Ferrous Industrial Wastes—Valuable Resources for Water and Wastewater Decontamination
Abstract
:1. Introduction
2. Iron—An Essential Element for Environmental Equilibrium
- −
- Sorption and/or stabilization technologies: the use of Fe as an immobilizing agent with the role of adsorbent and/or (co-)precipitant;
- −
- Reductive technologies: the use of Fe as an electron donor for the decomposition or degradation of pollutants to a less toxic or mobile form.
3. Industrial Ferrous Waste Sources as New-Valuable Raw Materials for Industrial and Water and Wastewater Treatment Applications
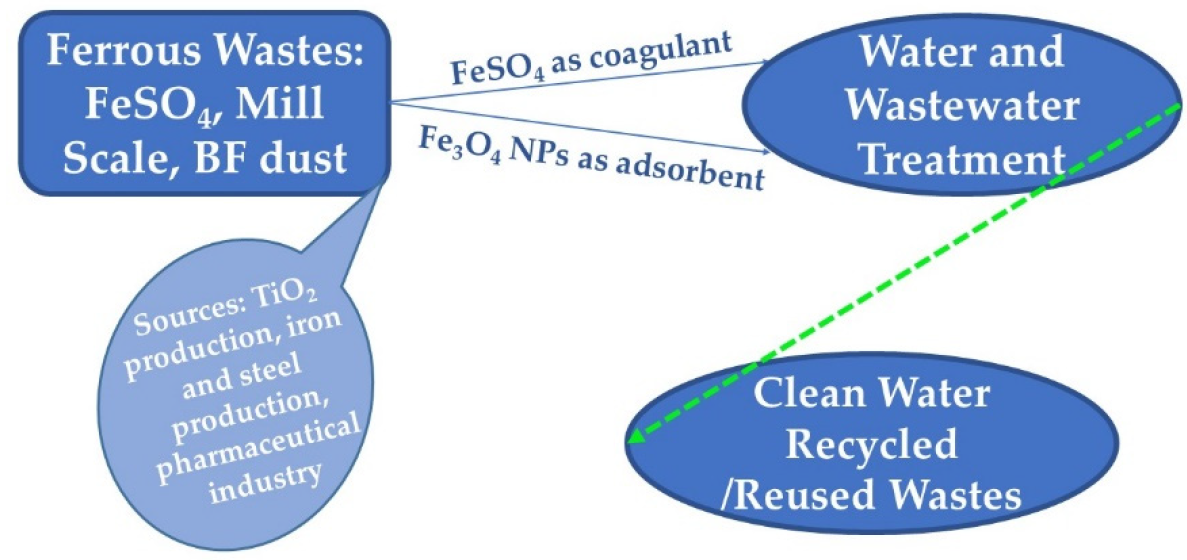
3.1. Ferrous Sulfate from Titanium Dioxide Production
3.2. Mill Scale from Rolling Process
3.3. Pickling Liquors from Metal Finishing Industry
3.4. Other Metallurgical Wastes
3.5. Acid Mine Drainage
3.6. Other Ferrous Wastes
4. Performances within Iron-Based Coagulants and Adsorbents from Ferrous Wastes in Water and Wastewater Treatment
5. Ferrous Wastes Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilarinho, I.S.; Lopes, A.L.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. A new added-value application for steel wire drawing mill scale waste in stoneware ceramic products. Metals 2021, 11, 661. [Google Scholar] [CrossRef]
- Eurostat. Waste Statistics. 2022. Available online: https://ec.europa.eu/eurostat/cros/content/waste-statistics-2022_en (accessed on 27 July 2022).
- Research, G.V. Steel Wire Market Size Analysis, Global Report, 2022–2030. In Market Analysis Report; 2022; 120p, Available online: https://www.grandviewresearch.com/industry-analysis/steel-wire-market-report/toc (accessed on 30 July 2022).
- Mercier, F.; Decarvalho, A.; Hijikata, T.; Ozturk, B.; Morenghi, D.; Mattera, G.; Giua, L. Steel Market developments: Q2 2022. OECD Organ. Econ. Co-Oper. Dev. Dir. Sci. Technol. Innov. (STI) 2022, 114. Available online: https://www.oecd.org/industry/ind/steel-market-developments-Q2-2021.pdf (accessed on 30 July 2022).
- Gade, N.; Verma, G.; Sen, R.; Pandel, U. Effect of calcium carbonate on the reduction behaviour of mill scale. Procedia Earth Planet. Sci. 2015, 11, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Bagatini, M.C.; Fernandes, T.; Silva, R.; Galvao, D.F.; Flores, I.V. Mill scale and flue dust briquettes as alternative burden to low height blast furnaces. J. Clean. Prod. 2020, 276, 124332. [Google Scholar] [CrossRef]
- Somova, Y.V.; Sviridova, T.; Alekseeva, P.; Nekerov, E.; Schwabecher, D. Analysis of methods for processing oily mill scale and oily sludge for iron and steel production. Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 042046. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and recycling of by-products in the steel sector: Recent achievements paving the way to circular economy and industrial symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Faisal, A.A.H.; Alquzweeni, S.S.; Naji, L.A.; Naushad, M. Predominant Mechanisms in the treatment of wastewater due to interaction of benzaldehyde and iron slag byproduct. Int. J. Environ. Res. Public Health 2020, 17, 226. [Google Scholar] [CrossRef] [Green Version]
- Association, W.S. Steel Industry Co-Products. 2020. Available online: https://worldsteel.org/wp-content/uploads/Fact-sheet-Steel-industry-co-products.pdf (accessed on 1 August 2022).
- Grillo, F.F.; Coleti, J.L.; Espinosa, D.C.R.; Oliveira, J.R.; Tenório, J.A.S. Zn and Fe recovery from electric arc furnace dusts. Mater. Trans. 2014, 55, M2013385. [Google Scholar] [CrossRef] [Green Version]
- Heal, K.; Younger, P.L.; Smith, K.; Glendinning, S.; Quinn, P.; Dobbie, K. Novel use of ochre from mine water treatment plants to reduce point and diffuse phosphorus pollution. Land Contam. Reclam. 2003, 11, 145–152. [Google Scholar] [CrossRef]
- Kumpiene, J.; Ore, S.; Renella, G.; Mench, M.; Lagerkvist, A.; Maurice, C. Assessment of zerovalent iron for stabilization of chromium, copper, and arsenic in soil. Environ. Pollut. 2006, 144, 62–69. [Google Scholar] [CrossRef]
- Naveau, A.; Monteil-Rivera, F.; Guillon, E.; Dumonceau, J. Interactions of aqueous selenium (−II) and (IV) with metallic sulfide surfaces. Environ. Sci. Technol. 2007, 41, 5376–5382. [Google Scholar] [CrossRef]
- Liang, L.; Gu, B.; Yin, X. Removal of technetium-99 from contaminated groundwater with sorbents and reductive materials. Sep. Technol. 1996, 6, 111–122. [Google Scholar] [CrossRef]
- Kim, J.S.; Shea, P.J.; Yang, J.E.; Kim, J.-E. Halide salts accelerate degradation of high explosives by zerovalent iron. Environ. Pollut. 2007, 147, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.D.; Su, C.; Lee, T.R.; Wilkin, R.T.; Acree, S.D.; Ross, R.R.; Keeley, A. In situ chemical reduction of Cr (VI) in groundwater using a combination of ferrous sulfate and sodium dithionite: A field investigation. Environ. Sci. Technol. 2007, 41, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Hu, S. Reductive dechlorination of chlorinated solvents on zerovalent iron surfaces. In Physicochemical Groundwater Remediation; Springer: Berlin/Heidelberg, Germany, 2002; pp. 139–159. [Google Scholar]
- Puls, R.W.; Paul, C.J.; Powell, R.M. The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chromate-contaminated groundwater: A field test. Appl. Geochem. 1999, 14, 989–1000. [Google Scholar] [CrossRef]
- Joshi, A.; Chaudhuri, M. Removal of arsenic from ground water by iron oxide-coated sand. J. Environ. Eng. 1996, 122, 769–771. [Google Scholar] [CrossRef]
- Rao, T.; Karthikeyan, J. Removal of As (V) from Water by Adsorption on to Low-cost and Waste Materials. In Progress in Environmental Science and Technology; Science Press: Beijing, China, 2007; p. 684e691. [Google Scholar]
- Mohan, D.; Pittman Jr, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Namasivayam, C.; Senthilkumar, S. Removal of arsenic (V) from aqueous solution using industrial solid waste: Adsorption rates and equilibrium studies. Ind. Eng. Chem. Res. 1998, 37, 4816–4822. [Google Scholar] [CrossRef]
- Sarkar, S.; Blaney, L.M.; Gupta, A.; Ghosh, D.; SenGupta, A.K. Use of ArsenXnp, a hybrid anion exchanger, for arsenic removal in remote villages in the Indian subcontinent. React. Funct. Polym. 2007, 67, 1599–1611. [Google Scholar] [CrossRef]
- Ţurcanu, A.A.; Matei, E.; Râpă, M.; Predescu, A.M.; Coman, G.; Predescu, C. Biowaste valorization using hydrothermal carbonization for potential wastewater treatment applications. Water 2022, 14, 2344. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of agri-food wastes as sustainable eco-materials for wastewater treatment: Current state and new perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, E.; Socalici, A.; Lupu, O.; Bistrian, D.; Dobrescu, C.; Constantin, N. Recovery of waste with a high iron content in the context of the circular economy. Materials 2022, 15, 4995. [Google Scholar] [CrossRef] [PubMed]
- Kanari, N.; Ostrosi, E.; Diliberto, C.; Filippova, I.; Shallari, S.; Allain, E.; Diot, F.; Patisson, F.; Yvon, J. Green process for industrial waste transformation into super-oxidizing materials named alkali metal ferrates (VI). Materials 2019, 12, 1977. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.europeanrecycle.com/it/millscale/ (accessed on 1 August 2022).
