Human Breast Milk Contamination with Aflatoxins, Impact on Children’s Health, and Possible Control Means: A Review
Abstract
:1. Introduction
2. Routes of Breast Milk Contamination with AFM1
2.1. Diet
2.2. Feed as an Indirect Source of Human Breast Milk Contamination with AFM1
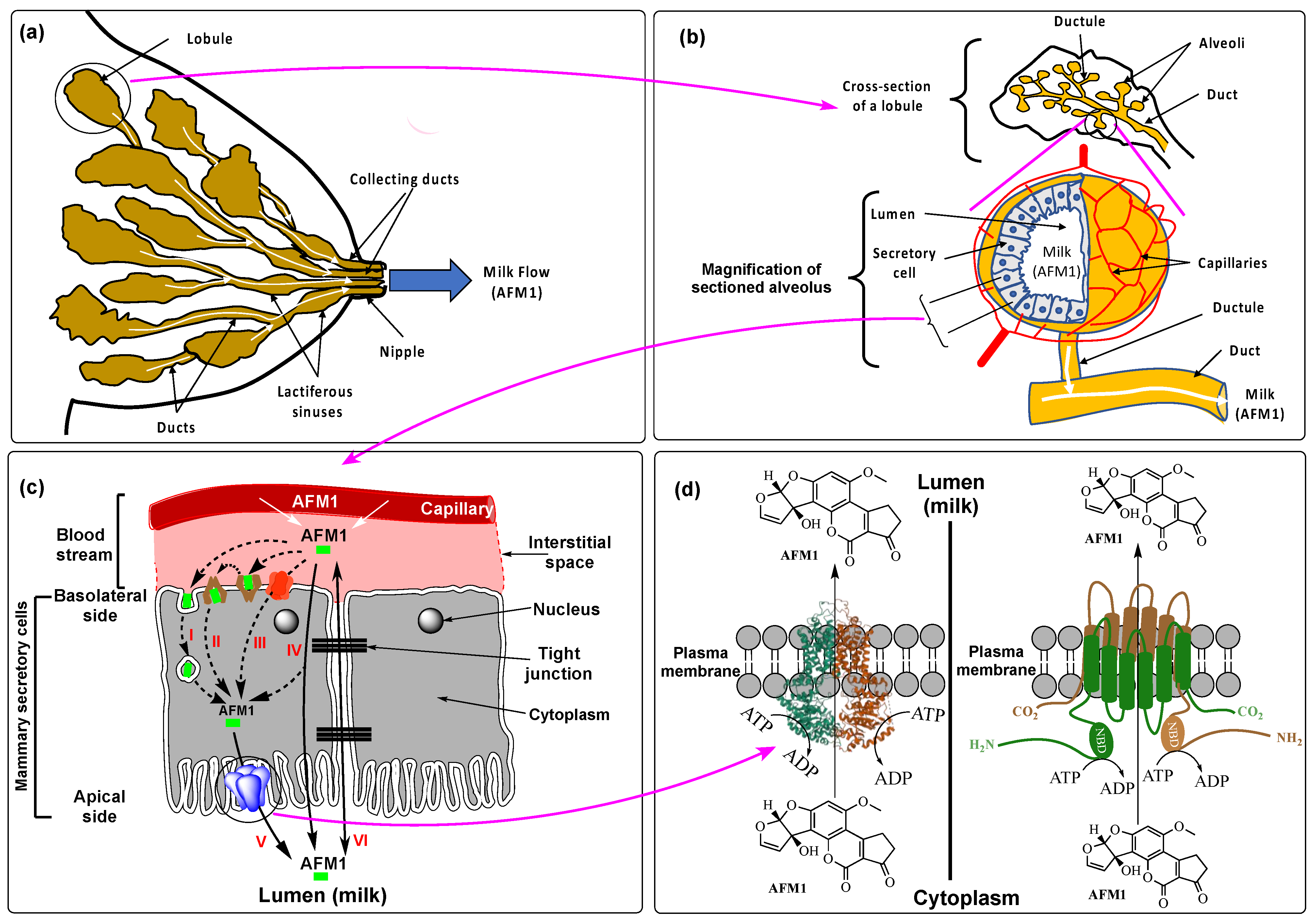
3. Physiological Mechanisms of AFM1 Secretion into Breast Milk
4. Adverse Health Effects of AFM1 on Infants and Young Children
4.1. Growth Impairment
4.2. Other AFM1-Related Health Issues
5. Risk Assessment of AFM1 on Infant and Children’s Health
5.1. Prevalence and Extent of Breast Milk Contamination with AFM1 around the World
| Country | Period of the Study (Month/Year) | Mean Concentration (Range) | Positive/Total Samples (%) | Analytical Technique | Clean-Up | LOD/LOQ (ng/L) | Reference |
|---|---|---|---|---|---|---|---|
| Africa | |||||||
| Egypt | 04/2000–05/2002 | 300.0 (20–2090.0) | 66/120 (55.0) | HPLC-FD | IAC | NS/NS | [155] |
| Egypt | 05–09/2003 | 13.5 1 (5.60–5131.0) | 138/388 (36.0) | HPLC-FD | SPE | NS/NS | [32] |
| Egypt | [159] | ||||||
| NS | NS | 248/443 (56.0) | HPLC-FD | SPE | 4/NS | |
| NS | 8.0 (4.2–108.0) | 12/50 (24.0) | HPLC-FD | SPE | 4/NS | |
| NS | 60.0 (6.3–497.0) | 24/26 (92.0) | HPLC-FD | SPE | 4/NS | |
| Egypt | 03–08/2010 | 74.0 (7.3–328.6) | 87/125 (69.7) | ELISA | ND | NS/NS | [49] |
| Morocco | 11–12/2017 | 5.8 (<LOD-13.3) | 43/82 (52.4) | ELISA | ND | 5/NS | [160] |
| Nigeria | 04–06/2006 | NS (<LOD-4000.0) | 2/10 (20.0) | TLC | ND | 2000 | [161] |
| Nigeria | [46] | ||||||
| 06–10/2010 | 35.0 (42.7–92.1) | 15/15 (100.0) | HPLC-FD | IAC | 10/50 | |
| 06–10/2010 | 6.5 (<LOD-18.6) | 12/18 (66.7) | HPLC-FD | IAC | 10/50 | |
| 06–10/2010 | 3.5 (<LOD-5.4) | 41/50 (82.0) | HPLC-FD | IAC | 10/50 | |
| Nigeria | NS | 3.9 (2.0–11.0) | 10/75 (13.3) | HPLC-MS/MS | SPE | 2.0/NS | [12] |
| Sudan | NS | 401.0 (13.0–2561.0) | 51/94 (54.0) | HPLC-FD | LLE | 13/NS | [156] |
| Kenya | [162] | ||||||
| NS | 8.46 (0.200–47.5) 10.83 (1.4–152.7) | 85/98 (86.7) 4/18 (22.2) | ELISA HPLC | LLE NS | 5/NS NS/NS | |
| NS | 0.02 (0.003–3.7) 0.06 (0.5–0.8) | 38/67 (56.7) 2/21 (9.5) | ELISA HPLC | LLE NS | 5/NS NS/NS | |
| Ethiopia | [151] | ||||||
| 08/2017–03/2018 | 1.1 1 (<LOD-143.3) | 360/232 (64.4) | ELISA | ND | 5/NS | |
| 08/2017–03/2018 | 2.6 1 (0.9–9.3) | 101/120 (84.2) | ELISA | ND | 5/NS | |
| 08/2017–03/2018 | 1.0 1 (<5.0–1.9) | 58/120 (48.3) | ELISA | ND | 5/NS | |
| 08/2017–03/2018 | 0.6 1 (<5.0–7.9) | 73/120 (60.8) | ELISA | ND | 5/NS | |
| 08/2017–03/2018 | 0.9 1 (<5.0–2.9) | 115/180 (63.9) | ELISA | ND | 5/NS | |
| 08/2017–03/2018 | 1.2 1 (<5.0–10.0) | 117/180 (65.0) | ELISA | ND | 5/NS | |
| Tanzania | 11/2011–02/2012 | 80.0 (10.0–550.0) | 143/143 (100.0) | HPLC-FD | IAC | 5/NS | [128] |
| Cameroun | NS/1991-NS/1995 | NS (5.0–625.0) | 3/62 (4.8) | HPLC-FD | LLE | NS | [158] |
| Angola | 8–9/2018–8/2019 | ND | 0/37 (0.0) | ELISA | ND | 5.0/NS | [150] |
| Asia | |||||||
| UAE | 01/1999–12/2000 | 560.0 1 (123.5–940.0) | 140/129 (92.1) | LC-UV | LLE | NS | [163] |
Lebanon
| 11/2015–12/2016 | 4.1 (0.2–7.9) 5.0 (2.2–7.8) | 41/43 (93.8) 63/68 (92.6) | ELISA | ND | NS/0.20 | [47] |
| Turkey | NS/2006-NS/2007 | 5.7 (5.1–6.9) | 8/61 (13.0) | HPLC-FD | ND | NS | [164] |
| Turkey | 10/2007–03/2008 | NS (61.0–300.0) | 75/75 (100.0) | HPLC-FD | LLE | 5/NS | [165] |
| Turkey | 12/2008–04/2009 | 3.0 (1.3–6.0) | 18/73 (25.0) | ELISA | ND | 10/NS | [166] |
| Turkey | 12/2014–06/2015 | 19.0 (9.6–80.0) | 66/74 (89.2) | ELISA | ND | 5/NS | [167] |
| Turkey | 10–11/2017 | 6.4 (5.1–8.3) | 53/100 (53.0) | ELISA | ND | 5/NS | [168] |
| Turkey | 06/2017–03/2018 | 3.1 1 (<2.0–5.5) | NA/122 | ELISA | ND | NS | [13] |
| Turkey | 12/2018–06/2019 | 12.2 (5.0–23.2) | 75/90 (83.3) | ELISA | ND | 5/NS | [153] |
| Iran | 11/2003–03/2004 | 9.5 (7.1–10.8) | 8/132 (6.1) | ELISA | ND | 5/NS | [169] |
| Iran | 05/NS-09/2006 | 8.0 (<LOD-27.0) | 157/160 (98.0) | ELISA | ND | 5/NS | [123] |
| Iran | 03–04/2007 | 7.00 (5.1–8.1) | 20/182 (11.0) | ELISA | SPE | 5\NS | [124] |
| Iran | NS | 6.8 4 | 1/80 (1.3) | ELISA | ND | 5/NS | [170] |
| Iran | 05–08/2011 | 20 4 | 1/136 (0.7) | HPLC-FD | LLE | NS | [171] |
| Iran | 06–07/2011 | 0.6 (0.1–5.0) | 24/87 (28.0) | ELISA | ND | NS | [172] |
| Iran | 04–10/2014 | 5.9 (2.0–10.0) | 85/85 (100.0) | ELISA | ND | NS | [173] |
| Iran | 09/2015–04/2016 | 14.7 (5.0–41.3) | 98/150 (65.0) | ELISA | ND | 10/NS | [174] |
| Iran | 04/2016–01/2017 | 4.1 (3.2–8.8) | 84/84 (100.0) | ELISA | ND | NS/25 | [175] |
| Iran | 06/2020–03/2021 | 7.1 (5.4–9.0) | 39/100 (39.0) | ELISA | ND | 5/NS | [176] |
