Antimony Immobilization in Primary-Explosives-Contaminated Soils by Fe–Al-Based Amendments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sb-Contaminated Soils and Adsorbents
2.2. Soil Analysis
2.3. Immobilization Experiment
2.3.1. Batch Experiments
2.3.2. Column Leachate Experiments
3. Results and Discussions
3.1. Soil Characteristics and Risk
3.2. Batch Experiments
3.2.1. Changes in pH and Sb Fractions
3.2.2. Effects of Fe- and Al-Based Sorbents on Sb
3.2.3. Effect of Fe- and Al-Based Sorbents on Zn and Cu
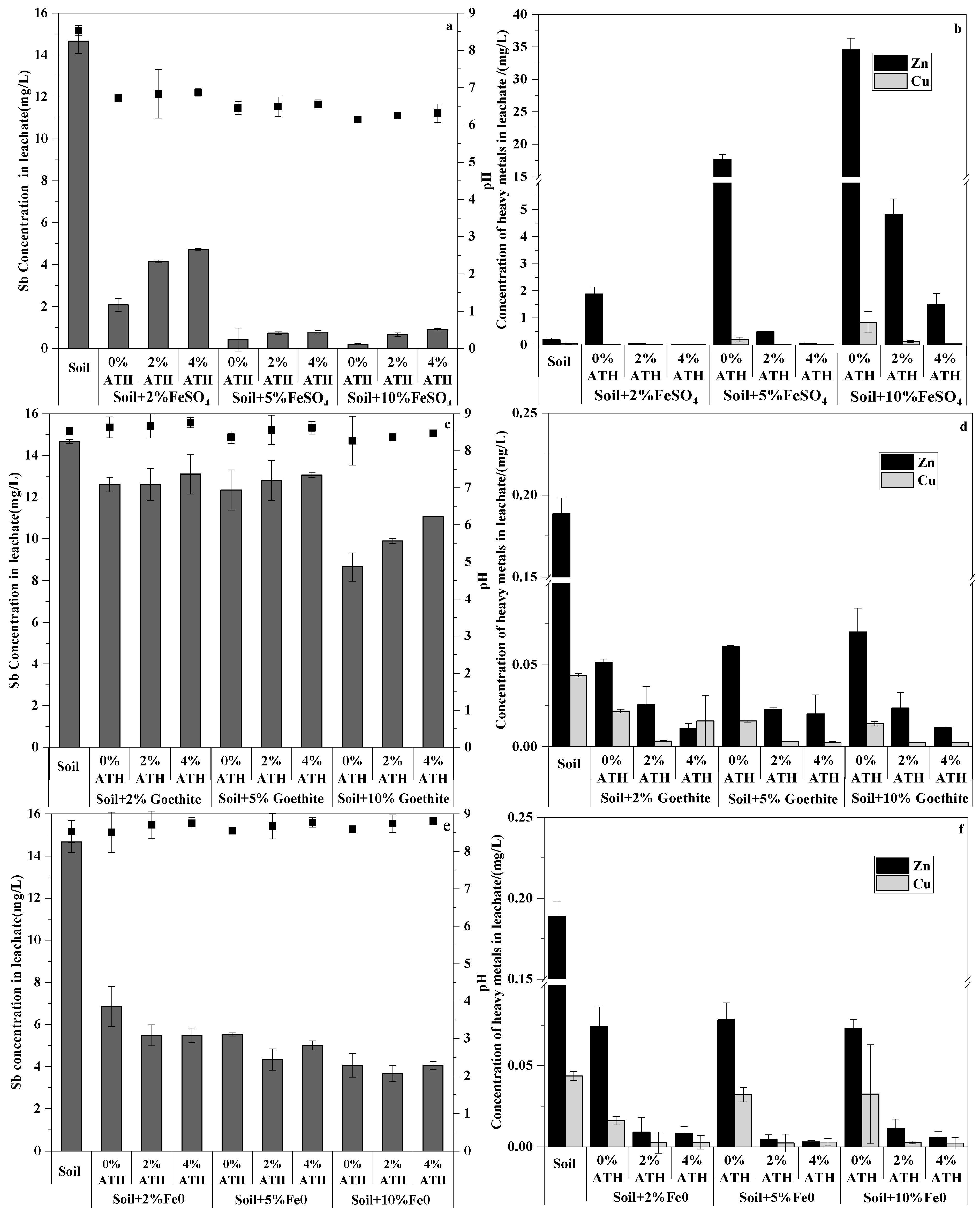
3.3. Column Experiments
3.3.1. Column Experiments for Sb Immobilization
3.3.2. Column Experiments for Zn and Cu Immobilization
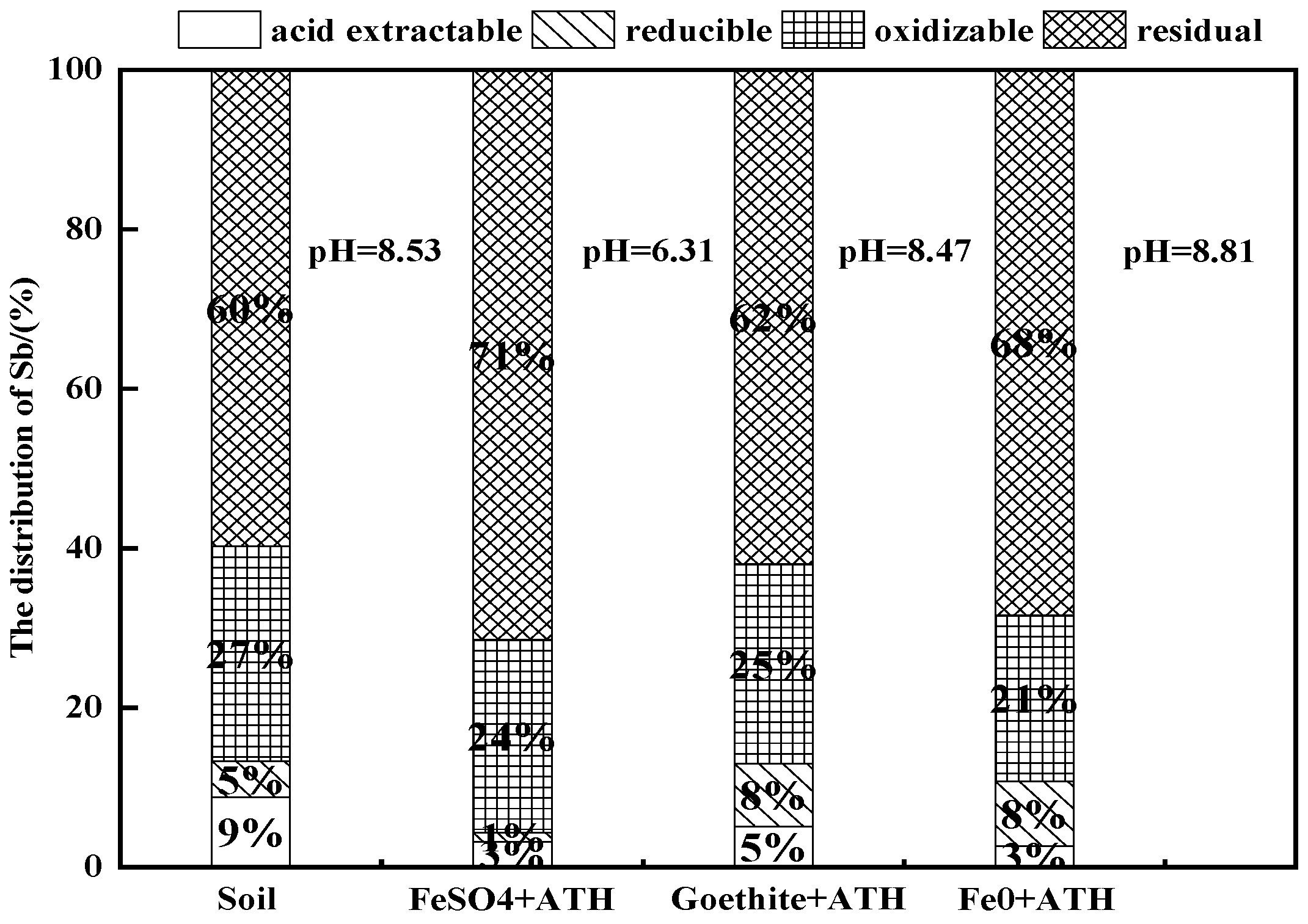
3.3.3. pH Effects on Sb and Co-Occurring Heavy Metals Immobilization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, X.; Kong, L.; He, M. Kinetics and mechanism of photopromoted oxidative dissolution of antimony trioxide. Environ. Sci. Technol. 2014, 48, 14266–14272. [Google Scholar] [CrossRef]
- Kong, L.; Hu, X.; He, M. Mechanisms of Sb(III) oxidation by pyrite-induced hydroxyl radicals and hydrogen peroxide. Environ. Sci. Technol. 2015, 49, 3499–3505. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters I. occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- He, M.; Wang, N.; Long, X.; Zhang, C.; Ma, C.; Zhong, Q.; Wang, A.; Wang, Y.; Pervaiz, A.; Shan, J. Antimony speciation in the environment: Recent advances in understanding the biogeochemical processes and ecological effects. J. Environ. Sci. 2019, 75, 14–39. [Google Scholar] [CrossRef] [PubMed]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem.-Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- Gerhardsson, L.; Brune, D.; Nordberg, G.F.; Wester, P.O. Antimony in lung, liver and kidney from deceased smelter workers. Scandinavian. J. Work. Environ. Health 1982, 8, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, A.; Kong, L.; He, M. Calculation and application of Sb toxicity coefficient for potential ecological risk assessment. Sci. Total Environ. 2018, 610-611, 167–174. [Google Scholar] [CrossRef]
