Genotoxicity and Reproductive Risk in Workers Exposed to Pesticides in Rural Areas of Curicó, Chile: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Fieldwork and Blood Sampling
2.3. Micronucleus Assay (MN)
2.4. Reproductive Risk
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic Description
3.2. Cytogenetic Damage
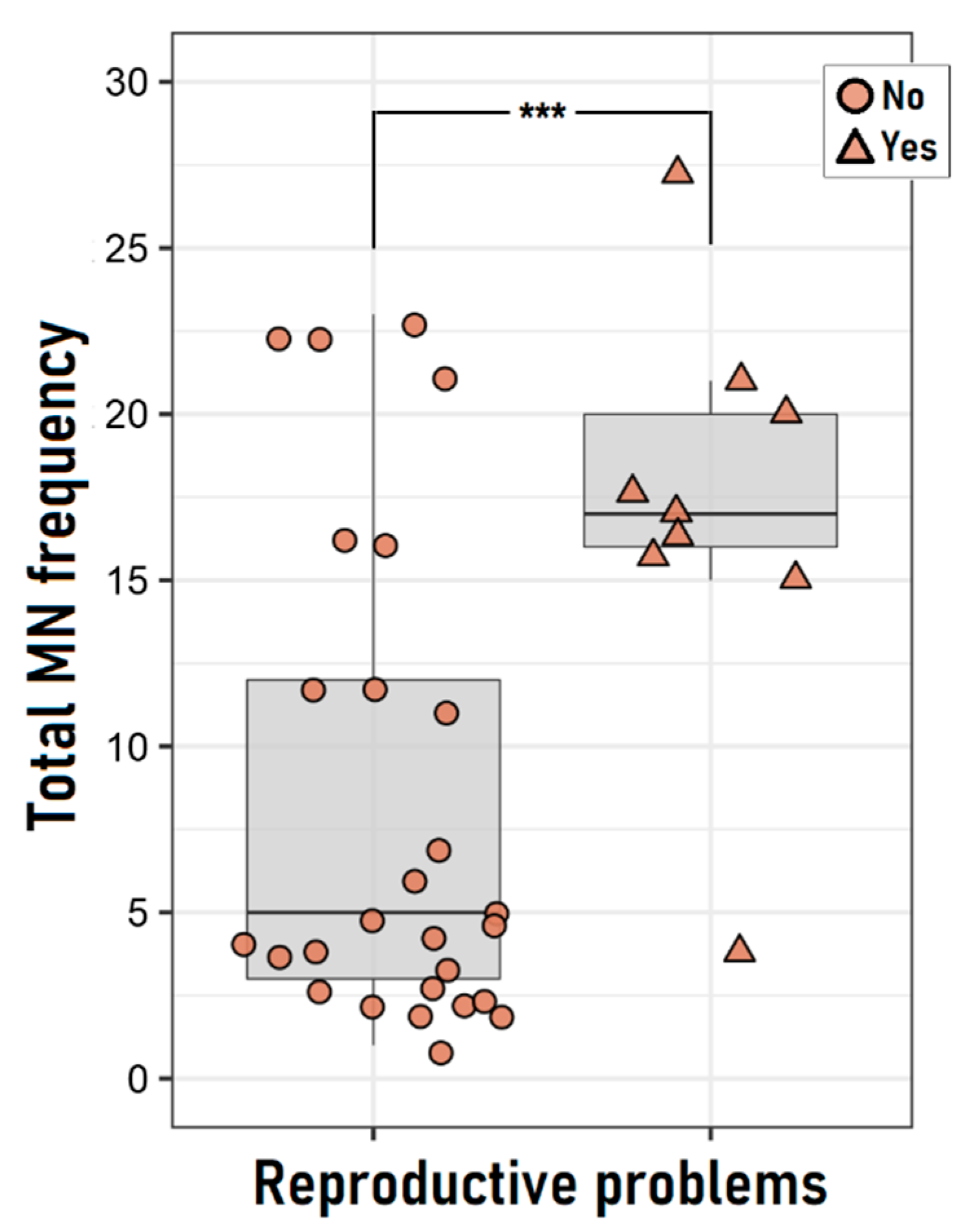
3.3. Reproductive Problems and Pesticide Exposure
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ Exposure to Pesticides: Toxicity Types and Ways of Prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M.; la Cecilia, D.; McBratney, A. PEST-CHEMGRIDS, global gridded maps of the top 20 crop-specific pesticide application rates from 2015 to 2025. Sci. Data 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Jepson, P.C.; Murray, K.; Bach, O.; Bonilla, M.A.; Neumeister, L. Selection of pesticides to reduce human and environmental health risks: A global guideline and minimum pesticides list. Lancet Planet. Health 2020, 4, e56–e63. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.P.; Poteser, M.; Lemmerer, K.; Wallner, P.; Sanavi, S.S.; Kundi, M.; Moshammer, H.; Weitensfelder, L. Indicators of Genotoxicity in Farmers and Laborers of Ecological and Conventional Banana Plantations in Ecuador. Int. J. Environ. Res. Public Health 2020, 17, 1435. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M.; McManus, I.C.; Harrison, V.; Mason, O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: A systematic and meta-analytic review. Crit. Rev. Toxicol. 2013, 43, 21–44. [Google Scholar] [CrossRef]
- Brender, J.D.; Maantay, J.A.; Chakraborty, J. Residential proximity to environmental hazards and adverse health outcomes. Am. J. Public Health 2011, 101 (Suppl. S1), S37–S52. [Google Scholar] [CrossRef]
- Gatto, M.P.; Cabella, R.; Gherardi, M. Climate change: The potential impact on occupational exposure to pesticides. Ann. Ist. Super. Sanita 2016, 52, 374–385. [Google Scholar]
- Noyes, P.D.; McElwee, M.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Weiss, F.T.; Leuzinger, M.; Zurbrügg, C.; Eggen, H.I. Chemical Pollution in Low- and Middle-Income Countries; Swiss Federal Institute of Aquatic Science and Technology: Dübendorf, Switzerland, 2016; p. 163. [Google Scholar]
- Bolognesi, C.; Creus, A.; Ostrosky-Wegman, P.; Marcos, R. Micronuclei and pesticide exposure. Mutagenesis 2011, 26, 19–26. [Google Scholar] [CrossRef]
- Ladeira, C.; Smajdova, L. The use of genotoxicity biomarkers in molecular epidemiology: Applications in environmental, occupational and dietary studies. AIMS Genet. 2017, 4, 166–191. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Venegas, L.A.; Hyland, C.; Muñoz-Quezada, M.T.; Quirós-Alcalá, L.; Butinof, M.; Buralli, R.; Cardenas, A.; Fernandez, R.A.; Foerster, C.; Gouveia, N.; et al. Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ. Health Perspect. 2022, 130, 96002. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Wigle, D.T.; Krewski, D. Residential pesticides and childhood leukemia: A systematic review and meta-analysis. Cien. Saude Colet. 2011, 16, 1915–1931. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Libotte, V.; Willems, J.; Lison, D. Review and meta-analysis of risk estimates for prostate cancer in pesticide manufacturing workers. Cancer Causes Control 2006, 17, 353–373. [Google Scholar] [CrossRef]
