Effects of Growing Rod Technique with Different Surgical Modes and Growth Phases on the Treatment Outcome of Early Onset Scoliosis: A 3-D Finite Element Analysis
Abstract
:1. Introduction
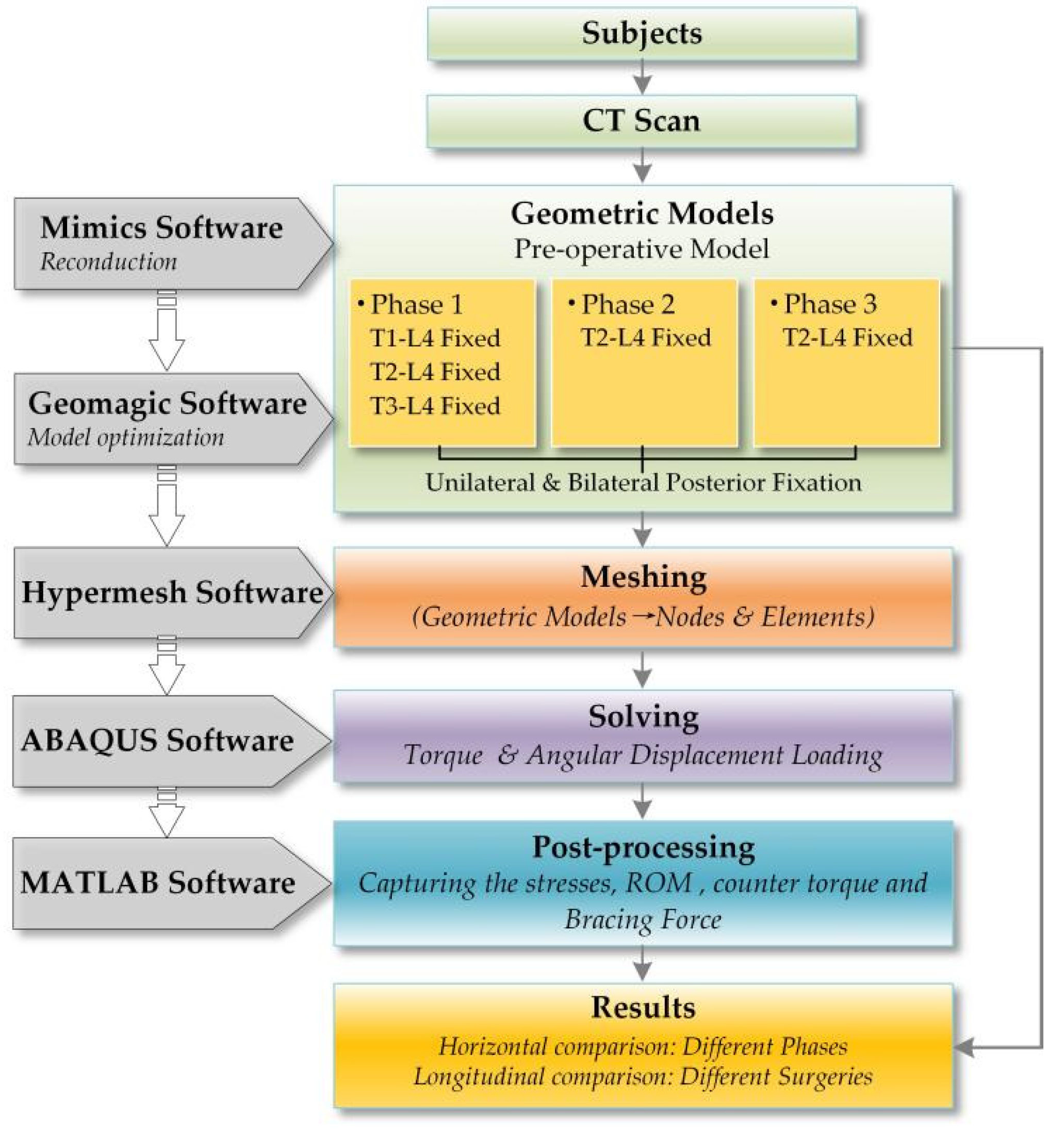
2. Materials and Methods
2.1. Subjects