- Mohammad Ilias, M.K.; Hossain, M.S.; Ngteni, R.; Al-Gheethi, A.; Ahmad, H.; Omar, F.M.; Naushad, M.; Pandey, S. Environmental remediation potential of ferrous sulfate waste as an eco-friendly coagulant for the removal of NH3-N and COD from the rubber processing effluent. Int. J. Environ. Res. Public Health 2021, 18, 12427. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.; Silva, R.; Arce, I.; Schneider, I. Production of a poly-alumino-iron sulphate coagulant by chemical precipitation of a coal mining acid drainage. Miner. Eng. 2010, 23, 249–251. [Google Scholar] [CrossRef]
- Cundy, A.B.; Hopkinson, L.; Whitby, R.L. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 2008, 400, 42–51. [Google Scholar] [CrossRef]
- Li, D.; Hou, H.; Liu, X.; Yao, Y.; Dai, Z.; Yu, C. The synchronous reutilization of the expired ferrous sulfate granules and waste Li foils for LiFePO4/C cathode. Int. J. Hydrogen Energy 2018, 43, 22419–22426. [Google Scholar] [CrossRef]
- Gavrilescu, M. Microbial recovery of critical metals from secondary sources. Bioresour. Technol. 2022, 344, 126208. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Omar, F.; Asis, A.J.; Bachmann, R.T.; Sarker, M.Z.I.; Ab Kadir, M.O. Effective treatment of palm oil mill effluent using FeSO4·7H2O waste from titanium oxide industry: Coagulation adsorption isotherm and kinetics studies. J. Clean. Prod. 2019, 219, 86–98. [Google Scholar] [CrossRef]
- Ngteni, R.; Hossain, M.S.; Ab Kadir, M.O.; Asis, A.J.; Tajudin, Z. Kinetics and isotherm modeling for the treatment of rubber processing effluent using iron (II) sulphate waste as a coagulant. Water 2020, 12, 1747. [Google Scholar] [CrossRef]
- Mat Yasin, N.M.F.; Hossain, M.S.; HPS, A.K.; Zulkifli, M.; Al-Gheethi, A.; Asis, A.J.; Yahaya, A.N.A. Treatment of palm oil refinery effluent using tannin as a polymeric coagulant: Isotherm, kinetics, and thermodynamics analyses. Polymers 2020, 12, 2353. [Google Scholar] [CrossRef]
- Cundy, A.B.; Croudace, I.W. Physical and chemical associations of radionuclides and trace metals in estuarine sediments: An example from Poole Harbour, Southern England. J. Environ. Radioact. 1995, 29, 191–211. [Google Scholar] [CrossRef]
- Cundy, A.; Hopkinson, L. Electrokinetic iron pan generation in unconsolidated sediments: Implications for contaminated land remediation and soil engineering. Appl. Geochem. 2005, 20, 841–848. [Google Scholar] [CrossRef]
- Kumar, K.; Srimurali, M.; Karthikeyan, J. Removal of colour from synthetic textile dyestuffs by adsorption onto preformed flocs. Prog. Environ. Sci. Technol. 2007, 1, 1278–1286. [Google Scholar]
- Read, H. Fluorine, Chlorine, Bromine, Iodine. In Rutley’s Elements of Mineralogy; Springer: Berlin/Heidelberg, Germany, 1970; pp. 511–512. [Google Scholar]
- Chen, X.-L.; Li, F.; Xie, X.J.; Li, Z.; Chen, L. Nanoscale zero-valent iron and chitosan functionalized Eichhornia crassipes biochar for efficient hexavalent chromium removal. Int. J. Environ. Res. Public Health 2019, 16, 3046. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Li, Q.; Yu, H.; Xiang, L.; Wei, J.; Lin, F. Pyrolysis behaviors and residue properties of iron-rich rolling sludge from steel smelting. Int. J. Environ. Res. Public Health 2022, 19, 2152. [Google Scholar] [CrossRef]
- Rodgers, K.; McLellan, I.; Cuthbert, S.; Masaguer Torres, V.; Hursthouse, A. The potential of remedial techniques for hazard reduction of steel process by products: Impact on steel processing, waste management, the environment and risk to human health. Int. J. Environ. Res. Public Health 2019, 16, 2093. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Yuan, T.; Yong Hu, J.; Ong, S.L.; Luo, Q.F.; Jun Ng, W. Arsenic removal from household drinking water by adsorption. J. Environ. Sci. Health Part A 2002, 37, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, R.T.; Su, C.; Ford, R.G.; Paul, C.J. Chromium-removal processes during groundwater remediation by a zerovalent iron permeable reactive barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G.; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef]
- Blodau, C. A review of acidity generation and consumption in acidic coal mine lakes and their watersheds. Sci. Total Environ. 2006, 369, 307–332. [Google Scholar] [CrossRef]
- Lloyd, J.R. Microbial reduction of metals and radionuclides. FEMS Microbiol. Rev. 2003, 27, 411–425. [Google Scholar] [CrossRef]
- Schirmer, M.; Butler, B.J. Transport behaviour and natural attenuation of organic contaminants at spill sites. Toxicology 2004, 205, 173–179. [Google Scholar] [CrossRef]
- Mwewa, B.; Stopić, S.; Ndlovu, S.; Simate, G.S.; Xakalashe, B.; Friedrich, B. Synthesis of poly-alumino-ferric sulphate coagulant from acid mine drainage by precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef] [Green Version]
- Moanţă, A.; Mohanu, I.; Paceagiu, J.; Năstac, D.C.; Petre, I.; Fechet, R.M. Valorificarea ţunderelor în materiale cu valoare adăugată. Rev. Romana Mater. 2017, 47, 276. [Google Scholar]
- Luo, L.; Zhang, Y.; Bao, S.; Chen, T. Utilization of iron ore tailings as raw material for Portland cement clinker production. Adv. Mater. Sci. Eng. 2016, 2016, 1596047. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, A.; Moussas, P.; Vasilakou, F. Polyferric sulphate: Preparation, characterisation and application in coagulation experiments. J. Hazard. Mater. 2008, 155, 459–468. [Google Scholar] [CrossRef]
- Croce, P.S.; Mousavi, A. A sustainable sulfate process to produce TiO2 pigments. Environ. Chem. Lett. 2013, 11, 325–328. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Ray, B.; Sahu, K. Extraction of acid and iron values from sulphate waste pickle liquor of a steel industry by solvent extraction route. Hydrometallurgy 2007, 88, 58–66. [Google Scholar] [CrossRef]