- Weichenthal, S.; Moase, C.; Chan, P. A review of pesticide exposure and cancer incidence in the agricultural health study cohort. Cien. Saude Colet. 2012, 17, 255–270. [Google Scholar] [CrossRef]
- Burns, C.J.; Juberg, D.R. Cancer and occupational exposure to pesticides: An umbrella review. Int. Arch. Occup. Environ. Health 2021, 94, 945–957. [Google Scholar] [CrossRef]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Czajka, M.; Matysiak-Kucharek, M.; Jodłowska-Jędrych, B.; Sawicki, K.; Fal, B.; Drop, B.; Kruszewski, M.; Kapka-Skrzypczak, L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ. Res. 2019, 178, 108685. [Google Scholar] [CrossRef]
- Singh, C.; Ahmad, I.; Kumar, A. Pesticides and metals induced Parkinson’s disease: Involvement of free radicals and oxidative stress. Cell Mol. Biol. (Noisy-le-grand) 2007, 53, 19–28. [Google Scholar]
- Song, C.; Kanthasamy, A.; Anantharam, V.; Sun, F.; Kanthasamy, A.G. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: Relevance to epigenetic mechanisms of neurodegeneration. Mol. Pharmacol. 2010, 77, 621–632. [Google Scholar] [CrossRef]
- Márquez, C.; Villalobos, C.; Poblete, S.; Villalobos, E.; de Los Angeles García, M.; Duk, S. Cytogenetic damage in female Chilean agricultural workers exposed to mixtures of pesticides. Environ. Mol. Mutagen. 2005, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Snijder, C.A.; Brouwers, M.M.; Jaddoe, V.W.; Hofman, A.; Roeleveld, N.; Burdorf, A. Occupational exposure to endocrine disruptors and time to pregnancy among couples in a large birth cohort study: The Generation R Study. Fertil. Steril. 2011, 95, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Kshetrimayum, C. Environmental & occupational exposure & female reproductive dysfunction. Indian J. Med. Res. 2019, 150, 532–545. [Google Scholar] [PubMed]
- Roberts, T.C. The MicroRNA Biology of the Mammalian Nucleus. Mol. Ther.-Nucleic Acids 2014, 3, e188. [Google Scholar] [CrossRef] [PubMed]
- Cortes, S.; Pozo, K.; Llanos, Y.; Martinez, N.; Foerster, C.; Leiva, C.; Ustáriz, J.; Přibylová, P.; Klánová, J.; Jorquera, H. First measurement of human exposure to current use pesticides (CUPs) in the atmosphere of central Chile: The case study of Mauco cohort. Atmos. Pollut. Res. 2020, 11, 776–784. [Google Scholar] [CrossRef]
- OECD/FAO (Organization for Economic Cooperation and Development, F.A.O.). AOCED-FAO Agricultural Outlook 2019–2028. In Special Focus: Latin America; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Servicio Agricola y Ganadaro (SAG). División Protección Agrícola y Forestal. Departamento Regulación y Control de Insumos Silvoagrícolas Subdepartamento de Plaguicidas y Fertilizantes. Ministerio de Agricultura. Declaración De Ventas De Plaguicidas De Uso Agrícola Año 2019; SAG. Santiago de Chile. 2019, p. 146. Available online: https://www.sag.gob.cl/sites/default/files/declaracion_de_ventas_de_plaguicidas_ano_2019_0.pdf (accessed on 21 October 2022).