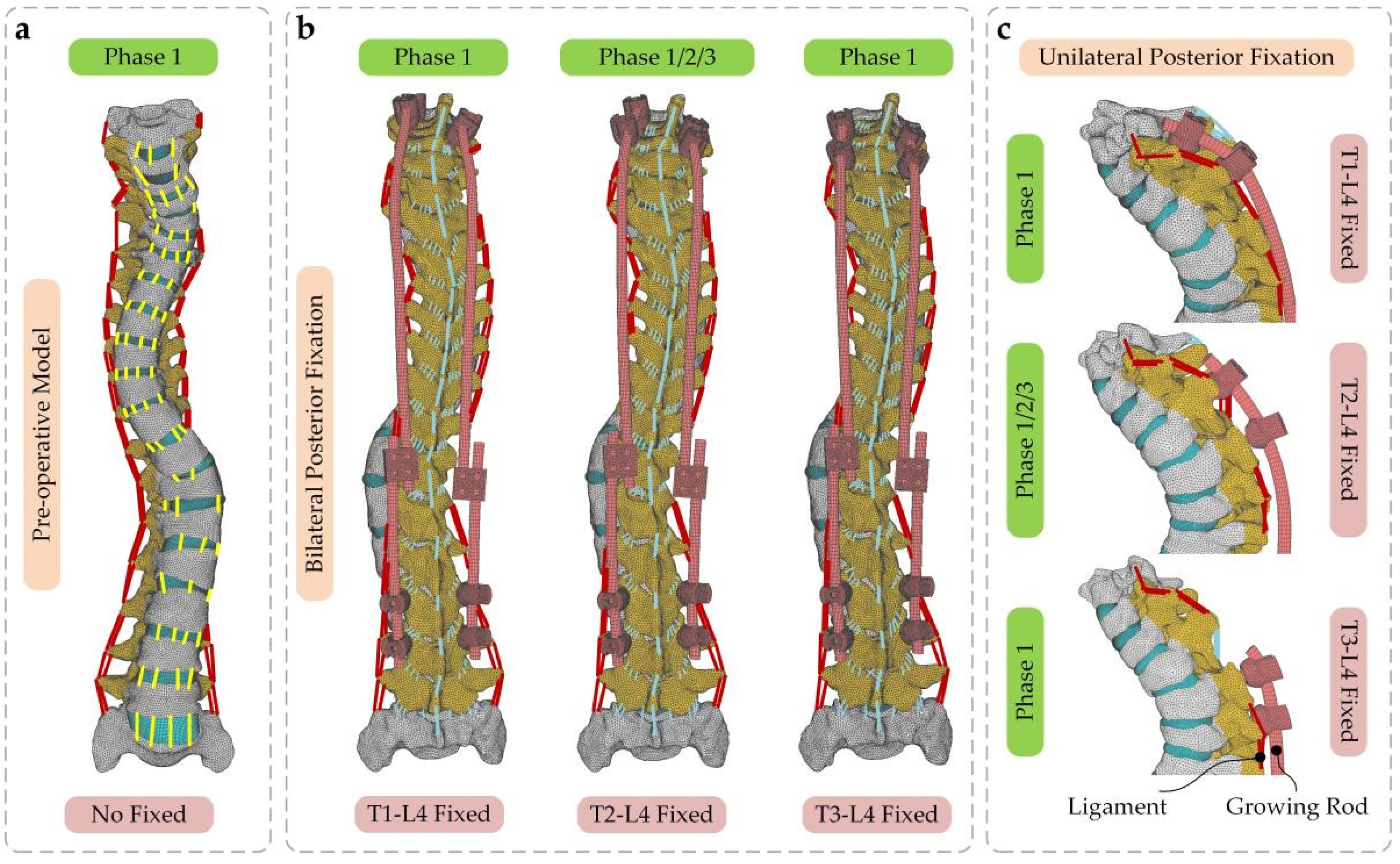
2.2. Creating the Base Model
2.3. Adopted Mesh and Material Properties
