef] [Green Version]
- Abdo, S.M.; Hetta, M.H.; Samhan, F.A.; El-Din, R.A.S.; Ali, G.H. Phytochemical and antibacterial study of five freshwater algal species. Asian J. Plant Sci. 2012, 11, 109–116. [Google Scholar] [CrossRef]
- Kim, H.; Lantvit, D.; Hwang, C.H.; Kroll, D.J.; Swanson, S.M.; Franzblau, S.G.; Orjala, J. Indole alkaloids from two cultured Cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorganic Med. Chem. 2012, 20, 5290–5295. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides c–f, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [Green Version]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Antimicrobial activities of microalgae: An invited review. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 1272–1284. [Google Scholar]
- Alsenani, F.; Tupally, K.R.; Chua, E.T.; Eltanahy, E.; Alsufyani, H.; Parekh, H.S.; Schenk, P.M. Evaluation of microalgae and Cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 2020, 28, 1834–1841. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Silprasit, K.; Sittipraneed, S. Antibacterial activity of crude extracts of Cyanobacteria Phormidium and Microcoleus species. Afr. J. Microbiol. Res. 2012, 6, 2574–2579. [Google Scholar] [CrossRef]
- El-Aty, A.M.A.; Mohamed, A.A.; Samhan, F.A. In vitro antioxidant and antibacterial activities of two fresh water cyanobacterial species, Oscillatoria agardhii and Anabaena sphaerica. J. Appl. Pharm. Sci. 2014, 4, 69. [Google Scholar]
- Ozdemir, G.; Karabay, N.U.; Dalay, M.C.; Pazarbasi, B. Antibacterial activity of volatile component and various extracts of Spirulina Platensis. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 754–757. [Google Scholar]
- Shaieb, F.A.; Issa, A.A.; Meragaa, A. Antimicrobial activity of crude extracts of Cyanobacteria Nostoc commune and Spirulina platensis. Arch. Biomed. Sci. 2014, 2, 34–41. [Google Scholar]
- del Pilar Sánchez-Saavedra, M.; Licea-Navarro, A.; Bernáldez-Sarabia, J. Evaluation of the antibacterial activity of different species of phytoplankton. Rev. Biol. Mar. Oceanogr. 2010, 45, 531–536. [Google Scholar] [CrossRef]
- Malathi, T.; Babu, M.R.; Mounika, T.; Snehalatha, D.; Rao, B.D. Screening of cyanobacterial strains for antibacterial activity. Phykos 2014, 44, 6–11. [Google Scholar]
- Anwer, S.S.; Abdulkareem, P.M. Antibacterial activity of Lyngbya and Chroococcus species isolated from koya (Hizoop River). J. Life Sci. 2014, 8, 925–930. [Google Scholar]
- Prakash, J.W.; Marimuthu, J.; Jeeva, S. Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pacific J. Trop. Biomed. 2011, 1, S170–S173. [Google Scholar] [CrossRef]
- Ostensvik, O.; Skulberg, O.M.; Underdal, B.; Hormazabal, V. Antibacterial properties of extracts from selected planktonic freshwater Cyanobacteria–a comparative study of bacterial bioassays. J. Appl. Microbiol. 1998, 84, 1117–1124. [Google Scholar] [CrossRef]
- Silva-Stenico, M.E.; Silva, C.S.P.; Lorenzi, A.S.; Shishido, T.K.; Etchegaray, A.; Lira, S.P.; Moraes, L.A.B.; Fiore, M.F. Non-ribosomal peptides produced by Brazilian cyanobacterial isolates with antimicrobial activity. Microbiol. Res. 2011, 166, 161–175. [Google Scholar] [CrossRef]
- Ibraheem, I.B.M.; Al-Othman, M.R.; Abdelraouf, N. Cyanobacterial extra-metabolites against some pathogenic bacteria. Afr. J. Microbiol. Res. 2012, 6, 6720–6725. [Google Scholar] [CrossRef]
- Gnanamani, A.; Hariharan, P.; Paul-Satyaseela, M. Staphylococcus aureus: Overview of bacteriology, clinical diseases, epidemiology, antibiotic resistance and therapeutic approach. Front. Staphylococcus aureus 2017, 4, 28. [Google Scholar]
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef]