- Wang, N.; Wang, A.; Xie, J.; He, M. Responses of soil fungal and archaeal communities to environmental factors in an ongoing antimony mine area. Sci. Total Environ. 2019, 652, 1030–1039. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Xi, J.; Lu, X.; Xie, J. Antimony, arsenic and other toxic elements in the topsoil of an antimony mine area. In Molecular Environmental Soil Science at the Interfaces in the Earth’s Critical Zone; Springer: Berlin/Heidelberg, Germany, 2010; pp. 58–61. [Google Scholar]
- Wu, F.; Fu, Z.; Liu, B.; Mo, C.; Chen, B.; Corns, W.; Liao, H. Health risk associated with dietary co-exposure to high levels of antimony and arsenic in the world’s largest antimony mine area. Sci. Total Environ. 2011, 409, 3344–3351. [Google Scholar] [CrossRef]
- Filella, M.; Philippo, S.; Belzile, N.; Chen, Y.W.; Quentel, F. Natural attenuation processes applying to antimony: A study in the abandoned antimony mine in Goesdorf, Luxembourg. Sci. Total Environ. 2009, 407, 6205–6216. [Google Scholar] [CrossRef]
- Amarasiriwardena, D.; Wu, F. Antimony: Emerging toxic contaminant in the environment Preface. Microchem. J. 2011, 97, 1–3. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, S.; He, M. Bacterial community profile of contaminated soils in a typical antimony mining site. Environ. Sci. Pollut. Res. 2018, 25, 141–152. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, G.; Amstatter, K.; Bue, H.L.; Cornelissen, G.; Breedveld, G.D.; Henriksen, T.; Mulder, J. Antimony (Sb) Contaminated Shooting Range Soil: Sb Mobility and Immobilization by Soil Amendments. Environ. Sci. Technol. 2013, 47, 6431–6439. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Xie, J.; Xi, J.; Lu, X. Heavy metal pollution of the world largest antimony mine-affected agricultural soils in Hunan province (China). J. Soils Sediments 2010, 10, 827–837. [Google Scholar] [CrossRef]
- Matyáš, R.; Pachman, B. Primary Explosives; Springer: Berlin/Heidelberg, Germany, 2013; p. 350. [Google Scholar]
- Hu, X.; He, M.; Kong, L. Photopromoted oxidative dissolution of stibnite. Appl. Geochem. 2015, 61, 53–61. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Coburn, M.D.; Meyer, T.J.; Wetzler, M. Green primary explosives: 5-Nitrotetrazolato-N2-ferrate hierarchies. Proc. Natl. Acad. Sci. USA 2006, 103, 10322–10327. [Google Scholar] [CrossRef] [Green Version]