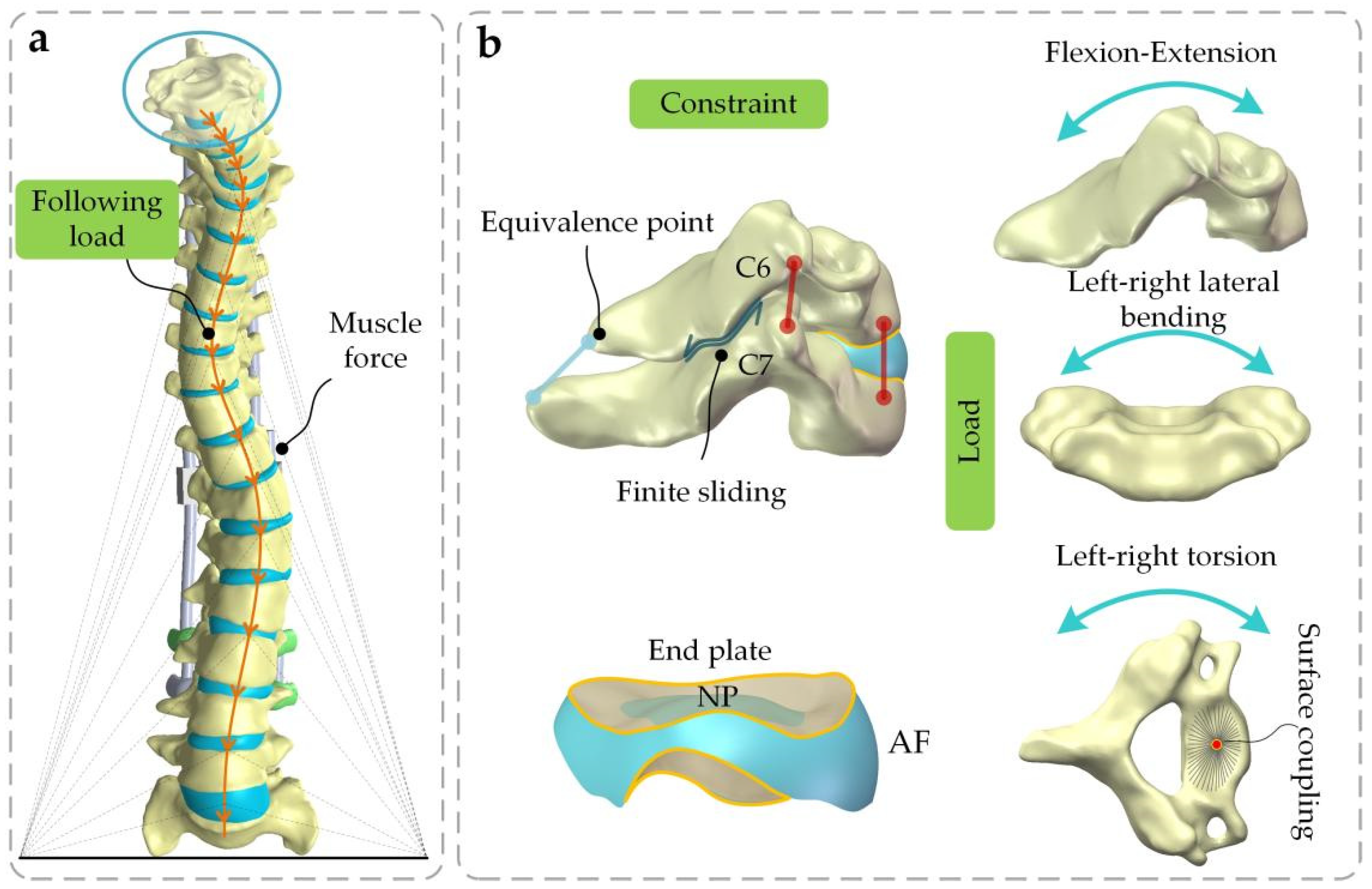
2.4. Boundary and Loading Conditions
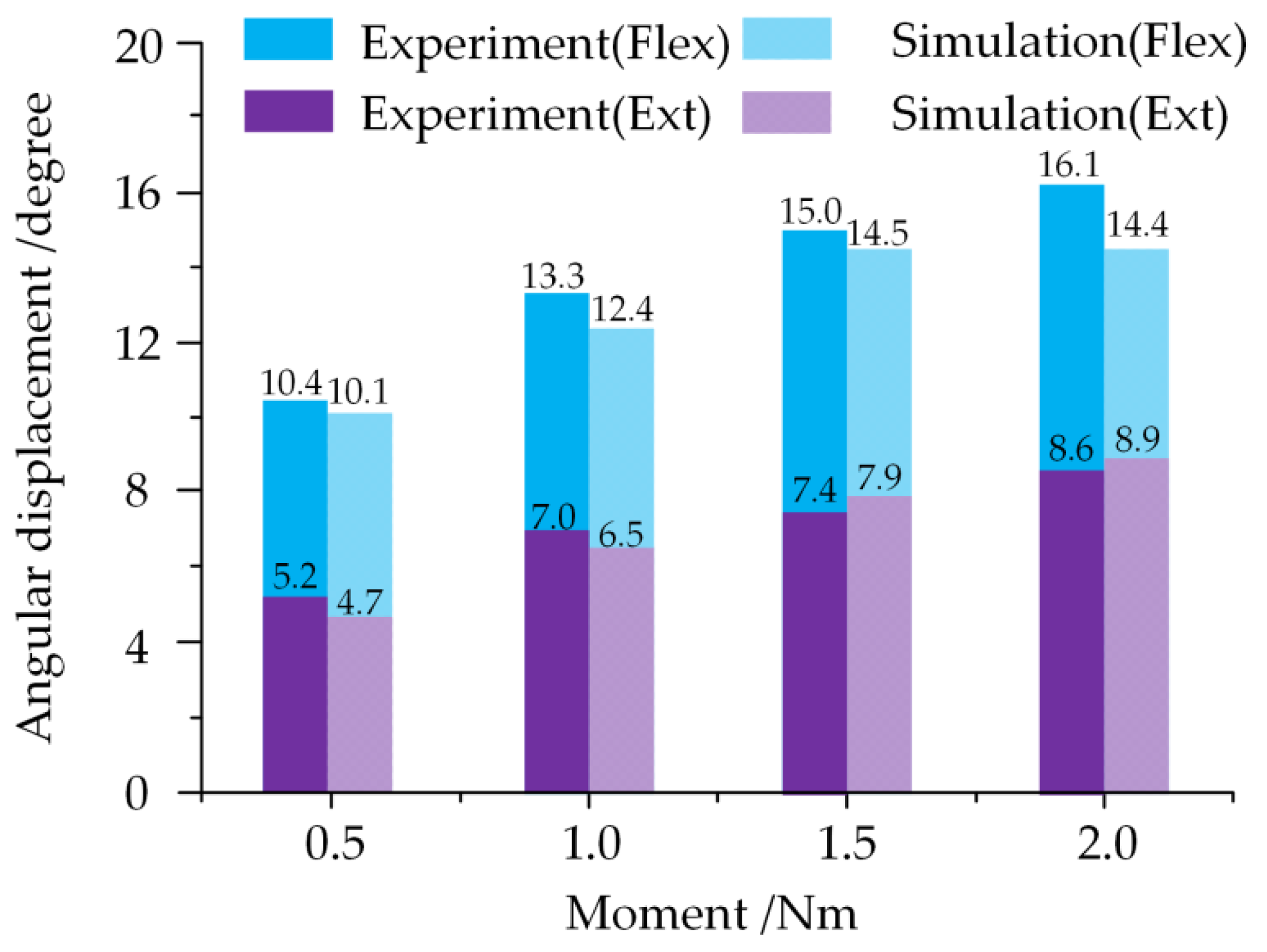
2.5. Validation of the Model