- de Buzin, P.; Vigânico, E.M.; Silva, R.d.A.; Heck, N.; Schneider, I.A.H.; Menezes, J. Prodution of ferrous sulfate from steelmaking mill scale. Int. J. Sci. Eng. Res. 2014, 5, 353–359. [Google Scholar]
- Tang, B.; Yuan, L.; Shi, T.; Yu, L.; Zhu, Y. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical co-precipitation. J. Hazard. Mater. 2009, 163, 1173–1178. [Google Scholar] [CrossRef]
- Huang, P.; Deng, S.; Zhang, Z.; Wang, X.; Chen, X.; Yang, X.; Yang, L. A sustainable process to utilize ferrous sulfate waste from titanium oxide industry by reductive decomposition reaction with pyrite. Thermochim. Acta 2015, 620, 18–27. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigments 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Kesavan, S.K.; Azad, A.-M. Conversion of steel mill waste into nanoscale zerovalent iron (nZVI) particles for hydrogen generation via metal-steam reforming. Int. J. Hydrogen Energy 2008, 33, 1232–1242. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xu, G.J.; Liu, C.H. Upgrading of China’s Titanium Dioxide industry from the perspective of clean production. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 8723–8726. [Google Scholar]
- Huang, P.; Jiang, B.; Zhang, Z.; Wang, X.; Chen, X.; Yang, X.; Yang, L. Recycling sulfur and iron resources in the waste ferrous sulfate. J. Therm. Anal. Calorim. 2015, 119, 2229–2237. [Google Scholar] [CrossRef]
- Vondruska, M.; Bednarik, V.; Sild, M. Stabilization/solidification of waste ferrous sulphate from titanium dioxide production by fluidized bed combustion product. Waste Manag. 2001, 21, 11–16. [Google Scholar] [CrossRef]
- Su, C.; Ludwig, R.D. Treatment of hexavalent chromium in chromite ore processing solid waste using a mixed reductant solution of ferrous sulfate and sodium dithionite. Environ. Sci. Technol. 2005, 39, 6208–6216. [Google Scholar] [CrossRef]
- Gázquez, M.J.; Contreras, M.; Pérez-Moreno, S.M.; Guerrero, J.L.; Casas-Ruiz, M.; Bolívar, J.P. A Review of the commercial uses of sulphate minerals from the titanium dioxide pigment industry: The case of Huelva (Spain). Minerals 2021, 11, 575. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T. A thermodynamic analysis on the roasting of pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.A.; Van Weert, G. On the catalysis of ferrous sulphate oxidation in autoclaves by nitrates and nitrites. Hydrometallurgy 1996, 42, 209–219. [Google Scholar] [CrossRef]
- Zhu, S.; Li, T.; Wu, Y.; Chen, Y.; Su, T.; Ri, K.; Huo, Y. Effective purification of cold-rolling sludge as iron concentrate powder via a coupled hydrothermal and calcination route: From laboratory-scale to pilot-scale. J. Clean. Prod. 2020, 276, 124274. [Google Scholar] [CrossRef]
- Kanari, N.; Filippova, I.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Utilization of a waste from titanium oxide industry for the synthesis of sodium ferrate by gas–solid reactions. Thermochim. Acta 2014, 575, 219–225. [Google Scholar] [CrossRef]
- Gázquez, M.; Bolívar, J.; García-Tenorio, R.; Vaca, F. Physicochemical characterization of raw materials and co-products from the titanium dioxide industry. J. Hazard. Mater. 2009, 166, 1429–1440. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Li, X.; Guo, H.; Li, L.; Wang, X.; Zheng, J. Cation-substituted LiFePO4 prepared from the FeSO4·7H2O waste slag as a potential Li battery cathode material. J. Alloys Compd. 2010, 497, 278–284. [Google Scholar] [CrossRef]
- Li, X.; Lei, Z.; Qu, J.; Li, Z.; Zhou, X.; Zhang, Q. Synthesizing slow-release fertilizers via mechanochemical processing for potentially recycling the waste ferrous sulfate from titanium dioxide production. J. Environ. Manag. 2017, 186, 120–126. [Google Scholar] [CrossRef]
- Frémy, E. Recherches sur les Acides Métalliques; Bachelier: Paris, France, 1844. [Google Scholar]
- Fremy, E. Recherches sur l’action des peroxides alcalins sur les oxides métalliques: Lettre de M. Ed. Fremy à M. Pelouze. Comptes Rendus L’académie Sci. 1841, 12, 23–24. [Google Scholar]
- Cici, M.; Cuci, Y. Production of some coagulant materials from galvanizing workshop waste. Waste Manag. 1998, 17, 407–410. [Google Scholar] [CrossRef]
- Leong, S.T.; Muttamara, S.; Laortanakul, P. Reutilization of wastewater in a rubber-based processing factory: A case study in Southern Thailand. Resour. Conserv. Recycl. 2003, 37, 159–172. [Google Scholar] [CrossRef]
- Rahman, A.; Habib, S.; Rahman, M.; Sajib, M.S.J.; Yousuf, A. A novel multi-phase treatment scheme for odorous rubber effluent. Environ. Technol. 2021, 42, 1366–1372. [Google Scholar] [CrossRef]
- Tanikawa, D.; Syutsubo, K.; Watari, T.; Miyaoka, Y.; Hatamoto, M.; Iijima, S.; Fukuda, M.; Nguyen, N.B.; Yamaguchi, T. Greenhouse gas emissions from open-type anaerobic wastewater treatment system in natural rubber processing factory. J. Clean. Prod. 2016, 119, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Changsong, W.; Hongliang, Q.; Yuanhui, W.; JIXiaohua, L. Discussion on current advance new methods of energy saving and emission reduction in titanium industry. Inorg. Chem. Ind. 2010, 4, 8–10. [Google Scholar]
- Nabiyouni, G.; Julaee, M.; Ghanbari, D.; Aliabadi, P.C.; Safaie, N. Room temperature synthesis and magnetic property studies of Fe3O4 nanoparticles prepared by a simple precipitation method. J. Ind. Eng. Chem. 2015, 21, 599–603. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, Z.; Li, S.; Zhang, X.; Ying, A. Fabrication and characterization of hollow Fe3O4 nanospheres in a microemulsion. Mater. Lett. 2008, 62, 4053–4055. [Google Scholar] [CrossRef]