| Jordan | 02/2011–02/2012 | 67.8 (9.7–137.2) | 80/80 (100.0) | ELISA | ND | NS/NS | [177] |
| Nepal | NS/2015–2017 5 | 4.5 (LOD-316.0) | 1355/1439 (94.0) | HPLC-FD | IAC | 0.04/NS | [178] |
| India | 07/2017–06/2018 | 13.7 1 (3.9–1200.0) | 41/100 (41.0) | UHPLC-MS/SRM | LLE | 7.8/15.6 | [157] |
| Bangladesh | 10/2019–03/2020 | 4.4 (LOD-6.7) | 32/62 (51.6) | ELISA | ND | 4.0/NS | [179] |
| Latin America | |||||||
| Brazil | NS/2012 | LOD > LOQ | 2/100 (2.0) | HPLC-FD | IAC | 0.3/0.8 | [180] |
| Brazil | NS. | 18.0 (<LOD-25.0) | 5/94 (5.3) | HPLC-FD | IAC | 4.0/21.0 | [79] |
| Colombia | 05–09/2013 | 5.2 (LOD-18.5) | 45/50 (90.0) | HPLC-FD | IAC | 1.0/2.0 | [181] |
Central Mexico
| 01–08/2014 | ELISA | ND | 0.92/2.79 | [154] | ||
| 10.9 (3.0–34.2) | 100/112 (89.3) | ||||||
| 12.8 (3.8–20.9) | 20/20 (100.0) | ||||||
| 12.1 (3.0–34.2) | 35/42 (83.3) | ||||||
| 7.9 (3.2–18.9) | 45/50 (90.0) | ||||||
| Mexico | NS 6/2012 | 17.0 (5.0–66.2) | 123/123 (100.0) | ELISA | ND | 5/NS | [182] |
| Guatemala | 06/NS–10/2014 | 13.0 (4.0–333.0) | 14/286 (4.9) | HPLC/MS | IAC | NS | [183] |
| Europe | |||||||
| Italy | 01/NS-12/2006 | 55.0 (<LOQ-140.0) | 4/82 (4.88) | HPLC-FD | IAC | 3/7 | [152] |
Portugal
| NS/2015-NS/2016 | ELISA | [184] | ||||
| 7.4 (5.1–10.6) | 22/37 (32.8) | ND | 5/NS | ||||
| 8.0 (>LOD-10.6) | 11/31 (35.5) | ND | |||||
| 6.8 (<LOD-8.9) | 10/25 (40.0) | ND | |||||
| 6.7 (<LOD-6.7) | 01/11 (9.1) | ND | |||||
| Serbia | 01/NS-05/2013 | 10 (6.0–22.0) | 10/10 (100.0) | ELISA | ND | 1.5/5 | [185] |
5.2. Exposure of Infants and Children to AFM1 via Breast Milk and Related Health Risks
6. Regulations
7. Measures to Control AFM1 Levels in Breast Milk
- The level of the mothers’ diet to reduce the intake of AFB1 and AFM1 during pregnancy and nursing to the lowest possible levels. Unfortunately, the socioeconomic considerations in many of developing countries, regulations on aflatoxins are lacking, too permissive, or loosely implemented due to poor administrative and analytical capabilities. On the other hand, setting strict regulations on aflatoxins in foods and enforcing them rigorously to protect consumers in general and pregnant and lactating women, may not be a realistic solution under the present economic and technological conditions in most developing countries. This issue has long been debated; and its opponents raise the argument that it would lead to food shortage with more dramatic health effects on the mother and child, as most of the agri-food production would be condemned because of deviations from the standards [120].
- At the level of breast milk itself to distinguish safe from unsafe breast milk on the basis AFM1 content as is the case for infant foods. Although this can be feasible for breast milk banks where milks exceeding a regulatory concentration (e.g., 0.025 ng/g) can be discarded, it is not technically possible at individual level of lactating mothers. No official control of the mothers’ milk to feed their children can be realistically applied.
- Organization of campaigns to raise awareness and sensitization of women before and after delivery about the health effect of aflatoxins.
- Adoption of biocontrol practices in agriculture by using atoxigenic strains of A. flavus.
- Actions to improve harvest and post-harvest practices, including the storage structures and use of hermetic bags.
- Application of dietary interventions aiming to reduce the dietary exposure of mothers and children to aflatoxins.
- Monitoring crops for aflatoxin contamination and communicating the results to farmers.
- Modern market development.
- Technology transfer for manufacturing and distribution.
- Policy developments and capacity building to monitor, regulate, and control food contamination with aflatoxins.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannan, M.A.; Faraji, B.; Tanguma, J.; Longoria, N.; Rodriguez, R.C. Maternal milk concentration of zinc, iron, selenium, and iodine and its relationship to dietary intakes. Biol. Trace Elem. Res. 2009, 127, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma. Immunol. 1998, 81, 523–533, quiz 533–534, 537. [Google Scholar] [CrossRef] [PubMed]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.H.; Zhang, G.; Doherty, D.A.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.J.; et al. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, e0117038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- CDC. Brestfeeding. 2021. Available online: https://www.cdc.gov/breastfeeding/faq/index.htm#howlong (accessed on 28 December 2021).