3. Results
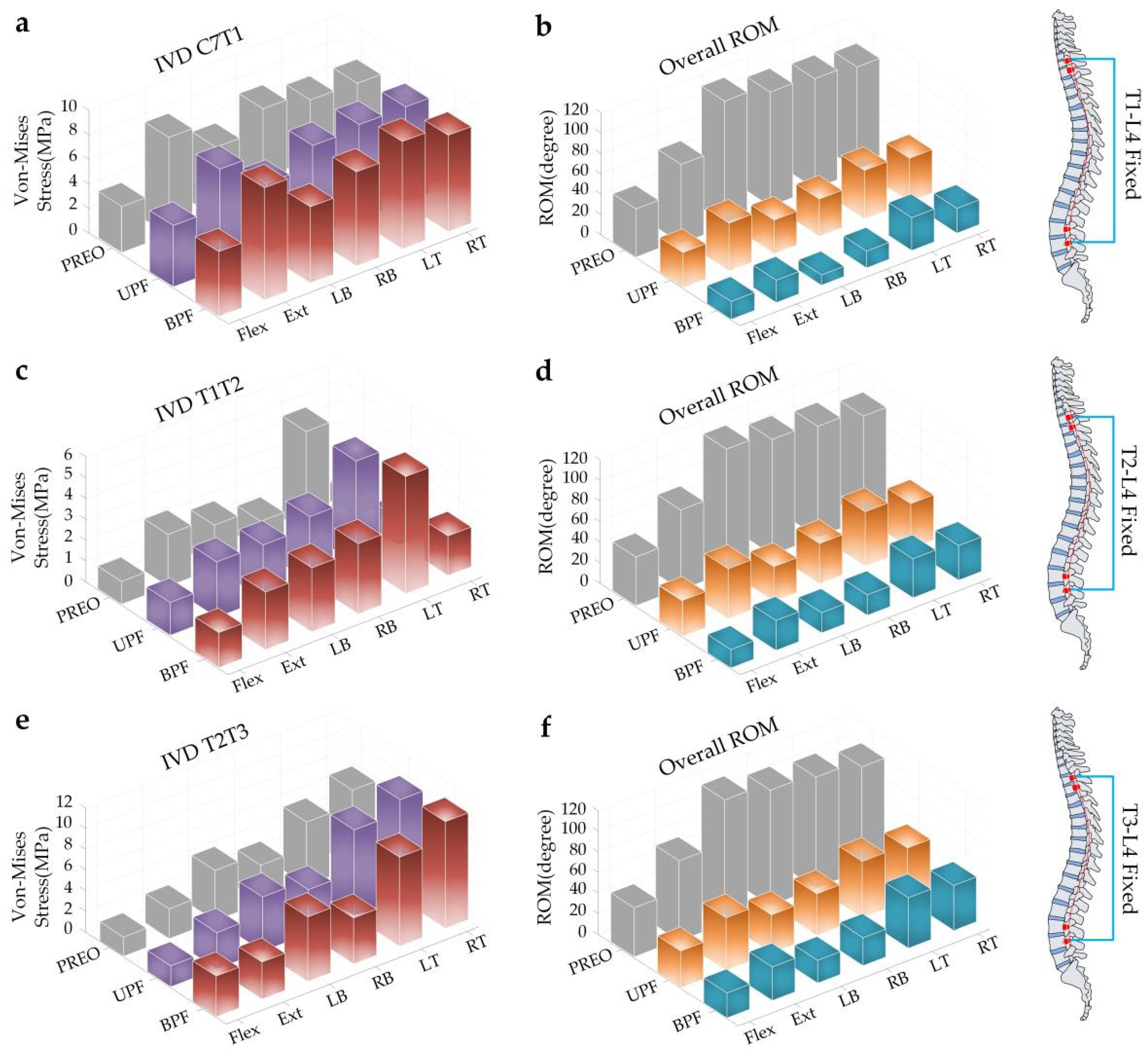
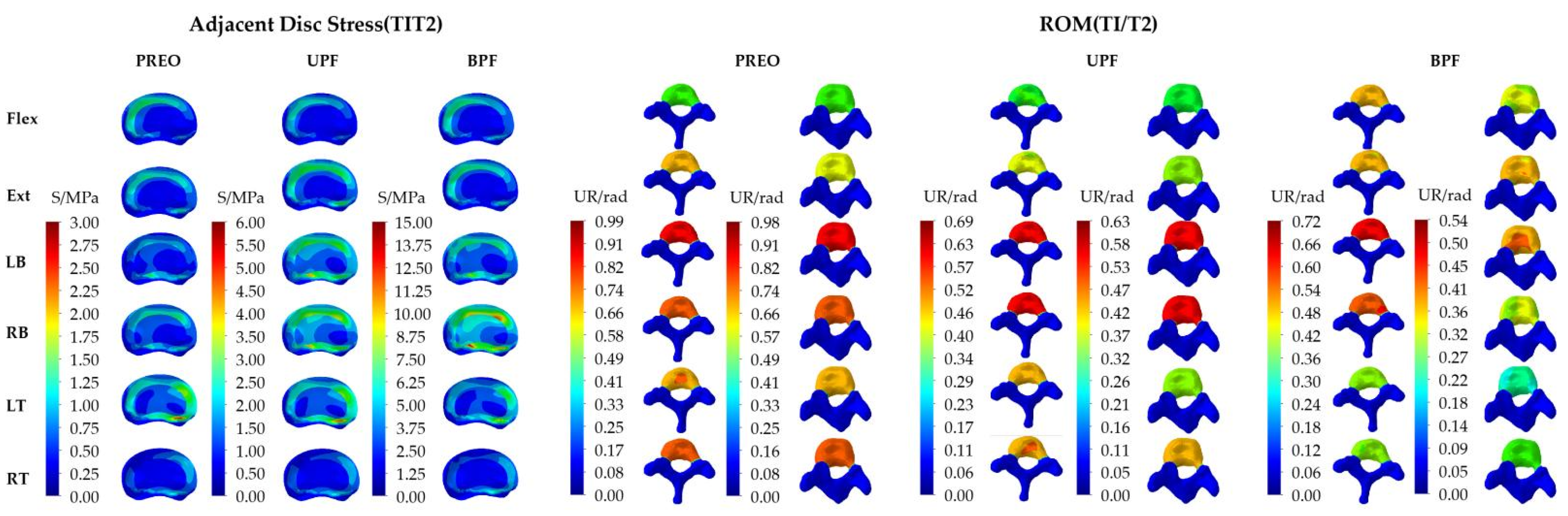
3.1. IVD Stress of SAS and Overall