- Karu, T. Primary and secondary mechanisms of action of visible to near-irradiation on cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Oron, U.; Yaakobi, T.; Oron, A.; Mordechovitz, D.; Shofti, R.; Hayam, G.; Dror, U.; Gepstein, L.; Wolf, T.; Haudenschild, C. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation 2001, 103, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Sommer, A.P.; Pinheiro, A.L.B.; Mester, A.R.; Franke, R.-P.; Whelan, H.T. Bio stimulatory windows in low-intensity laser activation: Lasers, scanners, and nasa’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001, 19, 29–33. [Google Scholar] [CrossRef]
- Hu, W.-P.; Wang, J.-J.; Yu, C.-L.; Lan, C.-C.E.; Chen, G.-S.; Yu, H.-S. Helium–neon laser irradiation stimulates cell proliferation through photo stimulatory effects in mitochondria. J. Investig. Dermatol. 2007, 127, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Ajlal, S. Applications of light amplification by stimulated emission of radiation (lasers) for restorative dentistry. Med. Princ. Pract. 2016, 25, 201–211. [Google Scholar] [CrossRef]
- Belykh, E.; Yagmurlu, K.; Martirosyan, N.L.; Lei, T.; Izadyyazdanabadi, H.M.; Malik, K.M.; Byvaltsev, V.A.; Nakaji, P.; Preul, M.C. Laser application in neurosurgery. Surg. Neurol. Int. 2017, 8, 274. [Google Scholar]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates 1, 2. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Allen, M.M.; Stanier, R.Y. Selective isolation of blue-green algae from water and soil. Microbiology 1968, 51, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R. [1] isolation and purification of Cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar]
- Cribier, B.; Prevost, G.; Couppie, P.; Finck-Barbancon, V.; Grosshans, E.; Piemont, Y. Staphylococcus aureus leukocidin: A new virulence factor in cutaneous infections? Dermatology 1992, 185, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Katz, E.B.S.; Sherertz, R.J.; Sarubbi, F.A. Environmental study of a methicillin-resistant Staphylococcus aureus epidemic in a burn unit. J. Clin. Microbiol. 1983, 18, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Aldayel, M.; El Semary, N. UV irradiation-promoting effect on the antibacterial activity of cyanobacterial extracts against plant pathogens: A first record. Egypt. J. Biol. Pest Control 2020, 30, 1–4. [Google Scholar] [CrossRef]
- Claus, D. A standardized gram staining procedure. World J. Microbiol. Biotechnol. 1992, 8, 451–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- El Semary, N.; Al Dayel, M.; Al Amer, K.; Al Ali, K.M. Investigating the applications of Chlorella vulgaris in agriculture and nanosilver production. J. Environ. Biol. 2020, 41, 1099–1104. [Google Scholar]
- Heidari, F.; Riahi, H.; Yousefzadi, M.; Asadi, M. Antimicrobial activity of Cyanobacteria isolated from hot spring of geno. Middle-East J. Sci. Res. 2012, 12, 336–339. [Google Scholar]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and Cyanobacteria 1. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Report. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Presting, G.G. Identification of conserved regions in the plastid genome: Implications for DNA barcoding and biological function. Botany 2006, 84, 1434–1443. [Google Scholar] [CrossRef]
- Hajnalka, H.; Kovács, A.W.; Riddick, C.; Présing, M. Extraction methods for phycocyanin determination in freshwater filamentous Cyanobacteria and their application in a shallow lake. Eur. J. Phycol. 2013, 48, 278–286. [Google Scholar]