- Haw, C.Y.; Mohamed, F.; Chia, C.; Radiman, S.; Zakaria, S.; Huang, N.; Lim, H. Hydrothermal synthesis of magnetite nanoparticles as MRI contrast agents. Ceram. Int. 2010, 36, 1417–1422. [Google Scholar] [CrossRef]
- Albornoz, C.; Jacobo, S.E. Preparation of a biocompatible magnetic film from an aqueous ferrofluid. J. Magn. Magn. Mater. 2006, 305, 12–15. [Google Scholar] [CrossRef]
- Azizi, N.; Bashipour, F. Demulsification of water-in-oil emulsions applying Fe3O4 magnetic nanoparticles for demulsifier modification: Experimental optimization via response surface methodology. J. Pet. Sci. Eng. 2022, 216, 110806. [Google Scholar] [CrossRef]
- Azadi, F.; Karimi-Jashni, A.; Zerafat, M.M. Green synthesis and optimization of nano-magnetite using Persicaria bistorta root extract and its application for rosewater distillation wastewater treatment. Ecotoxicol. Environ. Saf. 2018, 165, 467–475. [Google Scholar] [CrossRef]
- Valenzuela, R.; Fuentes, M.C.; Parra, C.; Baeza, J.; Duran, N.; Sharma, S.; Knobel, M.; Freer, J. Influence of stirring velocity on the synthesis of magnetite nanoparticles (Fe3O4) by the co-precipitation method. J. Alloys Compd. 2009, 488, 227–231. [Google Scholar] [CrossRef]
- Perez, G.; Romero, M.P.; Saitovitch, E.B.; Litterst, F.J.; Araujo, J.F.; Bell, D.C.; Solorzano, G. Alkali concentration effects on the composition, morphology and magnetic properties of magnetite, maghemite and iron oxyhydroxide nanoparticles. Solid State Sci. 2020, 106, 106295. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.-L.; Zheng, Y.-J. Recovery of iron from waste ferrous sulphate by co-precipitation and magnetic separation. Trans. Nonferrous Met. Soc. China 2017, 27, 211–219. [Google Scholar]
- Kumar, N.; Amritphale, S.S.; Matthews, J.C.; Lynam, J.G.; Alam, S.; Abdulkareem, O.A. Synergistic utilization of diverse industrial wastes for reutilization in steel production and their geopolymerization potential. Waste Manag. 2021, 126, 728–736. [Google Scholar] [CrossRef]
- Vigânico, E.M.; Colling, A.V.; de Almeida Silva, R.; Schneider, I.A.H. Biohydrometallurgical/UV production of ferrous sulphate heptahydrate crystals from pyrite present in coal tailings. Miner. Eng. 2011, 24, 1146–1148. [Google Scholar] [CrossRef]
- Alibrahim, M. Extraction of phosphoric acid from various aqueous solutions using tributyl phosphate (TBP). Period. Polytech. Chem. Eng. 2007, 51, 39–42. [Google Scholar] [CrossRef]
- Moyer, B.A. Ion Exchange and Solvent Extraction: A Series of Advances; CRC Press: Boca Raton, FL, USA, 2009; Volume 19. [Google Scholar]
- Alguacil, F.J.; López-Delgado, A.; Alonso, M.; Sastre, A.M.A. The phosphine oxides Cyanex 921 and Cyanex 923 as carriers for facilitated transport of chromium (VI)-chloride aqueous solutions. Chemosphere 2004, 57, 813–819. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Wei, G.; Zhao, H.; Wang, L.; Qi, T.; Meng, F.; Meng, L. Extraction of iron (III) from chloride leaching liquor with high acidity using tri-n-butyl phosphate and synergistic extraction combined with methyl isobutyl ketone. Sep. Purif. Technol. 2015, 150, 132–138. [Google Scholar] [CrossRef]
- Liu, Y.; Nam, S.-H.; Lee, M. Stripping of Fe (III) from the loaded mixture of D2EHPA and TBP with sulfuric acid containing reducing agents. Bull. Korean Chem. Soc. 2014, 35, 2109–2113. [Google Scholar] [CrossRef] [Green Version]
- Biswas, R.; Begum, D. Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene. Hydrometallurgy 1998, 50, 153–168. [Google Scholar] [CrossRef]
- Hirato, T.; Wu, Z.-C.; Yamada, Y.; Majima, H. Improvement of the stripping characteristics of Fe (III) utilizing a mixture of di (2-ethylhexyl) phosphoric acid and tri-n-butyl phosphate. Hydrometallurgy 1992, 28, 81–93. [Google Scholar] [CrossRef]
- Sahu, K.; Das, R. Mixed solvent systems for the extraction and stripping of iron (III) from concentrated acid chloride solutions. Metall. Mater. Trans. B 2000, 31, 1169–1174. [Google Scholar] [CrossRef]
- Meng, M.X.; Yu, S. Kinetics of iron (III) extraction with primary amine and TBP using a modified rotating diffusion cell. Hydrometallurgy 1996, 41, 55–70. [Google Scholar] [CrossRef]
- Hariharan, A.; Manasa, K.; Kalyani, J. Solvent extraction of iron (III) with tri methyl amine from mineral acid solutions. Chem. Sci. 2014, 3, 1427–1431. [Google Scholar]
- Maeda, M.; Narita, H.; Tokoro, C.; Tanaka, M.; Motokawa, R.; Shiwaku, H.; Yaita, T. Selective extraction of Pt (IV) over Fe (III) from HCl with an amide-containing tertiary amine compound. Sep. Purif. Technol. 2017, 177, 176–181. [Google Scholar] [CrossRef]
- Csicsovszki, G.; Kékesi, T.; Török, T.I. Selective recovery of Zn and Fe from spent pickling solutions by the combination of anion exchange and membrane electrowinning techniques. Hydrometallurgy 2005, 77, 19–28. [Google Scholar] [CrossRef]
- You, X.; Chen, J.; Pan, S.; Lu, G.; Teng, L.; Lin, X.; Zhao, S.; Lin, J. Piperazine-functionalized porous anion exchange membranes for efficient acid recovery by diffusion dialysis. J. Membr. Sci. 2022, 654, 120560. [Google Scholar] [CrossRef]
- Liu, M.; Iizuka, A.; Shibata, E. Acid mine drainage sludge as an alternative raw material for M-type hexaferrite preparation. J. Clean. Prod. 2019, 224, 284–291. [Google Scholar] [CrossRef]
- Paquay, E.; Clarinval, A.-M.; Delvaux, A.; Degrez, M.; Hurwitz, H.D. Applications of electrodialysis for acid pickling wastewater treatment. Chem. Eng. J. 2000, 79, 197–201. [Google Scholar] [CrossRef]