- Barness, L.A.; Mauer, A.M.; Holliday, M.A.; Anderson, A.S.; Dallman, P.R.; Forbes, G.B.; Goldbloom, R.B.; Haworth, J.C.; Jesse, M.J.; Scriver, C.R.; et al. Commentary on Breast-Feeding and Infant Formulas, Including Proposed Standards for Formulas. Pediatrics 1976, 57, 278–285. [Google Scholar] [CrossRef]
- Ekeanyanwu, C.L.; Alisi, C.S.; Ekeanyanwu, R.C. Levels of Aflatoxin M1 and selected heavy metals (Pb, Cd, Cr, Cu, Zn, Fe, As, and Hg) in the breast milk of lactating mothers in South Eastern, Nigeria. Food Control 2020, 112, 107150. [Google Scholar] [CrossRef]
- García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef] [Green Version]
- Mahnke, H.; Ballent, M.; Baumann, S.; Imperiale, F.; von Bergen, M.; Lanusse, C.; Lifschitz, A.L.; Honscha, W.; Halwachs, S. The ABCG2 Efflux Transporter in the Mammary Gland Mediates Veterinary Drug Secretion across the Blood-Milk Barrier into Milk of Dairy Cows. Drug Metab. Dispos. 2016, 44, 700. [Google Scholar] [CrossRef] [Green Version]
- Memis, E.Y.; Yalcın, S.S.; Yalcın, S. Mycotoxin carry-over in breast milk and weight of infant in exclusively-breastfed infants. Arch. Environ. Occup. Health 2021, 76, 313–318. [Google Scholar] [CrossRef]
- Warth, B.; Braun, D.; Ezekiel, C.N.; Turner, P.C.; Degen, G.H.; Marko, D. Biomonitoring of Mycotoxins in Human Breast Milk: Current State and Future Perspectives. Chem. Res. Toxicol. 2016, 29, 1087–1097. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.; Abia, W.A.; Šarkanj, B.; Sulyok, M.; Waldhoer, T.; Erber, A.C.; Krska, R.; Turner, P.C.; Marko, D.; Ezekiel, C.N.; et al. Mycotoxin-mixture assessment in mother-infant pairs in Nigeria: From mothers’ meal to infants’ urine. Chemosphere 2022, 287, 132226. [Google Scholar] [CrossRef] [PubMed]
- Memis, E.Y.; Yalcın, S.S. Human milk mycotoxin contamination: Smoking exposure and breastfeeding problems. J. Matern. Fetal Neonatal. Med. 2021, 34, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Augustyniak, J.; Buzanska, L.; Caloni, F. Species-specific models in toxicology: In vitro epithelial barriers. Environ. Toxicol. Pharmacol. 2019, 70, 103203. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Yiannikouris, A.; Jouany, J.-P. Mycotoxins in feeds and their fate in animals: A review. Anim. Res. 2002, 51, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Ghadiri, S.; Spalenza, V.; Dellafiora, L.; Badino, P.; Barbarossa, A.; Dall’Asta, C.; Nebbia, C.; Girolami, F. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicol. Vitr. 2019, 57, 174–183. [Google Scholar] [CrossRef]
- Smith, J.W.; Groopman, J.D. Aflatoxins. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 30–43. [Google Scholar]
- Loveland, P.M.; Wilcox, J.S.; Pawlowski, N.E.; Bailey, G.S. Metabolism and DNA binding of aflatoxicol and aflatoxin B1 in vivo and in isolated hepatocytes from rainbow trout (Salmo gairdneri). Carcinogenesis 1987, 8, 1065–1070. [Google Scholar] [CrossRef]
- Bailey, G.S.; Loveland, P.M.; Pereira, C.; Pierce, D.; Hendricks, J.D.; Groopman, J.D. Quantitative carcinogenesis and dosimetry in rainbow trout for aflatoxin B1 and aflatoxicol, two aflatoxins that form the same DNA adduct. Mutat. Res. 1994, 313, 25–38. [Google Scholar] [CrossRef]
- Carvajal-Moreno, M.; Vargas-Ortiz, M.; Hernández-Camarillo, E.; Ruiz-Velasco, S.; Rojo-Callejas, F. Presence of unreported carcinogens, Aflatoxins and their hydroxylated metabolites, in industrialized Oaxaca cheese from Mexico City. Food Chem. Toxicol. 2019, 124, 128–138. [Google Scholar] [CrossRef]
- Wu, Q.; Jezkova, A.; Yuan, Z.; Pavlikova, L.; Dohnal, V.; Kuca, K. Biological degradation of aflatoxins. Drug Metab. Rev. 2009, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Massey, T.E.; Guindon, K.A. Aflatoxins. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; p. 101. [Google Scholar]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, H.; Zhu, H.; Salminen, E.; Juvonen, R.O.; Ling, W.; Ma, J.; Polychronaki, N.; Kemiläinen, H.; Mykkänen, O.; Salminen, S.; et al. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N7-guanine) in young Chinese males. Int. J. Cancer 2005, 115, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Bailey, G.S.; Dashwood, R.; Loveland, P.M.; Pereira, C.; Hendricks, J.D. Molecular dosimetry in fish: Quantitative target organ DNA adduction and hepatocarcinogenicity for four aflatoxins by two exposure routes in rainbow trout. Mutat. Res. 1998, 399, 233–244. [Google Scholar] [CrossRef]
- Karabulut, S.; Paytakov, G.; Leszczynski, J. Reduction of aflatoxin B1 to aflatoxicol: A comprehensive DFT study provides clues to its toxicity. J. Sci. Food Agric. 2014, 94, 3134–3140. [Google Scholar] [CrossRef]
- Caruso, M.; Mariotti, A.; Zizzadoro, C.; Zaghini, A.; Ormas, P.; Altafini, A.; Belloli, C. A clonal cell line (BME-UV1) as a possible model to study bovine mammary epithelial metabolism: Metabolism and cytotoxicity of aflatoxin B1. Toxicon 2009, 53, 400–408. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations; A Review of Human Carcinogens, 100 F; IARC: Lyon, France, 2012. [Google Scholar]
- Polychronaki, N.; Turner, P.C.; Mykkänen, H.; Gong, Y.Y.; Amra, H.; Abdel-Wahhab, M.; El-Nezami, H. Determinants of aflatoxin M1 in breast milk in a selected group of Egyptian mothers. Food Addit. Contam. 2006, 23, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, M.; Rojo, F.; Méndez, I.; Bolaños, A. Aflatoxin B1 and its interconverting metabolite aflatoxicol in milk: The situation in Mexico. Food Addit. Contam. 2003, 20, 1077–1086. [Google Scholar] [CrossRef]
- Williams, J.A.; Phillips, D.H. Mammary Expression of Xenobiotic Metabolizing Enzymes and Their Potential Role in Breast Cancer. Cancer Res. 2000, 60, 4667–4677. [Google Scholar]
- Williams, J.A.; Stone, E.M.; Millar, B.C.; Gusterson, B.A.; Grover, P.L.; Phillips, D.H. Determination of the enzymes responsible for activation of the heterocyclic amine 2-amino-3-methylimidazo[4,5-f]quinoline in the human breast. Pharmacogenetics 1998, 8, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, M.E.M.; Maas, R.F.M.; Fink-Gremmels, J. Cytochrome P450-mediated metabolism and cytotoxicity of aflatoxin B1 in bovine hepatocytes. Toxicol. Vitr. 2000, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Héraud, F.; Barraj, L.M.; Moy, G.G. GEMS/Food Consumption Cluster Diets. In Total Diet Studies; Moy, G.G., Vannoort, R.W., Eds.; Springer: New York, NY, USA, 2013; pp. 427–434. [Google Scholar]
- Britzi, M.; Friedman, S.; Miron, J.; Solomon, R.; Cuneah, O.; Shimshoni, J.A.; Soback, S.; Ashkenazi, R.; Armer, S.; Shlosberg, A. Carry-over of aflatoxin B1 to aflatoxin M1 in high yielding Israeli cows in mid- and late-lactation. Toxins 2013, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ayar, A.; Sert, D.; Con, A.H. A study on the occurrence of aflatoxin in raw milk due to feeds. J. Food Safety 2007, 27, 199–207. [Google Scholar] [CrossRef]
- Costamagna, D.; Gaggiotti, M.; Chiericatti, C.A.; Costabel, L.; Audero, G.M.L.; Taverna, M.; Signorini, M.L. Quantification of aflatoxin M1 carry-over rate from feed to soft cheese. Toxicol. Rep. 2019, 6, 782–787. [Google Scholar] [CrossRef]
- Veldman, A.; Meijs, J.A.C.; Borggreve, G.J.; Heeres-van der Tol, J.J. Carry-over of aflatoxin from cows’ food to milk. Anim. Sci. 1992, 55, 163–168. [Google Scholar] [CrossRef]