- Brede, U.; Hagel, R.; Redecker, K.H.; Weuter, W. Primer compositions in the course of time: From black powder and SINOXID to SINTOX compositions and SINCO booster. Propellants Explos. Pyrotech. 1996, 21, 113–117. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, X.; Xia, T.; Li, N.; Zhang, W. Environmental Risk Assessment of Antimony in Contaminated Soil by Primary Explosives. Res. Environ. Sci. 2020, 33, 485–493. [Google Scholar]
- Okkenhaug, G.; Zhu, Y.G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, G.; Lin, J.; Zeng, X.; Ma, X.; Wang, X.; Wang, S.; Jia, Y. The stability of Fe(III)-As(V) co-precipitate in the presence of ascorbic acid: Effect of pH and Fe/As molar ratio. Chemosphere 2019, 218, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, S.; Gomez, M.A.; Wang, Y.; Jia, Y. Long-term stability of the Fe(III)-As(V) coprecipitates: Effects of neutralization mode and the addition of Fe(II) on arsenic retention. Chemosphere 2019, 237, 124503. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Meier, N.; Schulin, R. Use of soil amendments to immobilize antimony and lead in moderately contaminated shooting range soils. J. Hazard. Mater. 2017, 324, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Filella, M.; Belzile, N.; Chen, Y.W. Antimony in the environment: A review focused on natural waters II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Majs, F.; Barker, A.J.; Douglas, T.A.; Trainor, T.P. Oxidation and mobilization of metallic antimony in aqueous systems with simulated groundwater. Geochim. Cosmochim. Acta 2014, 132, 16–30. [Google Scholar] [CrossRef]
- Filella, M.; Williams, P.A.; Belzile, N. Antimony in the environment: Knowns and unknowns. Environ. Chem. 2009, 6, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Silvetti, M.; Vasileiadis, S.; Donner, E.; Diquattro, S.; Deiana, S.; Lombi, E.; Castaldi, P. Use of municipal solid wastes for chemical and microbiological recovery of soils contaminated with metal(loid)s. Soil Biol. Biochem. 2017, 111, 25–35. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Moench, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to goethite: Influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef]