- Muñoz-Quezada, M.T.; Lucero, B.; Iglesias, V.; Muñoz, M.P. Exposure pathways to pesticides in schoolchildren in the Province of Talca, Chile. Gac. Sanit. 2014, 28, 190–195. [Google Scholar] [CrossRef]
- Ramírez-Santana, M.; Farías-Gómez, C.; Zúñiga-Venegas, L.; Sandoval, R.; Roeleveld, N.; Van der Velden, K.; Scheepers, P.T.J.; Pancetti, F. Biomonitoring of blood cholinesterases and acylpeptide hydrolase activities in rural inhabitants exposed to pesticides in the Coquimbo Region of Chile. PLoS ONE 2018, 13, e0196084. [Google Scholar] [CrossRef]
- Ramírez-Santana, M.; Zúñiga-Venegas, L.; Corral, S.; Roeleveld, N.; Groenewoud, H.; van der Velden, K.; Scheepers, P.T.J.; Pancetti, F. Association between cholinesterase’s inhibition and cognitive impairment: A basis for prevention policies of environmental pollution by organophosphate and carbamate pesticides in Chile. Environ. Res. 2020, 186, 109539. [Google Scholar] [CrossRef]
- Muñoz-Quezada, M.T.; Lucero, B.; Iglesias, V.; Levy, K.; Muñoz, M.P.; Achú, E.; Cornejo, C.; Concha, C.; Brito, A.M.; Villalobos, M. Exposure to organophosphate (OP) pesticides and health conditions in agricultural and non-agricultural workers from Maule, Chile. Int. J. Environ. Health Res. 2017, 27, 82–93. [Google Scholar] [CrossRef]
- Cabello, G.; Valenzuela-Estrada, M.; Siques, P.; Brito, J.; Parra, E.; Valdivia, U.; Lavin, C.; Manríquez, A.; Ortega, A. Relation of Breast Cancer and Malathion Aerial Spraying in Arica, Chile. Int. J. Morphol. 2013, 31, 640–645. [Google Scholar] [CrossRef][Green Version]
- Venegas, L.A.Z.; Urrizola, C.G.M.; Palacios, M.S.D. Estudio citogenético y reproductivo en mujeres temporeras expuestas a pesticidas de la Vlll Región de Chile. Theoria 2007, 16, 77–87. [Google Scholar]
- Zúñiga-Venegas, L.; Pancetti, F.C. DNA damage in a Chilean population exposed to pesticides and its association with PON1 (Q192R and L55M) susceptibility biomarker. Environ. Mol. Mutagen. 2022, 63, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Knasmueller, S.; Nersesyan, A.; Thomas, P.; Fenech, M. The HUMNxl scoring criteria for different cell types and nuclear anomalies in the buccal micronucleus cytome assay—An update and expanded photogallery. Mutat. Res. 2013, 753, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Medina-Carrilo, L.; Rivas-Solis, F.; Fernández-Argüelles, R. Risk for congenital malformations in pregnant women exposed to pesticides in the state od Nayarit, Mexico. Ginecol. Obstet. Mex. 2002, 70, 538–544. [Google Scholar] [PubMed]
- Baldacci, S.; Gorini, F.; Santoro, M.; Pierini, A.; Minichilli, F.; Bianchi, F. Environmental and individual exposure and the risk of congenital anomalies: A review of recent epidemiological evidence. Epidemiol. Prev. 2018, 42, 1–34. [Google Scholar]
- Pagès, J. Analyse Factorielle de Donnees Mixtes. Revue. Stat. Appl. 2004, 4, 93–111. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 21 October 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Occupational pesticide exposures and respiratory health. Int. J. Environ. Res. Public Health 2013, 10, 6442–6471. [Google Scholar] [CrossRef]