- Phillips, E.; Turner, P.C.; Ngure, F.M.; Kassim, N.; Makule, E.; Smith, L.E.; Nelson, R.J.; Stoltzfus, R.J. Ethical considerations in the design and conduct of a cluster-randomised mycotoxin mitigation trial in Tanzania. World Mycotoxin J. 2022, 15, 213–222. [Google Scholar] [CrossRef]
- Phillips, E.; Ngure, F.; Smith, L.E.; Makule, E.; Turner, P.C.; Nelson, R.; Kimanya, M.; Stoltzfus, R.; Kassim, N. Protocol for the trial to establish a causal linkage between mycotoxin exposure and child stunting: A cluster randomized trial. BMC Public Health 2020, 20, 598. [Google Scholar] [CrossRef]
- Phillips, E.; Turner, P.C.; Kassim, N.; Makule, E.; Nelson, R.; Ngure, F.; Smith, L.; Stoltzfus, R. Ethical considerations of the trial to establish a causal linkage between mycotoxin exposure and child stunting. Curr. Dev. Nutr. 2021, 5 (Suppl. 2), 678. [Google Scholar] [CrossRef]
- El-Nezami, H.S.; Nicoletti, G.; Neal, G.E.; Donohue, D.C.; Ahokas, J.T. Aflatoxin M1 in human breast milk samples from Victoria, Australia and Thailand. Food Chem. Toxicol. 1995, 33, 173–179. [Google Scholar] [CrossRef]
- Adejumo, O.; Atanda, O.O.; Raiola, A.; Somorin, Y.; Bandyopadhyay, R.; Ritieni, A. Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem. Toxicol. 2013, 56, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Elaridi, J.; Bassil, M.; Kharma, J.A.; Daou, F.; Hassan, H.F. Analysis of Aflatoxin M1 in Breast Milk and Its Association with Nutritional and Socioeconomic Status of Lactating Mothers in Lebanon. J. Food Prot. 2017, 80, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- El-Tras, W.F.; El-Kady, N.N.; Tayel, A.A. Infants exposure to aflatoxin M1 as a novel foodborne zoonosis. Food Chem. Toxicol. 2011, 49, 2816–2819. [Google Scholar] [CrossRef]
- Benkerroum, N. Mycotoxins in dairy products: A review. Int. Dairy J. 2016, 62, 63–75. [Google Scholar] [CrossRef]
- Battacone, G.; Nudda, A.; Palomba, M.; Pascale, M.; Nicolussi, P.; Pulina, G. Transfer of aflatoxin B1 from feed to milk and from milk to curd and whey in dairy sheep fed artificially contaminated concentrates. J. Dairy Sci. 2005, 88, 3063–3069. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Camenzuli, L. Effects of Milk Yield, Feed Composition, and Feed Contamination with Aflatoxin B1 on the Aflatoxin M1 Concentration in Dairy Cows’ Milk Investigated Using Monte Carlo Simulation Modelling. Toxins 2016, 8, 290. [Google Scholar] [CrossRef] [Green Version]
- Frobish, R.A.; Bradley, B.D.; Wagner, D.D.; Long-Bradley, P.E.; Hairston, H. Aflatoxin Residues in Milk of Dairy Cows after Ingestion of Naturally Contaminated Grain. J. Food Prot. 1986, 49, 781–785. [Google Scholar] [CrossRef]
- Masoero, F.; Gallo, A.; Moschini, M.; Piva1, G.; Diaz, D. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 2007, 9, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- van Eijkeren, J.C.; Bakker, M.I.; Zeilmaker, M.J. A simple steady-state model for carry-over of aflatoxins from feed to cow’s milk. Food Addit. Contam. 2006, 23, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Zarba, A.; Wild, C.P.; Hall, A.J.; Montesano, R.; Hudson, G.J.; Groopman, J.D. Aflatoxin M1 in human breast milk from The Gambia, west Africa, quantified by combined monoclonal antibody immunoaffinity chromatography and HPLC. Carcinogenesis 1992, 13, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Battacone, G.; Nudda, A.; Rassu, S.P.G.; Decandia, M.; Pulina, G. Excretion pattern of aflatoxin M1 in milk of goats fed a single dose of aflatoxin B1. J. Dairy Sci. 2012, 95, 2656–2661. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Moschini, M.; Masoero, F. Aflatoxins absorption in the gastro-intestinal tract and in the vaginal mucosa in lactating dairy cows. Ital. J. Anim. Sci. 2008, 7, 53–63. [Google Scholar] [CrossRef]
- Caloni, F.; Stammati, A.; Friggè, G.; De Angelis, I. Aflatoxin M1 absorption and cytotoxicity on human intestinal in vitro model. Toxicon 2006, 47, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef]
- Moschini, M.; Masoero, F.; Gallo, A.; Diaz, D. Mucosal absorption of aflatoxin B1 in lactating dairy cows. Ital. J. Anim. Sci. 2007, 6 (Suppl. 1), 324–326. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Addit. Contam. Part A 2008, 25, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Caloni, F.; Cortinovis, C.; Pizzo, F.; De Angelis, I. Transport of Aflatoxin M(1) in Human Intestinal Caco-2/TC7 Cells. Front. Pharmacol. 2012, 3, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Balimane, P.V.; Johnson, S.R.; Chong, S. Development of an in silico model for predicting efflux substrates in Caco-2 cells. Int. J. Pharm. 2007, 343, 98–105. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X.-B. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Jani, M.; Ambrus, C.; Magnan, R.; Jakab, K.T.; Beéry, E.; Zolnerciks, J.K.; Krajcsi, P. Structure and function of BCRP, a broad specificity transporter of xenobiotics and endobiotics. Arch. Toxicol. 2014, 88, 1205–1248. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Morris, M.E. Chapter 3—Efflux transporters in cancer resistance: Molecular and functional characterization of breast cancer resistance protein. In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Sosnik, A., Bendayan, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 67–96. [Google Scholar]
- Dornetshuber, R.; Heffeter, P.; Sulyok, M.; Schumacher, R.; Chiba, P.; Kopp, S.; Koellensperger, G.; Micksche, M.; Lemmens-Gruber, R.; Berger, W. Interactions between ABC-transport proteins and the secondary Fusarium metabolites enniatin and beauvericin. Mol. Nutr. Food Res. 2009, 53, 904–920. [Google Scholar] [CrossRef] [PubMed]
- van Herwaarden, A.E.; Wagenaar, E.; Karnekamp, B.; Merino, G.; Jonker, J.W.; Schinkel, A.H. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis 2005, 27, 123–130. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Q.; Bircsak, K.M.; Wen, X.; Aleksunes, L.M. In Vitro Screening of Environmental Chemicals Identifies Zearalenone as a Novel Substrate of the Placental BCRP/ABCG2 Transporter. Toxicol. Res. 2015, 4, 695–706. [Google Scholar] [CrossRef] [Green Version]
- Manzini, L.; Halwachs, S.; Girolami, F.; Badino, P.; Honscha, W.; Nebbia, C. Interaction of mammary bovine ABCG2 with AFB1 and its metabolites and regulation by PCB 126 in a MDCKII in vitro model. J. Vet. Pharmacol. Ther. 2017, 40, 591–598. [Google Scholar] [CrossRef]
- Tuntiteerawit, P.; Jarukamjorn, K.; Porasuphatana, S. The effect of green tea catechins on breast cancer resistance protein activity and intestinal efflux of aflatoxin B1 via breast cancer resistance protein in Caco-2 cells. Toxicol. Res. 2020, 36, 293–300. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, Z.; Chen, D.; Huang, G.; Cao, H. Investigation of the transport of aflatoxin M1 by the transporter ABCG2 in bovine mammary epithelial cells. Med. Weter 2020, 76, 646–651. [Google Scholar] [CrossRef]
- González-Lobato, L.; Real, R.; Prieto, J.G.; Álvarez, A.I.; Merino, G. Differential inhibition of murine Bcrp1/Abcg2 and human BCRP/ABCG2 by the mycotoxin fumitremorgin C. Eur. J. Pharmacol. 2010, 644, 41–48. [Google Scholar] [CrossRef]