- Kong, L.; He, M. Mechanisms of Sb(III) photooxidation by the excitation of organic Fe(III) complexes. Environ. Sci. Technol. 2016, 50, 6974–6982. [Google Scholar] [CrossRef] [PubMed]
- Almas, A.R.; Pironin, E.; Okkenhaug, G. The partitioning of Sb in contaminated soils after being immobilization by Fe-based amendments is more dynamic compared to Pb. Appl. Geochem. 2019, 108, 104378. [Google Scholar] [CrossRef]
- Wang, Y.; Michel, F.M.; Levard, C.; Choi, Y.; Eng, P.J.; Brown, G.E., Jr. Competitive Sorption of Pb(II) and Zn(II) on Polyacrylic Acid-Coated Hydrated Aluminum-Oxide Surfaces. Environ. Sci. Technol. 2013, 47, 12131–12139. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Silvetti, M.; Castaldi, P.; Mele, E.; Deiana, P.; Deiana, S. Stabilising metal(loid)s in soil with iron and aluminium-based products: Microbial, biochemical and plant growth impact. J. Environ. Manag. 2014, 139, 146–153. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Peters, R.E.; Laird, B.D.; Ma, W.K.; Wickstrom, M.; Stephenson, G.L.; Siciliano, S.D. Human Exposure Assessment: A Case Study of 8 PAH Contaminated Soils Using in Vitro Digestors and the Juvenile Swine Model. Environ. Sci. Technol. 2011, 45, 4586–4593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Pervaiz, A.; Kong, L.; He, M. Comparison of diffusive gradients in thin-films (DGT) and chemical extraction methods for predicting bioavailability of antimony and arsenic to maize. Geoderma 2018, 332, 1–9. [Google Scholar] [CrossRef]
- Rauret, G.; Lopez-Sanchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Weng, H.-X.; Ma, X.-W.; Fu, F.-X.; Zhang, J.-J.; Liu, Z.; Tian, L.-X.; Liu, C. Transformation of heavy metal speciation during sludge drying: Mechanistic insights. J. Hazard. Mater. 2014, 265, 96–103. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Liu, F.; Shen, C.; Li, F.; Yang, B.; Huang, M.; Sang, W. Nanoscale iron (oxyhydr)oxide-modified carbon nanotube filter for rapid and effective Sb(III) removal. Rsc Adv. 2019, 9, 9. [Google Scholar]