- Foureaux, A.; Moreira, V.; Lebron, Y.; Santos, L.; Amaral, M. Direct contact membrane distillation as an alternative to the conventional methods for value-added compounds recovery from acidic effluents: A review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Passos, H.; Cruz, B.; Schaeffer, N.; Patinha, C.; da Silva, E.F.; Coutinho, J.A. Selective sequential recovery of zinc and copper from acid mine drainage. ACS Sustain. Chem. Eng. 2021, 9, 3647–3657. [Google Scholar] [CrossRef]
- Heras, F.; Dufour, J.; Lopez-Delgado, A.; Negro, C.; Lopez-Mateos, F. Feasibility study of metals recycling from nitric-hydrofluoric waste pickle baths. Environ. Eng. Sci. 2004, 21, 583–590. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, H.-C.; Yang, S.-Y.; Horng, H.-E.; Hung, J.; Chen, Y.; Hong, C.-Y. Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 2004, 283, 210–214. [Google Scholar] [CrossRef]
- Shaheen, K.; Shah, Z.; Marwat, R.; Arshad, T.; Khan, S.B.; Iqbal, N.; Khan, B.; Cui, J.; Ji, Y.T.; Ma, L. Synthesis of silver and aluminum doped magnetic nanoparticles: New fascinating materials with multipurpose applications. Chem. Phys. Lett. 2020, 742, 137167. [Google Scholar] [CrossRef]
- Ak, G.; Şanlıer, Ş.H. Erythrocyte membrane vesicles coated biomimetic and targeted doxorubicin nanocarrier: Development, characterization and in vitro studies. J. Mol. Struct. 2020, 1205, 127664. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of functional magnetic nanocomposites for bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, H.S.; Kwak, B.K.; Kim, B.-K. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J. Magn. Magn. Mater. 2005, 289, 328–330. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Han, H.; Wang, X.; Zhou, L.; Yi, Z.; Fu, Z.; Wu, X.; Li, G.; Zeng, L. Optical and magnetic properties of small-size core–shell Fe3O4@ C nanoparticles. Mater. Today Chem. 2021, 22, 100556. [Google Scholar] [CrossRef]
- Căpraru, A.; Moacă, E.-A.; Păcurariu, C.; Ianoş, R.; Lazău, R.; Barbu-Tudoran, L. Development and characterization of magnetic iron oxide nanoparticles using microwave for the combustion reaction ignition, as possible candidates for biomedical applications. Powder Technol. 2021, 394, 1026–1038. [Google Scholar] [CrossRef]
- Zhang, G.-Y.; Feng, Y.; Xu, Y.-Y.; Gao, D.-Z.; Sun, Y.-Q. Controlled synthesis of mesoporous α-Fe2O3 nanorods and visible light photocatalytic property. Mater. Res. Bull. 2012, 47, 625–630. [Google Scholar] [CrossRef]
- Hataminia, F.; Majidi, R.F.; Shabankareh, A.N.T.; Ghanbari, H. Green synthesis of oxidized starch with a novel catalyst based on Fe3O4 nanoparticles and H2O2 reagent to form thermoplastic as a stable gel coating on the cardiovascular stents. Int. J. Biol. Macromol. 2022, 219, 290–303. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. Green synthesis of NiO/Fe3O4@ chitosan composite catalyst based on graphite for urea electro-oxidation. Mater. Chem. Phys. 2022, 290, 126603. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Abdulridha, S.A.; Aljbory, E.H.; Alkhayatt, A.H.O.; Zabibah, R.S.; Alrubaie, A.J.; Rady, S.K.; Kizar, S.H.; Hariz, S.S.; Kadhem, M.J. Capping agent effect on optical properties of Fe2O3 nanoparticles. Mater. Today Proc. 2022, 56, 2010–2015. [Google Scholar] [CrossRef]
- Matos, R.; Monteiro, M.; Silva Jr, R.; Macêdo, M.; Paz, S.; Angélica, R.; Oliveira, R.; Ferreira, N. Novel Amapá latex-mediated synthesis of defective α-Fe2O3 nanoparticles with enhanced ferromagnetism and sunlight photocatalytic activity. Ceram. Int. 2022, 48, 28496–28511. [Google Scholar] [CrossRef]
- Shoorangiz, M.; Shariatifard, L.; Roshan, H.; Mirzaei, A. Selective ethanol sensor based on α-Fe2O3 nanoparticles. Inorg. Chem. Commun. 2021, 133, 108961. [Google Scholar] [CrossRef]
- Soleimani, F.; Nezamzadeh-Ejhieh, A. Study of the photocatalytic activity of CdS–ZnS nano-composite in the photodegradation of rifampin in aqueous solution. J. Mater. Res. Technol. 2020, 9, 16237–16251. [Google Scholar] [CrossRef]
- Devi, R.A.; Latha, M.; Velumani, S.; Oza, G.; Reyes-Figueroa, P.; Rohini, M.; Becerril-Juarez, I.; Lee, J.-H.; Yi, J. Synthesis and characterization of cadmium sulfide nanoparticles by chemical precipitation method. J. Nanosci. Nanotechnol. 2015, 15, 8434–8439. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.R.; Syed, R.; Adil, S.F.; Kuniyil, M.; Khan, M.; Alqahtani, M.S.; Shaik, J.P.; Siddiqui, M.R.H.; Al-Warthan, A.; Sharaf, M.A. Mn3O4 nanoparticles: Synthesis, characterization and their antimicrobial and anticancer activity against A549 and MCF-7 cell lines. Saudi J. Biol. Sci. 2021, 28, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, B.; Jiang, Y.; Zhang, Y.Z. Synthesis of Mn3O4 nanoparticles for catalytic application via ultrasound-assisted ball milling. ChemistrySelect 2018, 3, 3904–3908. [Google Scholar] [CrossRef]
- Dengo, N.; Vittadini, A.; Natile, M.M.; Gross, S. In-depth study of ZnS nanoparticle surface properties with a combined experimental and theoretical approach. J. Phys. Chem. C 2020, 124, 7777–7789. [Google Scholar] [CrossRef]
- Pathak, C.; Mishra, D.; Agarwala, V.; Mandal, M. Optical properties of ZnS nanoparticles produced by mechanochemical method. Ceram. Int. 2012, 38, 6191–6195. [Google Scholar] [CrossRef]
- Mukh-Qasem, R.A.; Gedanken, A. Sonochemical synthesis of stable hydrosol of Fe3O4 nanoparticles. J. Colloid Interface Sci. 2005, 284, 489–494. [Google Scholar] [CrossRef]
- Konishi, Y.; Nomura, T.; Mizoe, K. A new synthesis route from spent sulfuric acid pickling solution to ferrite nanoparticles. Hydrometallurgy 2004, 74, 57–65. [Google Scholar] [CrossRef]