- Susanto, J.; Lin, Y.-H.; Chen, Y.-N.; Shen, C.-R.; Yan, Y.-T.; Tsai, S.-T.; Chen, C.H.; Shen, C.N. Porphyrin homeostasis maintained by ABCG2 regulates self-renewal of embryonic stem cells. PLoS ONE 2008, 3, e4023. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.N.; Court, M.H.; Fink-Gremmels, J.; Mealey, K.L. Population variability in animal health: Influence on dose-exposure-response relationships: Part I: Drug metabolism and transporter systems. J. Vet. Pharmacol. Ther. 2018, 41, E57–E67. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Fink-Gremmels, J.; Li, D.; Tong, X.; Tang, J.; Nan, X.; Yu, Z.; Chen, W.; Wang, G. An overview of aflatoxin B1 biotransformation and aflatoxin M1 secretion in lactating dairy cows. Anim. Nutr. 2021, 7, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.T.; Takabayashi-Yamashita, C.R.; Ono, E.Y.S.; Bagatin, A.K.; Rigobello, F.F.; Kawamura, O.; Hirooka, E.Y.; Itano, E.N. Exposure Assessment of Infants to Aflatoxin M₁ through Consumption of Breast Milk and Infant Powdered Milk in Brazil. Toxins 2016, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- WHO. Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borst, P.; Elferink, R.O. Mammalian ABC Transporters in Health and Disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.P.; Alam, C.; Bendayan, R. Chapter 1—Efflux transporters in cancer resistance: Molecular and functional characterization of P-glycoprotein. In Drug Efflux Pumps in Cancer Resistance Pathways: From Molecular Recognition and Characterization to Possible Inhibition Strategies in Chemotherapy; Sosnik, A., Bendayan, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 1–30. [Google Scholar]
- Kumagai, S. Intestinal absorption and excretion of aflatoxin in rats. Toxicol. Appl. Pharmacol. 1989, 97, 88–97. [Google Scholar] [CrossRef]
- Shugarts, S.; Benet, L.Z. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 2009, 26, 2039–2054. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.M.; Cleal, J.K.; Godfrey, K.M. Chapter 78—The placental role in fetal programming. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1039–1049. [Google Scholar]
- Myllynen, P.; Pasanen, M.; Pelkonen, O. Human placenta: A human organ for developmental toxicology research and biomonitoring. Placenta 2005, 26, 361–371. [Google Scholar] [CrossRef]
- Partanen, H. Transplacental Transfer of Food Contaminants. Ph.D. Thesis, University of Eastern Finland, Faculty of Health Sciences, Kuopio, Finland, 2012; p. 71. [Google Scholar]
- Ventrella, D.; Forni, M.; Bacci, L.M.; Annaert, P. Non-clinical Models to Determine Drug Passage into Human Breast Milk. Curr. Pharm. Des. 2019, 25, 534–548. [Google Scholar] [CrossRef]
- Montalbetti, N.; Dalghi, M.G.; Albrecht, C.; Hediger, M.A. Nutrient transport in the mammary gland: Calcium, trace minerals and water soluble vitamins. J. Mammary Gland. Biol. Neoplasia 2014, 19, 73–90. [Google Scholar] [CrossRef]
- Roth, M.; Obaidat, A.; Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 2012, 165, 1260–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Wagenaar, E.; Xu, W.; Huang, K.; Schinkel, A.H. Ochratoxin A transport by the human breast cancer resistance protein (BCRP), multidrug resistance protein 2 (MRP2), and organic anion-transporting polypeptides 1A2, 1B1 and 2B1. Toxicol. Appl. Pharmacol. 2017, 329, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ito, K.; Ikebuchi, Y.; Kito, T.; Miyata, H.; Toyoda, Y.; Takada, T.; Hisaka, A.; Honma, M.; Oka, A.; et al. Organic cation transporter/solute carrier family 22a is involved in drug transfer into milk in mice. J. Pharm. Sci. 2014, 103, 3342–3348. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Reuther, S.; Meyer zu Schwabedissen, H.; Köck, K.; Draber, K.; Ritter, C.A.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Organic Anion Transporting Polypeptide 2B1 and Breast Cancer Resistance Protein Interact in the Transepithelial Transport of Steroid Sulfates in Human Placenta. Drug Metab. Dispos. 2007, 35, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlangwiset, P.; Shephard, G.S.; Wu, F. Aflatoxins and growth impairment: A review. Crit. Rev. Toxicol. 2011, 41, 740–755. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.V.; de Oliveira, C.A.; Ramalho, L.N. Effects of Prenatal Exposure to Aflatoxin B1: A Review. Molecules 2021, 26, 7312. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin exposure during pregnancy, maternal anemia, and adverse birth outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulrazzaq, Y.M.; Osman, N.; Yousif, Z.M.; Trad, O. Morbidity in neonates of mothers who have ingested aflatoxins. Ann. Trop. Paediatr. 2004, 24, 145–151. [Google Scholar] [CrossRef]
- Adhikari, M.; Gita, R.; Berjak, P. Aflatoxin, kwashiorkor, and morbidity. Nat. Toxins 1994, 2, 1–3. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Ortiz, J.; Jacxsens, L.; Astudillo, G.; Ballesteros, A.; Donoso, S.; Huybregts, L.; De Meulenaer, B. Multiple mycotoxin exposure of infants and young children via breastfeeding and complementary/weaning foods consumption in Ecuadorian highlands. Food Chem. Toxicol. 2018, 118, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Sherif, S.O.; Salama, E.E.; Abdel-Wahhab, M.A. Mycotoxins and child health: The need for health risk assessment. Int. J. Hyg. Environ. Health 2009, 212, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Hulin, M.; Bemrah, N.; Nougadère, A.; Volatier, J.L.; Sirot, V.; Leblanc, J.C. Assessment of infant exposure to food chemicals: The French Total Diet Study design. Food Addit. Contam. Part A 2014, 31, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.M.; Duggan, C.P.; Ausman, L.M.; Griffiths, J.K.; Webb, P.; Wang, J.S.; Xue, K.S.; Agaba, E.; Nshakira, N.; Ghosh, S. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern. Child Nutr. 2019, 15, e12701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.C.; Collinson, A.C.; Cheung, Y.B.; Gong, Y.Y.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007, 36, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- JME (UNICEF/WHO/World Bank). Levels and Trends in Child Malnutrition: Key Fndings of the 2021 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Boni, S.B.; Beed, F.; Kimanya, M.E.; Koyano, E.; Mponda, O.; Mamiro, D.; Kaoneka, B.; Bandyopadhyay, R.; Korie, S.; Mahuku, G. Aflatoxin contamination in Tanzania: Quantifying the problem in maize and groundnuts from rural households. World Mycotoxin J. 2021, 14, 553–564. [Google Scholar] [CrossRef]
- Ministry of Health, C.D. Gender, Elderly and Children (MoHCDGEC) [Tanzania Mainland]; Ministry of Health (MoH) [Zanzibar]; National Bureau of Statistics (NBS); Office of the Chief Government Statistician (OCGS); ICF; Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) 2015–2016. Dar es Salaam Rockville: MoHCDGEC, MoH, NBS, OCGS, and ICF. 2016, p. 591. Available online: https://dhsprogram.com/pubs/pdf/fr321/fr321.pdf (accessed on 12 June 2022).
- UNICEF. The Conceptual Framework of Under-Nutrition. 2022. Available online: https://forum.susana.org/244-theme-1-making-the-link-in-theory-and-practice-where-do-we-stand/17576-the-conceptual-framework-of-under-nutrition (accessed on 12 June 2022).