- Gao, F.; Ju, T.; Wu, X.; Wang, Y.; Li, X.; Fan, P.; Luan, T.; Zhou, J. Spatial Variability and Autocorrelation Analysis of pH in a Mollisol Tillage Area of Northeast China. Soils 2018, 50, 566–573. [Google Scholar]
- Shen, F.; Liao, R.; Ali, A.; Mahar, A.; Guo, D.; Li, R.; Xining, S.; Awasthi, M.K.; Wang, Q.; Zhang, Z. Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County, China. Ecotoxicol. Environ. Saf. 2017, 139, 254–262. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.-M.; Yang, Q.; Zeng, G.-M.; Zhang, Y.; Liao, D.-X.; Liu, J.-J.; Hu, J.-M.; Guo, L. Total concentrations and speciation of heavy metals in municipal sludge from Changsha, Zhuzhou and Xiangtan in middle-south region of China. J. Hazard. Mater. 2008, 160, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Vourlias, G.; Stavropoulos, G.; Mitrakas, M. Kilogram-scale synthesis of iron oxy-hydroxides with improved arsenic removal capacity: Study of Fe(II) oxidation-precipitation parameters. Water Res. 2012, 46, 5255–5267. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Bai, H.; Wang, W.; Zhang, Q. Enhanced arsenic removal from water and easy handling of the precipitate sludge by using FeSO4 with CaCO3 to Ca(OH)2. Chemosphere 2019, 231, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Dempsey, B.A.; Burgos, W.D. Solubilitv of hematite revisited: Effects of hydration. Environ. Sci. Technol. 2007, 41, 7303–7308. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Silvetti, M.; Deiana, S.; Deiana, P.; Castaldi, P. Long-term influence of red mud on As mobility and soil physico-chemical and microbial parameters in a polluted sub-acidic soil. J. Hazard. Mater. 2011, 185, 1241–1248. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.; Silvetti, M.; Garau, G.; Deiana, S.; Castaldi, P. Influence of iron-rich water treatment residues and compost on the mobility of metal(loid)s in mine soils. Geoderma 2016, 283, 1–9. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.-K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- Serafimovska, J.M.; Arpadjan, S.; Stafilov, T.; Tsekova, K. Study of the antimony species distribution in industrially contaminated soils. J. Soils Sediments 2013, 13, 294–303. [Google Scholar] [CrossRef]