- Zavřel, T.; Chmelík, D.; Sinetova, M.A.; Červený, J. Spectrophotometric determination of phycobiliprotein content in cyanobacterium synechocystis. J. Vis. Exp. 2018, 139, e58076. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusof, F.M. Optimization of the freezing-thawing method for extracting phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and Cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Fastner, J.; Heinze, R.; Humpage, A.R.; Mischke, U.; Eaglesham, G.K.; Chorus, I. Cylindrospermopsin occurrence in two german lakes and preliminary assessment of toxicity and toxin production of Cylindrospermopsis raciborskii Cyanobacteria isolates. Toxicon 2003, 42, 313–321. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Yunes, J.S.; Bianchini, A. Effects of Anabaena spiroides (Cyanobacteria) aqueous extracts on the acetylcholinesterase activity of aquatic species. Environ. Toxicol. Chem. Int. J. 2001, 20, 1228–1235. [Google Scholar] [CrossRef]
- El Semary, N.A.; Osman, M.; Botros, H.; Sabry, A.; Farag, A.T. Antibiosis towards human and plant pathogens via radiation-induced Synechococcus elongates nageli. Egypt. J. Biol. Pest Control 2015, 25, 393. [Google Scholar]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of enhanced ultraviolet-B radiation on algae and Cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV radiation. In Ecology of Cyanobacteria II; Springer: Berlin/Heidelberg, Germany, 2012; pp. 481–499. [Google Scholar]
- Ahmed, E.S.; Keyba, H.M.; Moussa, L.A. Gamma ray induced effects on new species of Streptomyces (aefo2) (hm775973. 1gi: 302495616) isolated from egyptian soil. Int. J. Bioassays 2014, 3, 3586–3593. [Google Scholar]
- Fatima, N.; Ahmad, I.Z.; Chaudhry, H. Alterations in the antibacterial potential of Synechococcus spp. pcc7942 under the influence of UV-B radiations on skin pathogens. Saudi J. Biol. Sci. 2017, 24, 1657–1662. [Google Scholar] [CrossRef]
- Doman, K.M.; El-Monem, M.A.; Gharieb, M.M. Effect of ultraviolet-B radiation on the biochemical composition and antibacterial activity of Spirulina platensis. J. Basic Environ. Sci. 2020, 7, 27–28. [Google Scholar]
- Kim, N.N.; Shin, H.S.; Park, H.G.; Lee, J.; Gyung-Suk, K.; Choi, C.Y. Profiles of photosynthetic pigment accumulation and expression of photosynthesis related genes in the marine Cyanobacteria Synechococcus sp.: Effects of led wavelengths. Biotechnol. Bioprocess Eng. 2014, 19, 250–256. [Google Scholar] [CrossRef]
- Wen-Tyng, L.; Yao-Chu, L.; Jia-Lung, W. Red-light light-emitting diode irradiation increases the proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. Photomed. Laser Surg. 2010, 28, S1–S157. [Google Scholar]
- Hsiao-Wei, C.; Tsung-Shi, Y.; Mao-Jing, C.; Yu-Ching, C.; Eugene, I.; Chen, W.; Chen-Lung, H.; Ying-Jang, L.; Chi-Cheng, Y.; Ju-Ching, C. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar]
- Sivasankari, S.; Vinoth, M.; Ravindran, D.; Baskar, K.; Alqarawi, A.A.; Abd-Allah, E.F. Efficacy of red light for enhanced cell disruption and fluorescence intensity of phycocyanin. Bioprocess Biosyst. Eng. 2021, 44, 141–150. [Google Scholar] [CrossRef]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of sensitivity of selected Candida strains on antimicrobial photodynamic therapy using diode laser 635 nm and toluidine blue–in vitro research. Photodiagnosis Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Couppé, C.; Chow, R.T.; Tunér, J.; Ljunggren, E.A. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003, 49, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Li, L.; Li, M.; Du, L.; Waleron, M.; Waleron, M.; Waleron, K.; Daroch, M. Description, taxonomy, comparative genomics of a novel Thermoleptolyngbya strain isolated from hot springs of Ganzi, Sichuan China. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ki-Seok, Y.; Nguyen, N.T.; Tran, K.T.; Tsuji, K.; Ogo, S. Nitrogen fixation genes and nitrogenase activity of the non-heterocystous cyanobacterium Thermoleptolyngbya sp. o-77. Microbes Environ. 2017, ME17015. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Jiang, D.; Luo, Y.; Liang, Y.; Li, L.; Shah, M.M.R.; Daroch, M. Potential new genera of cyanobacterial strains isolated from thermal springs of western Sichuan, China. Algal Res. 2018, 31, 14–20. [Google Scholar] [CrossRef]