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, S.M.; Familusi, J.B.; Sodeinde, O.; Chan, M.C.; Hendrickse, R.G. Detection of naphthols and aflatoxins in Nigerian cord blood. Ann. Trop. Paediatr. 1994, 14, 3–5. [Google Scholar] [CrossRef]
- Magoha, H.; Kimanya, M.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Risk of dietary exposure to aflatoxins and fumonisins in infants less than 6 months of age in Rombo, Northern Tanzania. Matern. Child Nutr. 2016, 12, 516–527. [Google Scholar] [CrossRef]
- Dewey, K.G.; Adu-Afarwuah, S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern. Child Nutr. 2008, 4 (Suppl. 1), 24–85. [Google Scholar] [CrossRef]
- Panjwani, A.; Heidkamp, R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2169s–2178s. [Google Scholar] [CrossRef] [Green Version]
- Bashiry, M.; Javanmardi, F.; Sadeghi, E.; Shokri, S.; Hossieni, H.; Oliveira, C.A.F.; Mousavi Khaneghah, A. The prevalence of aflatoxins in commercial baby food products: A global systematic review, meta-analysis, and risk assessment study. Trends Food Sci. Technol. 2021, 114, 100–115. [Google Scholar] [CrossRef]
- Humphrey, J.H.; Mbuya, M.N.N.; Ntozini, R.; Moulton, L.H.; Stoltzfus, R.J.; Tavengwa, N.V.; Mutasa, K.; Majo, F.; Mutasa, B.; Mangwadu, G.; et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: A cluster-randomised trial. Lancet Glob. Health 2019, 7, e132–e147. [Google Scholar] [CrossRef] [PubMed]
- Null, C.; Stewart, C.P.; Pickering, A.J.; Dentz, H.N.; Arnold, B.F.; Arnold, C.D.; Benjamin-Chung, J.; Clasen, T.; Dewey, K.G.; Fernald, L.C.; et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: A cluster-randomised controlled trial. Lancet Glob. Health 2018, 6, e316–e329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luby, S.P.; Rahman, M.; Arnold, B.F.; Unicomb, L.; Ashraf, S.; Winch, P.J.; Stewart, C.P.; Begum, F.; Hussain, F.; Benjamin-Chung, J.; et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: A cluster randomised controlled trial. Lancet Glob. Health 2018, 6, e302–e315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, V.; Jones, K.; Leroy, J.L. The impact of reducing dietary aflatoxin exposure on child linear growth: A cluster randomised controlled trial in Kenya. BMJ Glob. Health 2018, 3, e000983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkerroum, N. Retrospective and Prospective Look at Aflatoxin Research and Development from a Practical Standpoint. Int. J. Environ. Res. Public Health 2019, 16, 3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzmann, J.; Xu, Y.; Gong, Y.Y.; Lindahl, J.; Kersten, S.; Dänicke, S.; Routledge, M.N. Preliminary study on the relationship between aflatoxin-bovine serum albumin adducts in blood and aflatoxin M1 levels in milk of dairy cows. Mycot. Res. 2020, 36, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gong, Y.Y.; Kimanya, M.E.; Shirima, C.P.; Routledge, M.N. Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for assessing aflatoxin exposure in Tanzanian children. Biomarkers 2018, 23, 131–136. [Google Scholar] [CrossRef]
- Sadeghi, N.; Oveisi, M.R.; Jannat, B.; Hajimahmoodi, M.; Bonyani, H.; Jannat, F. Incidence of aflatoxin M1 in human breast milk in Tehran, Iran. Food Control 2009, 20, 75–78. [Google Scholar] [CrossRef]
- Mahdavi, R.; Nikniaz, L.; Arefhosseini, S.R.; Vahed Jabbari, M. Determination of aflatoxin M(1) in breast milk samples in Tabriz-Iran. Matern. Child Health J. 2010, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Hainaut, P.; Wild, C.P. Hepatocellular carcinoma: From gene to public health. J. Natl. Cancer Inst. 1997, 89, 1844–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.Y.; Egal, S.; Hounsa, A.; Turner, P.C.; Hall, A.J.; Cardwell, K.F.; Wild, C.P. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. Int. J. Epidemiol. 2003, 32, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Perspect 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Magoha, H.; Kimanya, M.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Association between aflatoxin M1 exposure through breast milk and growth impairment in infants from Northern Tanzania. World Mycotoxin J. 2014, 7, 277–284. [Google Scholar] [CrossRef]
- Shirima, C.P.; Kimanya, M.E.; Routledge, M.N.; Srey, C.; Kinabo, J.L.; Humpf, H.U.; Wild, C.P.; Tu, Y.K.; Gong, Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect 2015, 123, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, N.J.; Hsu, H.-H.; Chandyo, R.K.; Shrestha, B.; Bodhidatta, L.; Tu, Y.-K.; Gong, Y.Y.; Egner, P.A.; Ulak, M.; Groopman, J.D.; et al. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: An extension of the MAL-ED study. PLoS ONE 2017, 12, e0172124. [Google Scholar] [CrossRef] [Green Version]
- IARC (International Agency for Research on Cancer). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 1993; Volume V56, pp. 51–72. [Google Scholar]
- NIH. Mycotoxin Mitigation Trial. 2022. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03940547 (accessed on 31 August 2022).
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.E.; Stoltzfus, R.J.; Prendergast, A. Food chain mycotoxin exposure, gut health, and impaired growth: A conceptual framework. Adv. Nutr. 2012, 3, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Tessema, M.; De Groote, H.; Brouwer, I.D.; De Boevre, M.; Corominas, A.V.; Stoecker, B.J.; Stoecker, B.J.; Feskens, E.J.; Belachew, T.; Karakitsou, A.; et al. Exposure to aflatoxins and fumonisins and linear growth of children in rural Ethiopia: A longitudinal study. Public Health Nutr. 2021, 24, 3662–3673. [Google Scholar] [CrossRef]
- Mbuya, M.N.; Humphrey, J.H. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: An opportunity for stunting reduction in developing countries. Matern. Child Nutr. 2016, 12 (Suppl. 1), 106–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, K.M.; Mutasa, M.; Prendergast, A.J.; Humphrey, J.H.; Manges, A.R. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keusch, G.T.; Rosenberg, I.H.; Denno, D.M.; Duggan, C.; Guerrant, R.L.; Lavery, J.V.; Tarr, P.I.; Ward, H.D.; Black, R.E.; Nataro, J.P.; et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr. Bull. 2013, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.H.; Jones, A.D.; Manges, A.; Mangwadu, G.; Maluccio, J.A.; Mbuya, M.N.; Moulton, L.H.; Ntozini, R.; Prendergast, A.J. The sanitation hygiene infant nutrition efficacy (SHINE) trial: Rationale, design, and methods. Clin. Infect. Dis. 2015, 61 (Suppl. 7), S685–S702. [Google Scholar] [PubMed] [Green Version]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Mbuya, M.N.; Mutasa, K.; Kembo, G.; Stoltzfus, R.J. The Potential Role of Mycotoxins as a Contributor to Stunting in the SHINE Trial. Clin. Infect. Dis. 2015, 61 (Suppl. 7), S733–S737. [Google Scholar] [CrossRef] [Green Version]
- Weber, T.K.; Polanco, I. Gastrointestinal microbiota and some children diseases: A review. Gastroenterol. Res. Pract. 2012, 2012, 676585. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Obuseh, F.A.; Jolly, P.E.; Kulczycki, A.; Ehiri, J.; Waterbor, J.; Desmond, R.A.; Preko, P.O.; Jiang, Y.; Piyathilake, C.J. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: A cross-sectional study. J. Int. AIDS Soc. 2011, 14, 53. [Google Scholar] [CrossRef]
- Onyemelukwe, G.; Ogoina, D.; Ibiam, G.E.; Ogbadu, G.H. Aflatoxins in body fluids and food of Nigerian children with protein-energy malnutrition. Afr. J. Food Agric. Nutr. Dev. 2012, 12, 6553–6566. [Google Scholar]