- Kong, L.; He, M.; Hu, X. Rapid photooxidation of Sb(III) in the presence of different Fe(III) species. Geochim. Cosmochim. Acta 2016, 180, 214–226. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Leverett, P.; Reynolds, J.K.; Roper, A.J.; Williams, P.A. Tripuhyite and schafarzikite: Two of the ultimate sinks for antimony in the natural environment. Mineral. Mag. 2012, 76, 891–902. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, Z.; Yang, K.; Wang, Z.; Zhou, C.; Li, Y.; Cao, Z.; Xu, X. Coagulation removal of Sb(V) from textile wastewater matrix with enhanced strategy: Comparison study and mechanism analysis. Chemosphere 2019, 237, 124494. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Sharp, E.; Pidou, M.; Molinder, R.; Parsons, S.A.; Jefferson, B. Comparison of coagulation performance and floc properties using a novel zirconium coagulant against traditional ferric and alum coagulants. Water Res. 2012, 46, 4179–4187. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.H.; Karimi, B.; Rajaei, M.S. The effect of aeration on advanced coagulation, flotation and advanced oxidation processes for color removal from wastewater. J. Mol. Liq. 2016, 223, 75–80. [Google Scholar] [CrossRef]
- Essington, M.E.; Stewart, M.A. Influence of Temperature and pH on Antimonate Adsorption by Gibbsite, Goethite, and Kaolinite. Soil Sci. 2015, 180, 54–66. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, J.; Kumar, A.; Sankararamakrishnan, N. Removal of antimonite (Sb(III)) and antimonate (Sb(V)) using zerovalent iron decorated functionalized carbon nanotubes. RSC Adv. 2016, 6, 95865–95878. [Google Scholar] [CrossRef]
- Dai, C.; Zhou, Z.; Zhou, X.; Zhang, Y. Removal of Sb (III) and Sb(V) from Aqueous Solutions Using nZVI. Water Air Soil Pollut. 2014, 225, 1799. [Google Scholar] [CrossRef]
- Daoyong, Z.; Xiangliang, P.A.N.; Guijin, M.U.; Huiming, G.U. Removal of antimony from water by adsorption to red mud. Technol. Water Treat. 2008, 34, 34–37. [Google Scholar]