- Lopez, F.; Lopez-Delgado, A.; de Vidales, J.M.; Vila, E. Synthesis of nanocrystalline zinc ferrite powders from sulphuric pickling waste water. J. Alloys Compd. 1998, 265, 291–296. [Google Scholar] [CrossRef]
- Chandrika, M.; Ravindra, A.; Rajesh, C.; Ramarao, S.; Ju, S. Studies on structural and optical properties of nano ZnFe2O4 and ZnFe2O4-TiO2 composite synthesized by co-precipitation route. Mater. Chem. Phys. 2019, 230, 107–113. [Google Scholar] [CrossRef]
- Pellerin, C.; Booker, S.M. Reflections on hexavalent chromium: Health hazards of an industrial heavyweight. Environ. Health Perspect. 2000, 108, A402–A407. [Google Scholar] [CrossRef]
- Wang, G.-H.; Cheng, C.-Y.; Tsai, T.-H.; Chiang, P.-K.; Chung, Y.-C. Highly sensitive luminescent bioassay using recombinant Escherichia coli biosensor for rapid detection of low Cr (VI) concentration in environmental water. Biosensors 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Bandara, P.; Peña-Bahamonde, J.; Rodrigues, D. Redox mechanisms of conversion of Cr (VI) to Cr (III) by graphene oxide-polymer composite. Sci. Rep. 2020, 10, 9237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and removal of chromium VI in water by powdered activated carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao-Feng, N.; Yong, L.; Xin-Hua, X.; Zhang-Hua, L. Removal of hexavalent chromium from aqueous solution by iron nanoparticles. J. Zhejiang Univ. Sci. B 2005, 6, 1022–1027. [Google Scholar]
- Farooqi, Z.H.; Akram, M.W.; Begum, R.; Wu, W.; Irfan, A. Inorganic nanoparticles for reduction of hexavalent chromium: Physicochemical aspects. J. Hazard. Mater. 2021, 402, 123535. [Google Scholar] [CrossRef]
- Papassiopi, N.; Vaxevanidou, K.; Christou, C.; Karagianni, E.; Antipas, G. Synthesis, characterization and stability of Cr (III) and Fe (III) hydroxides. J. Hazard. Mater. 2014, 264, 490–497. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Xu, R.; Wang, Y.-N.; Sun, Y.; Bian, R.; Li, W. Remediation of Cr (VI)-contaminated soil by combined chemical reduction and microbial stabilization: The role of biogas solid residue (BSR). Ecotoxicol. Environ. Saf. 2022, 231, 113198. [Google Scholar] [CrossRef]
- Cantrell, K.J.; Kaplan, D.I.; Wietsma, T.W. Zero-valent iron for the in situ remediation of selected metals in groundwater. J. Hazard. Mater. 1995, 42, 201–212. [Google Scholar] [CrossRef]
- Gheju, M.; Iovi, A. Kinetics of hexavalent chromium reduction by scrap iron. J. Hazard. Mater. 2006, 135, 66–73. [Google Scholar] [CrossRef]
- Abdo, M.; Sedahmed, G. New technique for removing hexavalent chromium from waste water and energy generation via galvanic reduction with scrap iron. Energy Convers. Manag. 1998, 39, 943–951. [Google Scholar]
- Krogh, H.; Myhre, L.; Häkkinen, T.; Tattari, K.; Jönsson, Å.; Björklund, T. Environmental data for production of reinforcement bars from scrap iron and for production of steel products from iron ore in the Nordic countries. Build. Environ. 2001, 36, 109–119. [Google Scholar] [CrossRef]
- Innocenzi, V.; Cantarini, F.; Amato, A.; Morico, B.; Ippolito, N.M.; Beolchini, F.; Prisciandaro, M.; Vegliò, F. Case study on technical feasibility of galvanic wastewater treatment plant based on life cycle assessment and costing approach. J. Environ. Chem. Eng. 2020, 8, 104535. [Google Scholar] [CrossRef]
- García, V.; Steeghs, W.; Bouten, M.; Ortiz, I.; Urtiaga, A. Implementation of an eco-innovative separation process for a cleaner chromium passivation in the galvanic industry. J. Clean. Prod. 2013, 59, 274–283. [Google Scholar] [CrossRef]
- Özer, A.; Altundoğan, H.; Erdem, M.; Tümen, F. A study on the Cr (VI) removal from aqueous solutions by steel wool. Environ. Pollut. 1997, 97, 107–112. [Google Scholar] [CrossRef]
- Kontopoulos, A. Acid Mine Drainage Control. In Proceedings of the International Conference on Clean Technologies for the Mining Industry, Santiago, Chile, 13–15 May 1998. [Google Scholar]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Rao, S.; Gehr, R.; Riendeau, M.; Lu, D.; Finch, J. Acid mine drainage as a coagulant. Miner. Eng. 1992, 5, 1011–1020. [Google Scholar] [CrossRef]
- Galvão, J.L.B.; Andrade, H.D.; Brigolini, G.J.; Peixoto, R.A.F.; Mendes, J.C. Reuse of iron ore tailings from tailings dams as pigment for sustainable paints. J. Clean. Prod. 2018, 200, 412–422. [Google Scholar] [CrossRef]
- Ryu, S.; Naidu, G.; Johir, M.A.H.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid mine drainage treatment by integrated submerged membrane distillation–sorption system. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef]
- de Almeida Silva, R.; Secco, M.P.; Lermen, R.T.; Schneider, I.A.H.; Hidalgo, G.E.N.; Sampaio, C.H. Optimizing the selective precipitation of iron to produce yellow pigment from acid mine drainage. Miner. Eng. 2019, 135, 111–117. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C., Jr. Synthesis of magnetite nanoparticles with ferric iron recovered from acid mine drainage: Implications for environmental engineering. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 280–286. [Google Scholar] [CrossRef]
- Agrawal, A.; Kumari, S.; Sahu, K. Iron and copper recovery/removal from industrial wastes: A review. Ind. Eng. Chem. Res. 2009, 48, 6145–6161. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Shu, C.-M. Energy generation mechanisms for a Li-ion cell in case of thermal explosion: A review. J. Energy Storage 2022, 55, 105501. [Google Scholar] [CrossRef]