- Hatem, N.L.; Hassab, H.M.; Abd Al-Rahman, E.M.; El-Deeb, S.A.; El-Sayed Ahmed, R.L. Prevalence of aflatoxins in blood and urine of Egyptian infants with protein-energy malnutrition. Food Nutr. Bull. 2005, 26, 49–56. [Google Scholar] [CrossRef]
- Coulter, J.B.; Hendrickse, R.G.; Lamplugh, S.M.; Macfarlane, S.B.; Moody, J.B.; Omer, M.I.; Suliman, G.I.; Williams, T.E.; Liverpool/Khartoum Aflatoxin Study Group. Aflatoxins and kwashiorkor: Clinical studies in Sudanese children. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Hendrickse, R.G.; Coulter, J.B.S.; Lamplugh, S.M.; Macfarlane, S.B.J.; Williams, T.E.; Omer, M.I.A.; Suliman, G.I. Aflatoxins and kwashiorkor: A study In Sudanese children. Br. Med. J. (Clin. Res. Ed.) 1982, 285, 843–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Beltran, A.; Bandyopadhyay, R. Contributions of integrated aflatoxin management strategies to achieve the sustainable development goals in various African countries. Glob. Food Secur. 2021, 30, 100559. [Google Scholar] [CrossRef]
- Fakhri, Y.; Rahmani, J.; Oliveira, C.A.F.; Franco, L.T.; Corassin, C.H.; Saba, S.; Rafique, J.; Khaneghah, A.M. Aflatoxin M1 in human breast milk: A global systematic review, meta-analysis, and risk assessment study (Monte Carlo simulation). Trends Food Sci. Technol. 2019, 88, 333–342. [Google Scholar] [CrossRef]
- Duarte, S.; Silva, L.J.G.; Pereira, A.M.S.; Gimbi, M.; Cesar, C.; Vidal, V.; Basílio, R.; Almeida, A.; Lino, C.; Pena, A. Mycotoxins Exposure in Cabinda, Angola-A Pilot Biomonitoring Survey of Breastmilk. Toxins 2022, 14, 204. [Google Scholar] [CrossRef]
- Eshete, M.; Gebremedhin, S.; Alemayehu, F.R.; Taye, M.; Boshe, B.; Stoecker, B.J. Aflatoxin contamination of human breast milk and complementary foods in southern Ethiopia. Matern. Child Nutr. 2021, 17, e13081. [Google Scholar] [CrossRef]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Gagliardi, L.; Ciotti, S.; Luisi, S.; Bognanno, M.; La Fauci, L.; Iacopino, A.M.; Nigro, F.; et al. Maternal dietary habits and mycotoxin occurrence in human mature milk. Mol. Nutr. Food Res. 2008, 52, 496–501. [Google Scholar] [CrossRef]
- Dogan, R.A.; Afacan, M.; Ozdemir, M. Determination of aflatoxin M1 in breast milk and related factors. Rev. Da Assoc. Médica Bras. 2022, 68, 1000–1005. [Google Scholar] [CrossRef]
- Cantú-Cornelio, F.; Aguilar-Toalá, J.E.; de León-Rodríguez, C.I.; Esparza-Romero, J.; Vallejo-Cordoba, B.; González-Córdova, A.F.; García, H.S.; Hernández-Mendoza, A. Occurrence and factors associated with the presence of aflatoxin M1 in breast milk samples of nursing mothers in central Mexico. Food Control 2016, 62, 16–22. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Soher, E.A.; Neamat-Allah, A. Human exposure to mycotoxins in Egypt. Mycotoxin Res. 2002, 18, 23–30. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Abas, A.R.; Fadul, M.H.; Modwi, A.K.; Ali, N.M.; Jadian, A.F.; Adam, S.Y.; Ahmed, N.A.; Khairy, A.A.; Khalil, E.A. Aflatoxin M₁ in breast milk of nursing Sudanese mothers. Mycotoxin Res. 2012, 28, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.V.; Wenndt, A.J.; Girard, A.W.; Taneja, S.; Ranjan, S.; Ramakrishnan, U.; Martorell, R.; Ryan, P.B.; Rangiah, K.; Young, M.F. Risk of dietary and breastmilk exposure to mycotoxins among lactating women and infants 2–4 months in northern India. Matern. Child Nutr. 2021, 17, e13100. [Google Scholar] [CrossRef] [PubMed]
- Tchana, A.N.; Moundipa, P.F.; Tchouanguep, F.M. Aflatoxin contamination in food and body fluids in relation to malnutrition and cancer status in Cameroon. Int. J. Environ. Res. Public Health 2010, 7, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polychronaki, N.; West, R.M.; Turner, P.C.; Amra, H.; Abdel-Wahhab, M.; Mykkänen, H.; El-Nezami, H. A longitudinal assessment of aflatoxin M1 excretion in breast milk of selected Egyptian mothers. Food Chem. Toxicol. 2007, 45, 1210–1215. [Google Scholar] [CrossRef]
- Cherkani-Hassani, A.; Ghanname, I.; Zinedine, A.; Sefrioui, H.; Qmichou, Z.; Mouane, N. Aflatoxin M1 prevalence in breast milk in Morocco: Associated factors and health risk assessment of newborns “CONTAMILK study”. Toxicon 2020, 187, 203–208. [Google Scholar] [CrossRef]
- Atanda, O.; Oguntubo, A.; Adejumo, O.; Ikeorah, J.; Akpan, I. Aflatoxin M1 contamination of milk and ice cream in Abeokuta and Odeda local governments of Ogun State, Nigeria. Chemosphere 2007, 68, 1455–1458. [Google Scholar] [CrossRef]
- Kang’ethe, E.K.; Gatwiri, M.; Sirma, A.J.; Ouko, E.O.; Mburugu-Musoti, C.K.; Kitala, P.M.; Nduhiu, G.J.; Nderitu, J.G.; Mungatu, J.K.; Hietaniemi, V.; et al. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Qual. Saf. 2017, 1, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Abdulrazzaq, Y.M.; Osman, N.; Yousif, Z.M.; Al-Falahi, S. Aflatoxin M1 in breast-milk of UAE women. Ann. Trop. Paediatr. 2003, 23, 173–179. [Google Scholar] [CrossRef]
- Keskin, Y.; Başkaya, R.; Karsli, S.; Yurdun, T.; Ozyaral, O. Detection of aflatoxin M1 in human breast milk and raw cow’s milk in Istanbul, Turkey. J. Food Prot. 2009, 72, 885–889. [Google Scholar] [CrossRef]
- Gürbay, A.; Sabuncuoğlu, S.A.; Girgin, G.; Sahin, G.; Yiğit, S.; Yurdakök, M.; Tekinalp, G. Exposure of newborns to aflatoxin M1 and B1 from mothers’ breast milk in Ankara, Turkey. Food Chem. Toxicol. 2010, 48, 314–319. [Google Scholar] [CrossRef]
- Atasever, M.; Yildirim, Y.; Atasever, M.; Tastekin, A. Assessment of aflatoxin M1 in maternal breast milk in Eastern Turkey. Food Chem. Toxicol. 2014, 66, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Kilic Altun, S.; Gurbuz, S.; Ayag, E. Aflatoxin M(1) in human breast milk in southeastern Turkey. Mycotoxin Res. 2017, 33, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Karayagiz Muslu, G.; Ozdemir, M. Occurrence of and factors associated with the presence of aflatoxin M(1) in breast milk of mothers in Fethiye, Turkey. Biol. Res. Nurs. 2020, 22, 362–368. [Google Scholar] [CrossRef]
- Ghiasian, S.; Maghsood, A. Infants’ Exposure to Aflatoxin M1 from Mother’s Breast Milk in Iran. Iran J. Public Health 2012, 41, 119–126. [Google Scholar]
- Jafarian-Dehkordi, A.; Pourradi, N. Aflatoxin M1 contamination of human breast milk in Isfahan, Iran. Adv. Biomed. Res. 2013, 2, 86. [Google Scholar] [PubMed]
- Afshar, P.; Shokrzadeh, M.; Kalhori, S.; Babaee, Z.; Saeedi Saravi, S.S. Occurrence of Ochratoxin A and Aflatoxin M1 in human breast milk in Sari, Iran. Food Control 2013, 31, 525–529. [Google Scholar] [CrossRef]
- Rafiei, H.; Dehghan, P.; Pakshir, K.; Pour, M.C.; Akbari, M. The concentration of aflatoxin M1 in the mothers’ milk in Khorrambid City, Fars, Iran. Adv. Biomed. Res. 2014, 3, 152. [Google Scholar]
- Maleki, F.; Abdi, S.; Davodian, E.; Haghani, K.; Bakhtiyari, S. Exposure of Infants to Aflatoxin M1 from Mother’s Breast Milk in Ilam, Western Iran. Osong Public Health Res. Perspect. 2015, 6, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Pourtalebi, S.; Ayatollahi Mousavi, S.A.; Assadollahi, Z.; Mousavi, S.M. Assessment of Aflatoxin M1 in human breast milk in Rafsanjan, Iran. Curr. Med. Mycol. 2021, 7, 6–11. [Google Scholar] [CrossRef]
- Kamali, M.; Kamali, A. Evaluation of Aflatoxin M1 Level in Human Breast Milk samples from Jiroft, South of Iran. Med. Lab. J. 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Seifi, S. Is aflatoxin more in the milk of lactating mothers who have previously had COVID-19? J. Nutr. Food Secur. 2022, 7, 220–226. [Google Scholar] [CrossRef]