- Kameda, T.; Yagihashi, N.; Park, K.-S.; Grause, G.; Yoshioka, T. Preparetation of Fe-Al layered double hydroxide and its application in Sb removal. Fresenius Environ. Bull. 2009, 18, 1006–1010. [Google Scholar]
- Hai-ying, Z.; You-cai, Z.; Guo-xin, Z.; Jing-yu, Q.I.; Cui-xiang, G.U.O.; Li-jie, S. Leaching Behavior of Heavy Metals from MSWI Fly Ash. Environ. Sci. Technol. 2010, 33, 130–132. [Google Scholar]
- Li, J.; Chen, J.; Cao, H.; He, P. Influence of pH on leaching of pollutants from sewage sludge. Chin. J. Environ. Eng. 2013, 7, 4983–4989. [Google Scholar]
- Cui, H.; Yang, X.; Xu, L.; Fan, Y.; Yi, Q.; Lia, R.; Zhou, J. Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil. Rsc. Adv. 2017, 7, 45869–45877. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; He, M. Organic ligand-induced dissolution kinetics of antimony trioxide. J. Environ. Sci. 2017, 56, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Guo, X.; He, M.; Li, S. pH-dependent release characteristics of antimony and arsenic from typical antimony-bearing ores. J. Environ. Sci. 2016, 44, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hockmann, K.; Tandy, S.; Lenz, M.; Reiser, R.; Conesa, H.M.; Keller, M.; Studer, B.; Schulin, R. Antimony retention and release from drained and waterlogged shooting range soil under field conditions. Chemosphere 2015, 134, 536–543. [Google Scholar] [CrossRef]
- Klitzke, S.; Lang, F. Mobilization of Soluble and Dispersible Lead, Arsenic, and Antimony in a Polluted, Organic-rich Soil—Effects of pH Increase and Counterion Valency. J. Environ. Qual. 2009, 38, 933–939. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, F.J.; Zhou, Y.; Zhang, H.J.; Guo, G.L.; Tian, Y. Removal of Cd, Pb, Zn, Cu in smelter soil by citric acid leaching. Chemosphere 2020, 255, 126690. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, S.; Takahashi, Y.; Terada, Y.; Sakata, M. Antimony(V) Incorporation into Synthetic Ferrihydrite, Goethite, and Natural Iron Oxyhydroxides. Environ. Sci. Technol. 2010, 44, 3712–3718. [Google Scholar] [CrossRef]
- Ma, X.; Qin, J.; Zhang, Y. A Comparison of Desorption Behaviors of Sb in Different Soils. J. Agro-Environ. Sci. 2015, 34, 1528–1534. [Google Scholar]