- Zúñiga-Venegas, L.; Muñoz-Quezada, M.T.; Hyland, C.; Butinof, M.; Calaf, G.; Handal, A.J.; Quirós-Alcalá, L.; Cortés, S.; Mora, A.M. Pesticide exposure and health effects in Latin American and the Caribbean populations: A systematic review. Environ. Health Perspect. 2022, 130, 96002. [Google Scholar] [CrossRef] [PubMed]
- Kahl, V.F.S.; da Silva, F.R.; Alves, J.D.S.; da Silva, G.F.; Picinini, J.; Dhillon, V.S.; Fenech, M.; de Souza, M.R.; Dias, J.F.; de Souza, C.T.; et al. Role of PON1, SOD2, OGG1, XRCC1, and XRCC4 polymorphisms on modulation of DNA damage in workers occupationally exposed to pesticides. Ecotoxicol. Environ. Saf. 2018, 159, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-López, Y.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Calderón-Segura, M.E.; Martínez-Arroyo, A. Biomonitoring of agricultural workers exposed to pesticide mixtures in Guerrero state, Mexico, with comet assay and micronucleus test. Environ. Sci. Pollut Res. 2016, 23, 2513–2520. [Google Scholar] [CrossRef]
- How, V.; Hashim, Z.; Ismail, P.; Omar, D.; Said, S.M.; Tamrin, S.B. Characterization of risk factors for DNA damage among paddy farm worker exposed to mixtures of organophosphates. Arch. Environ. Occup. Health 2015, 70, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Venegas, L.; Saracini, C.; Pancetti, F.; Muñoz-Quezada, M.T.; Lucero, B.; Foerster, C.; Cortés, S. Pesticide exposure in Chile and population health: Urgency for decision making. Gac. Sanit. 2021, 35, 480–487. [Google Scholar] [CrossRef]
- Doss, C.R. Women and agricultural productivity: Reframing the Issues. Dev. Policy Rev. 2018, 36, 35–50. [Google Scholar] [CrossRef]
- Fucic, A.; Duca, R.C.; Galea, K.S.; Maric, T.; Garcia, K.; Bloom, M.S.; Andersen, H.R.; Vena, J.E. Reproductive Health Risks Associated with Occupational and Environmental Exposure to Pesticides. Int. J. Environ. Res. Public Health 2021, 18, 6576. [Google Scholar] [CrossRef]
- Rahimi, T.; Rafati, F.; Sharifi, H.; Seyedi, F. General and reproductive health outcomes among female greenhouse workers: A comparative study. BMC Women’s Health 2020, 20, 103. [Google Scholar] [CrossRef]
- Llanos, Y.; Cortés, S.; Martínez, A.; Pozo, K.; Přibylová, P.; Klánová, J.; Jorquera, H. Local and regional sources of organochlorine pesticides in a rural zone in central Chile. Atmos. Pollut. Res. 2022, 13, 101411. [Google Scholar] [CrossRef]
- Anguiano-Vega, G.A.; Cazares-Ramirez, L.H.; Osten, J.R.-V.; Santillan-Sidon, A.P.; Vazquez-Boucard, C.G. Risk of genotoxic damage in schoolchildren exposed to organochloride pesticides. Sci. Rep. 2020, 10, 17584. [Google Scholar] [CrossRef]
- Montes, L.P.B.; Waliszewski, S.; Hernández-Valero, M.; Sanín-Aguirre, L.; Infanzón-Ruiz, R.M.; Jañas, A.G. Prenatal exposure to organochlorine pesticides and cryptorchidism. Cien. Saude Colet. 2010, 15 (Suppl. S1), 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Souza, M.O.C.; Filho, G.L.A.; Krauss, T.M.; Pavesi, T.; Silva, L.E. Organochlorine compound levels in fertile and infertile women from Rio de Janeiro, Brazil. Arq. Bras. Endocrinol. Metabol. 2013, 57, 346–353. [Google Scholar] [CrossRef][Green Version]