- Lundtorp, K.; Jensen, D.L.; Sørensen, M.A.; Christensen, T.H.; Mogensen, E. Treatment of waste incinerator air-pollution-control residues with FeSO4: Concept and product characterisation. Waste Manag. Res. 2002, 20, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, I.; Garelick, H.; Valsami-Jones, E. An investigation into arsenic (V) removal from aqueous solutions by hydroxylapatite and bone-char. Mineral. Mag. 2005, 69, 769–780. [Google Scholar] [CrossRef]
- Chen, S.; Long, F.; Gao, G.; Belver, C.; Li, Z.; Li, Z.; Guan, J.; Guo, Y.; Bedia, J. Zero-valent iron-copper bimetallic catalyst supported on graphite from spent lithium-ion battery anodes and mill scale waste for the degradation of 4-chlorophenol in aqueous phase. Sep. Purif. Technol. 2022, 286, 120466. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Râpă, M.; Sohaciu, M.G.; Predescu, C.; Vidu, R. An Innovative method of converting ferrous mill scale wastes into superparamagnetic nanoadsorbents for water decontamination. Materials 2021, 14, 2539. [Google Scholar] [CrossRef]
- Ovčačíková, H.; Vlček, J.; Matějka, V.; Juřica, J.; Maierová, P.; Mlčoch, P. The effect of temperature and milling process on steel scale utilized as a pigment for ceramic glaze. Materials 2020, 13, 1814. [Google Scholar] [CrossRef] [Green Version]
- EUROFER. Available online: https://www.eurofer.eu/press-releases/ongoing-covid-pandemic-piles-pressure-on-steel-sector-in-mid-2020 (accessed on 1 August 2022).
- Mousa, E. Modern blast furnace ironmaking technology: Potentials to meet the demand of high hot metal production and lower energy consumption. Metall. Mater. Eng. 2019, 25, 69–104. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.; Kumar, M.S. An overview of environmental sustainability in cement and steel production. J. Clean. Prod. 2019, 231, 856–871. [Google Scholar] [CrossRef]
- Ma, N.; Sammon, W.J. Enhancement of in-plant recycling of integrated steel mill off gas solid wastes by reallocating crucial zinc-bearing materials. J. Clean. Prod. 2020, 251, 119783. [Google Scholar] [CrossRef]
- Sali, L. Stop Waste and Scrap Export to Countries Not Meeting EU Environment Standards, Asks EUROFER; EUROFER: Brussels, Belgium, 2022. [Google Scholar]
- Umadevi, T.; Brahmacharyulu, A.; Karthik, P.; Mahapatra, P.; Prabhu, M.; Ranjan, M. Recycling of steel plant mill scale via iron ore sintering plant. Ironmak. Steelmak. 2012, 39, 222–227. [Google Scholar] [CrossRef]
- Rieger, J.; Schenk, J. Residual processing in the European steel industry: A technological overview. J. Sustain. Metall. 2019, 5, 295–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Xiong, L.; Li, G.; Jiang, T. Recycling of carbonaceous iron-bearing dusts from iron & steel plants by composite agglomeration process (CAP). Ironmak. Steelmak. 2017, 44, 532–543. [Google Scholar]
- De Gisi, S.; Romaniello, L.; Dalessandro, M.; Todaro, F.; Notarnicola, M. Recovery of iron rich residues from integrated steel making process by hydrated lime/molasses pressurised cold agglomeration. J. Clean. Prod. 2019, 233, 830–840. [Google Scholar] [CrossRef]
- Strezov, V. Iron ore reduction using sawdust: Experimental analysis and kinetic modelling. Renew. Energy 2006, 31, 1892–1905. [Google Scholar] [CrossRef]
- Streltsova, T.P.; Lesovik, V.S.; Frolova, M.A.; Perkova, M.V.; Belikov, D.A. Natural iron oxide pigments for the construction industry. World Appl. Sci. J. 2013, 25, 193–201. [Google Scholar]
- Rabajczyk, A.; El Yamani, N.; Dusinska, M. The effect of time on the stability of iron oxide nanoparticles in environmental acids. Water Environ. Res. 2017, 89, 416–423. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]


| Waste Source | Waste Valorization Method | Type of Product | Reference |
|---|---|---|---|
| Acidic ferrous solutions from steel pickling | Extraction with binary solvent (methyl isobutyl ketone—MIBK) and (di(2-ethylhexyl) phosphoric acid—D2EHPA). | Fe(III) extracted from concentrated iron solution in a sulfate medium. | [62] |
| Coal AMD | Oxidation/selective chemical precipitation, solid–liquid separation, 24 h aeration, pH 2.5–3.0 (conversion of Fe2+ to Fe3+), 4 M NaOH, precipitation of Fe and Al. | Coagulant: Fe(III): 72% and Al(III): 27.5%, low concentrations of Mn, Zn, Ca, Mg. | [33] |
| Waste Li foils and expired ferrous sulfate granules | 2 wastes type: Li from spent CR2025 Li-coin-cells and expired ferrous sulfate granules collected from the households, hydrothermal preparation for LiFePO4/C nanoparticles cathode. | LiFePO4/C cathode. | [35] |
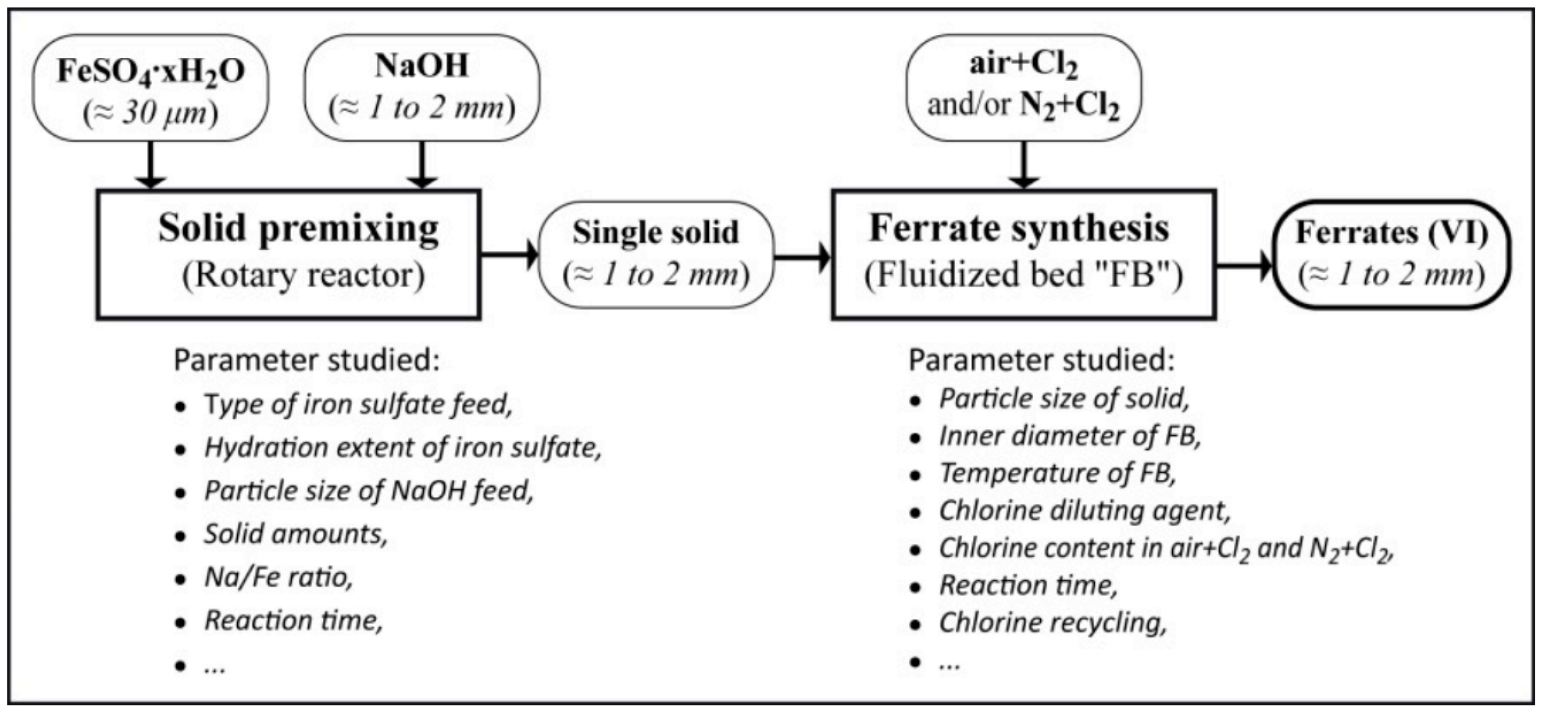
| FeSO4·7H2O from TiO2 industrial production. | Premixing of two solids (NaOH with FeSO4·H2O) for single solid production as iron sulfate coated with alkali hydroxides (80 to 90 g of Fe/kg of iron), oxidation with diluted chlorine in the fluidized bed. Fe(VI): 30–55%. | Alkali ferrates ~1 mm (A2FeO4, A: Na, K) with Fe(VI), for cleaning waters, wastewater, and other effluents. | [30] |
| MS and blast furnace flue dust | MS and BF dust dried at 105 °C for 12 h, mixed with Portland cement as binder and water, 3 different C/O mass ratios (0.25, 0.5, and 0.75), high-temperature and reducing atmosphere conditions. | Cylindrical briquettes for blast furnaces. Average weight and height: 2.7 ± 0.02 g and 10 ± 0.1 mm. | [6] |
| MS for colored ceramic pastes | 1, 3, 5, and 10 wt.% MS pretreatments: milling, 212 µm sieving, maximum firing temperature (1043 ± 1165 °C). | Dark grey hue ceramic pastes with MS incorporation. | [1] |
| Spent pickle liquor from steel manufacturers | MS leaching with H2SO4 (at concentrations of 5%, 10%, and 15%), liquor sample rich in Fe2+ formation, 80 °C evaporation, and crystallization of ferrous sulfate. | Melanterite (FeSO4⋅7H2O) main compound, szomolnokite (FeSO4⋅H2O), Rozenite (FeSO4⋅4H2O). | [63] |