- Du, X.; Cui, S.; Wang, Q.; Han, Q.; Liu, G. Non-competitive and competitive adsorption of Zn (II), Cu (II), and Cd (II) by a granular Fe-Mn binary oxide in aqueous solution. Environ. Prog. Sustain. Energy 2021, 40, e13611. [Google Scholar] [CrossRef]

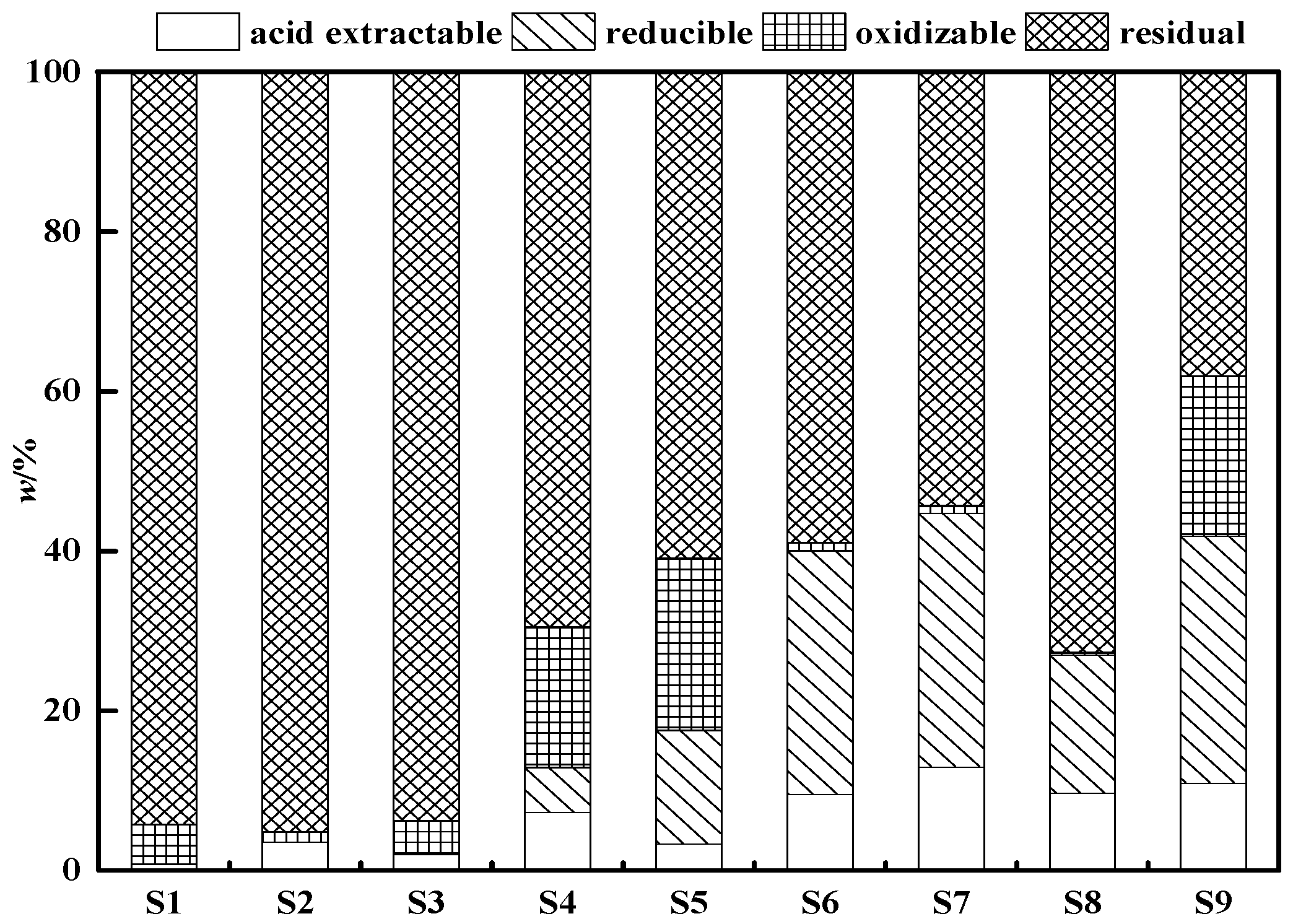


| Soil | pH | Concentrations (mg/kg) | ||||
|---|---|---|---|---|---|---|
| Mn | Fe | Cu | Zn | Sbtot | ||
| S1 | 8.37 ± 1.70 | 499 ± 66.54 | 32,200 ± 876.43 | 25.37 ± 0.17 | 71.19 ± 14.81 | 26.73 ± 2.31 |
| S2 | 7.8 ± 0.27 | 573 ± 36.67 | 30,000 ± 156.47 | 24.29 ± 3.12 | 67.62 ± 0.52 | 61.32 ± 4.27 |
| S3 | 8.11 ± 0.75 | 545 ± 20.82 | 28,900 ± 488.27 | 33.55 ± 1.57 | 83.80 ± 3.50 | 108.01 ± 6.43 |
| S4 | 7.96 ± 0.19 | 413 ± 41.93 | 24,200 ± 893.30 | 232.85 ± 5.87 | 402.75 ± 95.65 | 216.60 ± 9.64 |
| S5 | 7.69 ± 0.40 | 628 ± 16.05 | 31,200 ± 366.70 | 186.74 ± 2.75 | 868.02 ± 78.23 | 267.26 ± 172.5 |
| S6 | 7.93 ± 1.41 | 396 ± 14.83 | 23,000 ± 259.53 | 103.76 ± 5.44 | 331.36 ± 11.83 | 512.09 ± 19.52 |
| S7 | 8.15 ± 1.58 | 513 ± 37.53 | 25,000 ± 456.95 | 312.30 ± 14.83 | 981.60 ± 10.42 | 719.36 ± 23.34 |
| S8 | 7.91 ± 0.08 | 554 ± 52.51 | 23,600 ± 99.28 | 35.26 ± 1.56 | 215.88 ± 7.25 | 953.91 ± 39.72 |
| S9 | 8.25 ± 1.87 | 719 ± 189.57 | 20,000 ± 127.71 | 80.16 ± 3.05 | 1330.05 ± 36.67 | 4255.03 ± 231.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Jiang, Y.; Xia, T.; Xu, F.; Zhang, C.; Zhang, D.; Wu, Z. Antimony Immobilization in Primary-Explosives-Contaminated Soils by Fe–Al-Based Amendments. Int. J. Environ. Res. Public Health 2022, 19, 1979. https://doi.org/10.3390/ijerph19041979
Wang N, Jiang Y, Xia T, Xu F, Zhang C, Zhang D, Wu Z. Antimony Immobilization in Primary-Explosives-Contaminated Soils by Fe–Al-Based Amendments. International Journal of Environmental Research and Public Health. 2022; 19(4):1979. https://doi.org/10.3390/ijerph19041979
Chicago/Turabian StyleWang, Ningning, Yucong Jiang, Tianxiang Xia, Feng Xu, Chengjun Zhang, Dan Zhang, and Zhiyuan Wu. 2022. "Antimony Immobilization in Primary-Explosives-Contaminated Soils by Fe–Al-Based Amendments" International Journal of Environmental Research and Public Health 19, no. 4: 1979. https://doi.org/10.3390/ijerph19041979