- Blanco-Muñoz, J.; Lacasaña, M.; Aguilar-Garduño, C.; Rodríguez-Barranco, M.; Bassol, S.; Cebrián, M.E.; López-Flores, I.; Ruiz-Pérez, I. Effect of exposure to p, p′-DDE on male hormone profile in Mexican flower growers. Occup. Environ. Med. 2012, 69, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Koifman, R.J.; Sarcinelli, P.N.; Rosa, A.C.; Clapauch, R.; Koifman, S. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int. J. Hyg. Environ. Health 2014, 217, 370–378. [Google Scholar] [CrossRef] [PubMed]



| Sociodemographic | Unexposed (n = 30) | Exposed (n = 30) | p-Value |
|---|---|---|---|
| Age (years ± SD) a | 39.6 ± 12.0 | 39.8 ± 12.7 | 0.93 |
| Exposure time (years ± SD) | - | 9.7 ± 9.1 | - |
| Sex b | |||
| Men (n, %) | 10 (33) | 12 (40) | 0.40 |
| Women (n, %) | 20 (67) | 18 (60) | |
| Smoke habit b | |||
| No (n, %) | 22 (73) | 13 (43) | 0.02 |
| Yes (n, %) | 8 (27) | 17 (57) |
| Cytogenetic Damage a | Unexposed (n = 30) | Exposed (n = 30) | p-Value |
| Total MN | 4.2 ± 2.5 | 17.1 ± 4.9 | <0.001 |
| NDI | 1.9 ± 0.1 | 1.9 ± 0.1 | 0.690 |
| NPB | 0.1 ± 0.5 | 1.3 ± 1.2 | <0.001 |
| NBUD | 0.1 ± 0.3 | 0.6 ± 0.9 | 0.002 |
| Reproductive Problems b,c | Unexposed (n = 20) | Exposed (n = 16) | p-Value |
| Women with reproductive problems | 1 (5.0) | 8 (50) | 0.003 |
| Women with spontaneous abortion | 1 (5.0) | 5 (31.3) | 0.049 |
| Women with children with malformation | 0 (0) | 3 (18.8) | 0.078 |
| Infertility | 0 (0) | 2 (12.5) | 0.190 |
| Explanatory Variables | Coefficient | Model | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | R2 | Adjusted R2 | p-Value | |
| Group | 13.27 | 11.08–15.470 | <0.001 | 0.75 | 0.74 | 0.002 |
| Age | 0.05 | −0.04–0.13 | 0.279 | |||
| Sex | 1.09 | −1.10–3.28 | 0.321 | |||
| Smoker | −0.97 | −3.26–1.31 | 0.397 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landeros, N.; Duk, S.; Márquez, C.; Inzunza, B.; Acuña-Rodríguez, I.S.; Zúñiga-Venegas, L.A. Genotoxicity and Reproductive Risk in Workers Exposed to Pesticides in Rural Areas of Curicó, Chile: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 16608. https://doi.org/10.3390/ijerph192416608
Landeros N, Duk S, Márquez C, Inzunza B, Acuña-Rodríguez IS, Zúñiga-Venegas LA. Genotoxicity and Reproductive Risk in Workers Exposed to Pesticides in Rural Areas of Curicó, Chile: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(24):16608. https://doi.org/10.3390/ijerph192416608
Chicago/Turabian StyleLanderos, Natalia, Soledad Duk, Carolina Márquez, Bárbara Inzunza, Ian S. Acuña-Rodríguez, and Liliana A. Zúñiga-Venegas. 2022. "Genotoxicity and Reproductive Risk in Workers Exposed to Pesticides in Rural Areas of Curicó, Chile: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 24: 16608. https://doi.org/10.3390/ijerph192416608
APA StyleLanderos, N., Duk, S., Márquez, C., Inzunza, B., Acuña-Rodríguez, I. S., & Zúñiga-Venegas, L. A. (2022). Genotoxicity and Reproductive Risk in Workers Exposed to Pesticides in Rural Areas of Curicó, Chile: A Pilot Study. International Journal of Environmental Research and Public Health, 19(24), 16608. https://doi.org/10.3390/ijerph192416608