- Omar, S.S. Incidence of Aflatoxin M1 in Human and Animal Milk in Jordan. J. Toxicol. Environ. Health Part A 2012, 75, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, A.; Webb, P.; Andrews-Trevino, J.; Lamichhane, A.; Shrestha, R.; Acharya, S.; Davis, D.; Baral, K.; Wang, J.S.; Xue, K.; et al. Prevalence and associated factors of breastmilk aflatoxin M1 levels in mothers from Banke, Nepal. Food Control 2021, 126, 108069. [Google Scholar] [CrossRef]
- Islam, F.; Das Trisha, A.; Hafsa, J.M.; Hasan, A.; Degen, G.H.; Ali, N. Occurrence of aflatoxin M(1) in human breast milk in Bangladesh. Mycotoxin Res. 2021, 37, 241–248. [Google Scholar] [CrossRef]
- Iha, M.H.; Barbosa, C.B.; Heck, A.R.; Trucksess, M.W. Aflatoxin M1 and ochratoxin A in human milk in Ribeirão Preto-SP, Brazil. Food Control 2014, 40, 310–313. [Google Scholar] [CrossRef] [Green Version]
- Diaz, G.J.; Sánchez, M.P. Determination of aflatoxin M1 in breast milk as a biomarker of maternal and infant exposure in Colombia. Food Addit. Contam. Part A 2015, 32, 1192–1198. [Google Scholar] [CrossRef]
- Salas, R.; Acosta, N.; Garza, A.J.; Tijerina, A.; Dávila, R.; Jiménez-Salas, Z.; Otero, L.; Santos, M.; Trujillo, A.J. Levels of Aflatoxin M1 in Breast Milk of Lactating Mothers in Monterrey, Mexico: Exposure and Health Risk Assessment of Newborns. Toxins 2022, 14, 194. [Google Scholar] [CrossRef]
- Jolly, P.E.; Mazariegos, M.; Contreras, H.; Balas, N.; Junkins, A.; Aina, I.O.; Minott, S.; Wang, M.; Phillips, T.D. Aflatoxin Exposure Among Mothers and Their Infants from the Western Highlands of Guatemala. Matern. Child Health J. 2021, 25, 1316–1325. [Google Scholar] [CrossRef]
- Bogalho, F.; Duarte, S.; Cardoso, M.; Almeida, A.; Cabeças, R.; Lino, C.; Pena, A. Exposure assessment of Portuguese infants to Aflatoxin M1 in breast milk and maternal social-demographical and food consumption determinants. Food Control 2018, 90, 140–145. [Google Scholar] [CrossRef]
- Kos, J.; Lević, J.; Đuragić, O.; Kokić, B.; Miladinović, I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Saha Turna, N.; Wu, F. Estimation of Tolerable Daily Intake (TDI) for Immunological Effects of Aflatoxin. Risk Anal. 2021, 42, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, B.; Beghin, J.C. The political economy of food standard determination: International evidence from maximum residue limits. Econ. Work. Pap. 2014, 47, 239–267. [Google Scholar]

| Country | Type of the Study | Period of the Study | Age in Months | Dietary Status | Mean (Range) of AFM1 Concentration in Breast Milk or Suitable Biomarker | Growth Impairment 2 | Observations and limitations | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Biomarker 1 | Breast Milk AFM1 | ||||||||
| Benin and Togo | Cross-sectional | NS | 9–60 |
| 32.8 3 (5–1064) | - |
| No direct causal effect:
| [110,126] |
| Benin | Longitudinal (Prospective cohort) | February–October 2004 | 16–37 |
| 11.8–119.3 5 (9.2–148.1) | - | Stunting
| No direct causal effect:
| [127] |
| Iran (Teheran) | Cross-sectional | May–September 2006 | <21 | NS | - | 8.2 3 (0.3–26.7) | Stunting
| No direct link between AFM1 in breast milk and stunting after birth.
| [123] |
| Iran (Tabriz) | Cross-sectional | March–April 2007 | 3–4 | Exclusively breastfed | - | 6.96 (5.1–8.1) | Stunting
| Confounding factors not eliminated: Deficiency of mothers’ milk in growth-promoting micronutrients Occurrence of gastrointestinal infections
| [124] |
| Tanzania | Longitudinal (Prospective cohort) | November 2011–February 2012 | <6 |
| - | 0.07 2 (0.01–0.55) | Stunting
| Good evidence for the link between AFM1 intake and stunting and underweight in infants (<5 months of age) fed on contaminated breast milk at levels exceeding the EU MTL of 0.025 ng/g. | [128] |
| Tanzania | Longitudinal (Prospective cohort) | NS | 6–14 | Partially breastfed | 3.0–48.8 6 (2.1–69.1) | - | No significant inverse association between AF-Alb and stunting, underweight, or wasting | No direct link
| [129] |
| Tanzania | Longitudinal (Prospective cohort) | November 2011–February 2012 | <6 |
| - | 0.08 (0.01–0.55) 3 | Stunting and underweight
| Lack of evidence for causal link
| [112] |
| Nepal | Longitudinal (Prospective cohort) | May 2010–February 2012 | <36 |
| 3.62 8 | - |
| Growth impairement was associated with confoundersrather than to AF exposure:
| [130] |
| Kenya | Longitudinal (Cluster randomized controlled trial) | February 2013–November 2016 | <24 | Partially breastfed | 18.1 (4.5–8.3) 9 | - | Intervention with “aflatoxin-safe” complementary foods in chidren (0–22 months): No correlation between stunting and serum AF-Alb | Lack of sound evidence for causal link: Dietary interventions did not improve linear growth or the improvement did not correlate with aflatoxin exposure
| [119] |
| Turkey | Cross-sectional | June 2017–March 2018 | <4 | Exclusively breastfed | - | 3.03 (2.59–3.82) | No significant correlation between WAZ and AFM1 in breast milk | The lack of inverse correlation between AFM1 intake and WAS in infants does not exclude the stunting effect at higher levels of exposure to AFM1 | [13] |
| Country | Age | Breast Milk Intake (mL/day) | Exposure (ng/kg bw/day) | Hazard Index (HI) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Min | Max | Average | Min | Max | ||||
| Africa | |||||||||
| Egypt | 1–6 M | 708 | 52.68 | NA | NA | 0.053 | NA | NA | [49] |
| Tanzania | 1 M | 510 | 11.08 | 1.13 | 66.79 | 0.110 | 0.01 | 0.67 | [128] |
| 3 M | 690 | 11.94 | 0.81 | 58.96 | 0.119 | 0.008 | 0.590 | ||
| 6 M | 770 | 10.91 | 1.08 | 34.90 | 0.109 | 0.011 | 0.349 | ||
| Nigeria | 1–6 M | 750–1300 | 73.00 | NS | NS | 0.730 | NS | NS | [12] |
| Morocco | 3–5 D | 200 | 0.35 1 | NA | 1.16 | 0.004 | NA | 0.01 | [160] |
| Latin America | |||||||||
| Brazil 2 | 1 W | 590 | 0.069 | NS | NS | 0.001 | NS | NS | [79] |
| 1M | 642 | 0.057 | NS | NS | 0.001 | NS | NS | ||
| 6 M | 560 | 0.029 | NS | NS | 0.0003 | NS | NS | ||
| 12 M | 452 | 0.019 | NS | NS | 0.0002 | NS | NS | ||
| Mexico | 0–6 M | 1980 | 5.08 | 1.52 | 20.18 | 0.051 | 0.015 | 0.202 | [182] |
| 7–12 M | 2350 | 4.68 | 1.31 | 9.98 | 0.047 | 0.013 | 0.100 | ||
| 7–12 M | 2370 | 4.10 | 1.08 | 6.33 | 0.041 | 0.011 | 0.063 | ||
| 25–36 M | 2020 | 1.81 | 1.25 | 2.28 | 0.018 | 0.013 | 0.023 | ||
| Central Mexico | 0–6 M | 750 | 2.35 | 0.92 | 6.28 | 0.024 | 0.009 | 0.063 | [154] |
| Europe | |||||||||
| Portugal | NS | NS | 1.06 3 | NS | NS | 0.011 | NS | NS | [184] |
| NS | NS | 0.86 4 | NS | 1.25 | 0.009 | NS | 0.013 | ||
| Asia | |||||||||
| Lebanon | 3–8 W | 750 | 0.69 | 0.65 | 0.80 | 0.007 | 0.007 | 0.008 | [47] |
| UAE | 1 W | 500 | 80.00 | 7.57 | 378 | 0.800 | 0.076 | 3.780 | [163] |
| India | 2–4 M | 750 | 3.04 | 0.26 | 80.7 | 0.030 | 0.003 | 0.807 | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkerroum, N.; Ismail, A. Human Breast Milk Contamination with Aflatoxins, Impact on Children’s Health, and Possible Control Means: A Review. Int. J. Environ. Res. Public Health 2022, 19, 16792. https://doi.org/10.3390/ijerph192416792
Benkerroum N, Ismail A. Human Breast Milk Contamination with Aflatoxins, Impact on Children’s Health, and Possible Control Means: A Review. International Journal of Environmental Research and Public Health. 2022; 19(24):16792. https://doi.org/10.3390/ijerph192416792
Chicago/Turabian StyleBenkerroum, Noreddine, and Amir Ismail. 2022. "Human Breast Milk Contamination with Aflatoxins, Impact on Children’s Health, and Possible Control Means: A Review" International Journal of Environmental Research and Public Health 19, no. 24: 16792. https://doi.org/10.3390/ijerph192416792