- Sciuto, K.; Moro, I. Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, leptolyngbyaceae) using the 16S rRNA gene and 16S–23S its region. Mol. Phylogenetics Evol. 2016, 105, 15–35. [Google Scholar] [CrossRef]
- Momper, L.; Hu, E.; Moore, K.R.; Skoog, E.J.; Tyler, M.; Evans, A.J.; Bosak, T. Metabolic versatility in a modern lineage of Cyanobacteria from terrestrial hot springs. Free Radic. Biol. Med. 2019, 140, 224–232. [Google Scholar] [CrossRef]

| Cyanobacterial Strain | Distance | Exposure Time | Inhibition Zone of Staphylococcus aureus | |
|---|---|---|---|---|
| Extracts from Biomass Pre-Exposed to Laser | Extracts Directly Exposed to Laser | |||
| Thermoleptolyngbya sp. | 5 cm | 4 min | 7.0 ± 0.0 | 15.5 ± 7.7 |
| 16 min | 14.0 ± 1.4 | 15.0 ± 0.0 | ||
| 32 min | 10.0 ± 1.4 | 15.0 ± 7.0 | ||
| 10 cm | 4 min | 12.0 ± 1.4 | 20.0 ± 4.2 | |
| 16 min | 8.5.0 ± 0.7 | 19.0 ± 4.2 | ||
| 32 min | 14.5 ± 3.5 | 22.0 ± 4.2 | ||
| Control, no laser treatment | 11 ± 0.0 | 11.0 ± 0.0 | ||
| Leptolyngbya sp. | 5 cm | 4 min | 8.0 ± 0.0 | 21.5 ± 3.5 |
| 16 min | 12.5 ± 3.5 | 11.5 ± 2.1 | ||
| 32 min | 8.5 ± 0.7 | 9.0 ± 0.0 | ||
| 10 cm | 4 min | 8.0 ± 1.4 | 11.0 ± 2.8 | |
| 16 min | 15.0 ± 2.8 | 10.0 ± 4.2 | ||
| 32 min | 9.5 ± 0.7 | 14.0 ± 1.4 | ||
| Control, no laser treatment | 9.5 ± 0.0 | 9.5 ± 0.0 | ||
| Cyanobacterial Strain | Distance | Exposure Time | Inhibition zone of MRSA-Staphylococcus aureus | |
|---|---|---|---|---|
| Extracts from Biomass Pre-Exposed to Laser | Extracts Directly Exposed to Laser | |||
| Thermoleptolyngbya sp. | 5 cm | 4 min | 6.5 ± 0.7 | 13.5 ± 3.5 |
| 16 min | 10.0 ± 1.4 | 19.0 ± 1.4 | ||
| 32 min | 12.0 ± 4.9 | 13.5 ± 2.1 | ||
| 10 cm | 4 min | 8.5 ± 2.1 | 13.5 ± 3.5 | |
| 16 min | 9.0 ± 0.0 | 14.5 ± 4.9 | ||
| 32 min | 9.5 ± 0.7 | 16.0 ± 1.4 | ||
| Control, no Laser treatment | - | - | 9.0 ± 0.0 | 9.0 ± 0.0 |
| Leptolyngbya sp. | 5 cm | 4 min | 8.5 ± 0.7 | 13.0 ± 0.0 |
| 16 min | 8.5 ± 0.7 | 8.5 ± 0.7 | ||
| 32 min | 10.5 ± 2.1 | 11.0 ± 2.8 | ||
| 10 cm | 4 min | 11.0 ± 2.3 | 10.0 ± 2.1 | |
| 16 min | 8.5 ± 2.1 | 11.0 ± 2.8 | ||
| 32 min | 9.0 ± 0.0 | 9.0 ± 0.0 | ||
| Control, no Laser treatment | 6.5 ± 0.0 | 6.5 ± 0.0 | ||
| Species | Distance | Time | Phycocyanin (mg/mL) | Allophycocyanin (mg/mL) | Phycoerythrin (mg/mL) |
|---|---|---|---|---|---|
| Thermoleptolyngbya sp. | 0 (Control, no laser treatment) | 0 (Control, no laser treatment) | 0.0624704 | 0.0380794 | 0.205086 |
| 10 | 32 | 0.824993 | 0.118537 | 0.690715 | |
| Leptolyngbya sp. | 0 (Control, no laser treatment) | 0 (Control, no laser treatment) | 0.0244599 | 0.0329147 | 0.206482 |
| 5 | 4 | 0.106869 | 0.0971033 | 0.527876 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Naim, H.M.; El Semary, N. Laser Treatment Increases the Antimicrobial Efficacy of Cyanobacterial Extracts against Staphylococcusaureus (SA) and Methicillin-resistantStaphylococcus aureus (MRSA). Int. J. Environ. Res. Public Health 2022, 19, 13305. https://doi.org/10.3390/ijerph192013305
Al Naim HM, El Semary N. Laser Treatment Increases the Antimicrobial Efficacy of Cyanobacterial Extracts against Staphylococcusaureus (SA) and Methicillin-resistantStaphylococcus aureus (MRSA). International Journal of Environmental Research and Public Health. 2022; 19(20):13305. https://doi.org/10.3390/ijerph192013305
Chicago/Turabian StyleAl Naim, Haifa M., and Nermin El Semary. 2022. "Laser Treatment Increases the Antimicrobial Efficacy of Cyanobacterial Extracts against Staphylococcusaureus (SA) and Methicillin-resistantStaphylococcus aureus (MRSA)" International Journal of Environmental Research and Public Health 19, no. 20: 13305. https://doi.org/10.3390/ijerph192013305