| Spent pickling liquors from a steel surface treatment factory | Ultrasonic-assisted chemical co-precipitation, molar ratios of Fe(III)/Fe(II), pH 0.3–0.5, Fe total 105.6 g/L, HCl 10.6 g/L, heavy metals traces, suspended solids (SS) filtration, alkaline buffer solution (pH 13) add, reaction time: 20–30 min, separation by sedimentation, washing solid, ultrasonication. | Fe3O4 cubic nanoparticles, 13–23 nm diameter, super-paramagnetic. | [64] |
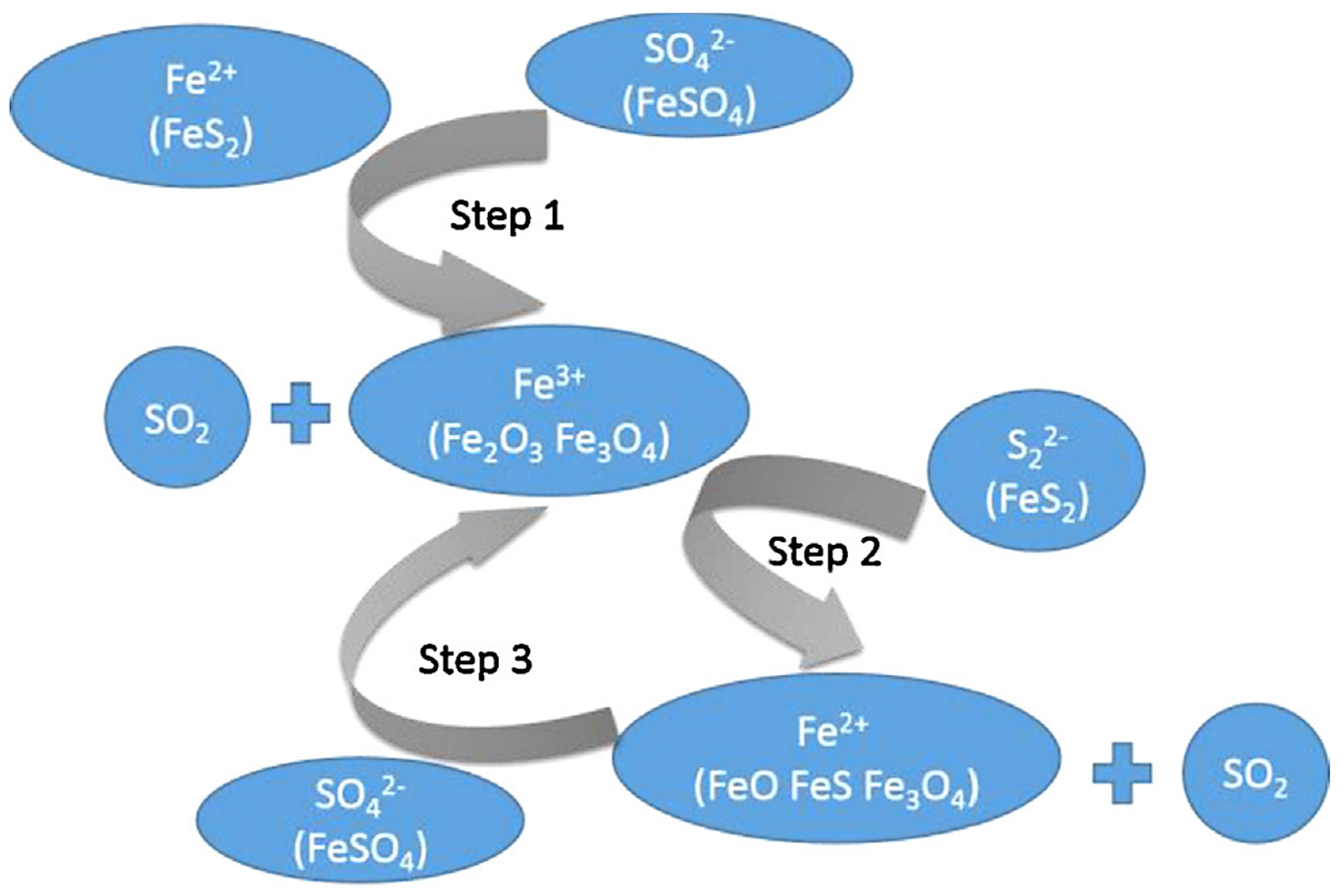
| Ferrous sulfate waste from TiO2 production | Ferrous sulfate reductive decomposition with pyrite, reaction temperature: 580–770 K. The desulfurization rate: 98.55%. | Fe3O4 nano-sized and SO2. | [65] |
| MS iron waste | Fe(II) and Fe(III) precursors from raw MS leached with H2SO4 conc, heating to dryness, solid products used as starting material. Fe3O4: 10 g Fe(II) in 120 mL distilled water, 25% NH4OH, pH 11–12, room temperature, 20 h, black precipitate/γ Fe2O3: 200 °C thermal treatment of Fe3O4/α FeO(OH): 20 g Fe(III) in 500 mL distilled water, 1 M NaHCO3, pH: 5–7, 100 °C, 1 h, room temperature/α Fe2O3: 600–900 °C calcination of α FeO(OH), 5 h. | Fe3O4, α and γ Fe2O3, α FeO(OH) (<0.1 mm), high surface area, various colors orange–brown, brown–red, bright red, maroon, purple and gray. | [66] |
| MS samples | Raw samples pretreatment: attrition milling, sieving, aqua regia digestion: sodium borohydride (NaBH4) and NaOH solution as pH stabilizer: 30–40 nm NPs formation. cetyltrimethylammonium bromide (CTAB) as a cationic surfactant, butanol as cosurfactant, octane as non-aqueous oil phase: 5 nm Fe spherical NPs formation. Hydrazine as stable reducing reagent: 5 nm body-centered cubic Fe NPs. | Nanoscale zerovalent Fe (nZVI), between 5 and 40 nm. | [67] |
| Pollutant | Waste Type | Application, Efficiency | References |
|---|---|---|---|
| Solid residues from air pollution control system (APC) | FeSO4 from TiO2 production as coagulant. | Leaching tests: liquid–solid ratio (L/S): 3 L/kg, stirring, aeration 24 h. 84–123% removal efficiencies for salts (Cl, K, Na); ≤0.001% for heavy metals (Pb: 27–39 μg/L, Cd: 2.6–4.6 μg/L), and 1–5% Cr. | [165] |
| N-NH3, COD | FeSO4⋅7H2O waste from the TiO2 manufacturing industry as a coagulant. | SRPE: 98.19% and 93.86% removal efficiencies for NH3-N and COD, 70 min, 900 mg/L coagulant doses, 62 °C. | [32] |
| pH, suspended solids, turbidity, color, conductivity, metals, hardness, sulfate | poly-alumino-iron sulfate (PAFS) as a coagulant based on iron and aluminum recovered from AMD. | 1000 mL water sample, 0.4 mM (Fe + Al) PAFS-SP/AMD, pH 7.0, 100 rpm, 5 min, 10 min solids settling. All analyzed pollutants were removed and concentrations were under Brazilian standards for drinking water. | [33] |
| As(V) | Fe NPs from local waste materials: iron-coated sand, cast iron filings, steel wool, amended blast furnace slags. | As(V) adsorption from sludge or contaminated water. | [34] |
| As(V) and As(III) | Cast-iron filings (wastes from mechanical workshops, lathes) and steel wool (commercially available, used for cleaning wood surfaces prior to polishing). | 90–95% As removal, increased with increased sorbent dosage from 2 to 20 g/L pH favorable conditions, data fitted with the Langmuir model, sorption reduced progressively from pH 3.0 to 9.0 and decreased beyond 9.0. | [21] |
| As(V) | Fe(III)/Cr(III) hydroxide sludge as waste: Cr(VI) as a corrosion inhibitor in cooling water and electrolytic Fe(II) as a reductive agent for Cr(VI) to Cr(III), pH acid. | As(V) adsorption followed a first-order rate independent of pH (3–10). Desorption with NaOH solutions. pH 4, 20–100 mg/L As, 500 mg waste/50 mL aqueous solution, 5 h, 32 °C, 11.02 mg/g. | [22,23,166] |
| 4-chlorophenol (4-CP) | Graphite-supported zero-valent iron–copper bimetallic catalyst (ZVI-Cu/C): MS waste with spent lithium-ion battery (LIB) anode by carbothermic reduction, 1:4 mass ratios. | 4-CP degradation on the catalyst in water (reduction and heterogeneous Fenton reactions). Spent LIB anode powders (0.5 g, size < 0.15 mm) mixed with MS (0.5, 1.0, 1.5, 2.0 g) of MS, 1000 °C, 2 h, N2 atmosphere (120 cm3·min−1) in a tubular furnace; 100% degradation with higher Fe in ZVI-Cu/C. | [167] |
| Cr(VI) | Scrap with iron air-formed oxides on surfaces. | Batch system, aqueous solutions, pH: 2.10–7.10, temperature: 10–40 °C, Cr(VI): 19.2–576.9 M. | [149] |
| Cd, Ni, Cu | MS as a precursor for Fe3O4, γ Fe2O3 NPs. | ≥90% removal efficiency, after 10 min, Langmuir isotherm data. | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, E.; Predescu, A.M.; Șăulean, A.A.; Râpă, M.; Sohaciu, M.G.; Coman, G.; Berbecaru, A.-C.; Predescu, C.; Vâju, D.; Vlad, G. Ferrous Industrial Wastes—Valuable Resources for Water and Wastewater Decontamination. Int. J. Environ. Res. Public Health 2022, 19, 13951. https://doi.org/10.3390/ijerph192113951
Matei E, Predescu AM, Șăulean AA, Râpă M, Sohaciu MG, Coman G, Berbecaru A-C, Predescu C, Vâju D, Vlad G. Ferrous Industrial Wastes—Valuable Resources for Water and Wastewater Decontamination. International Journal of Environmental Research and Public Health. 2022; 19(21):13951. https://doi.org/10.3390/ijerph192113951
Chicago/Turabian StyleMatei, Ecaterina, Andra Mihaela Predescu, Anca Andreea Șăulean, Maria Râpă, Mirela Gabriela Sohaciu, George Coman, Andrei-Constantin Berbecaru, Cristian Predescu, Dumitru Vâju, and Grigore Vlad. 2022. "Ferrous Industrial Wastes—Valuable Resources for Water and Wastewater Decontamination" International Journal of Environmental Research and Public Health 19, no. 21: 13951. https://doi.org/10.3390/ijerph192113951