ROM of Spine under Torque Loading
3.2. IVD Stress and ROM of SAS under Angular Displacement
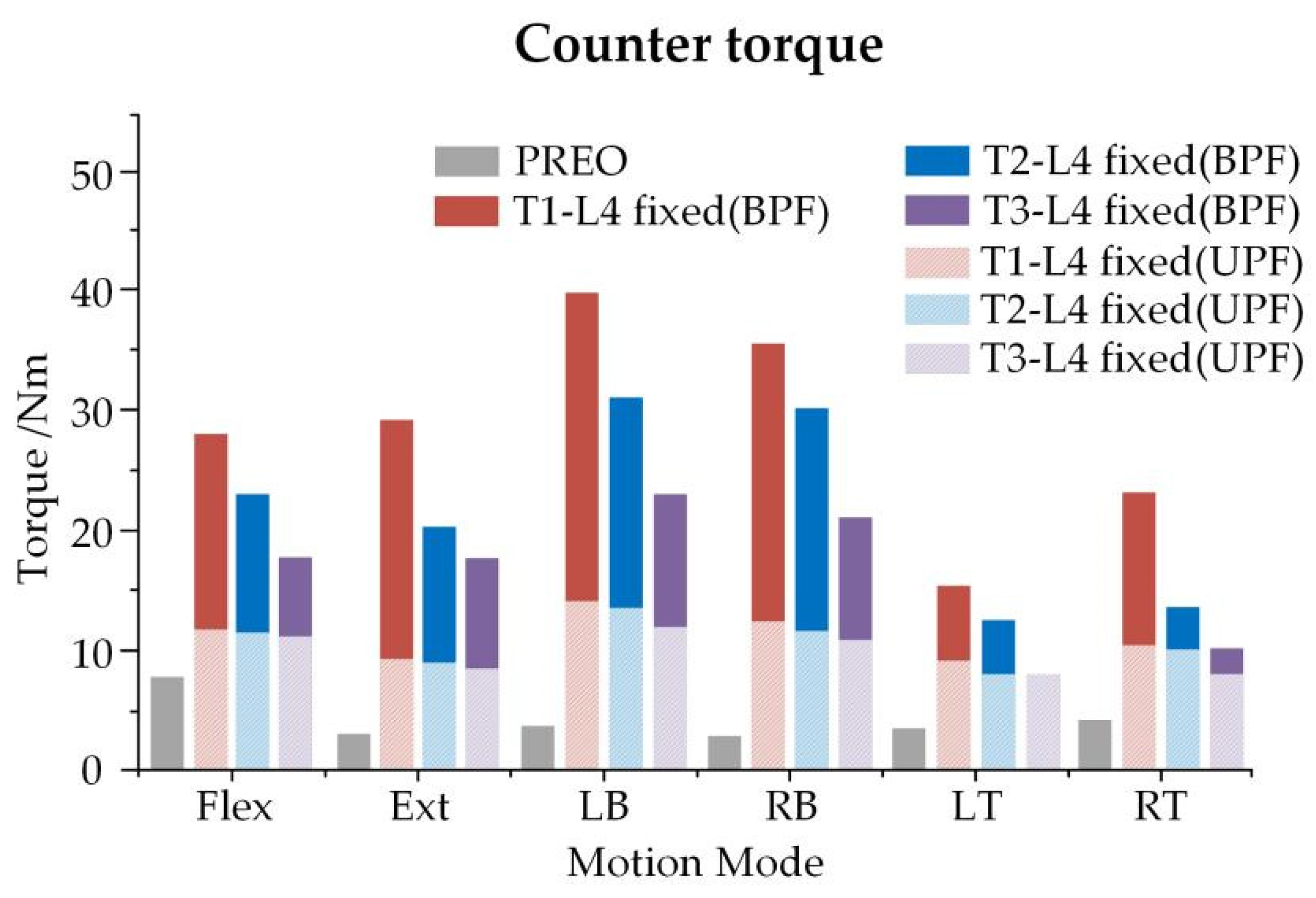
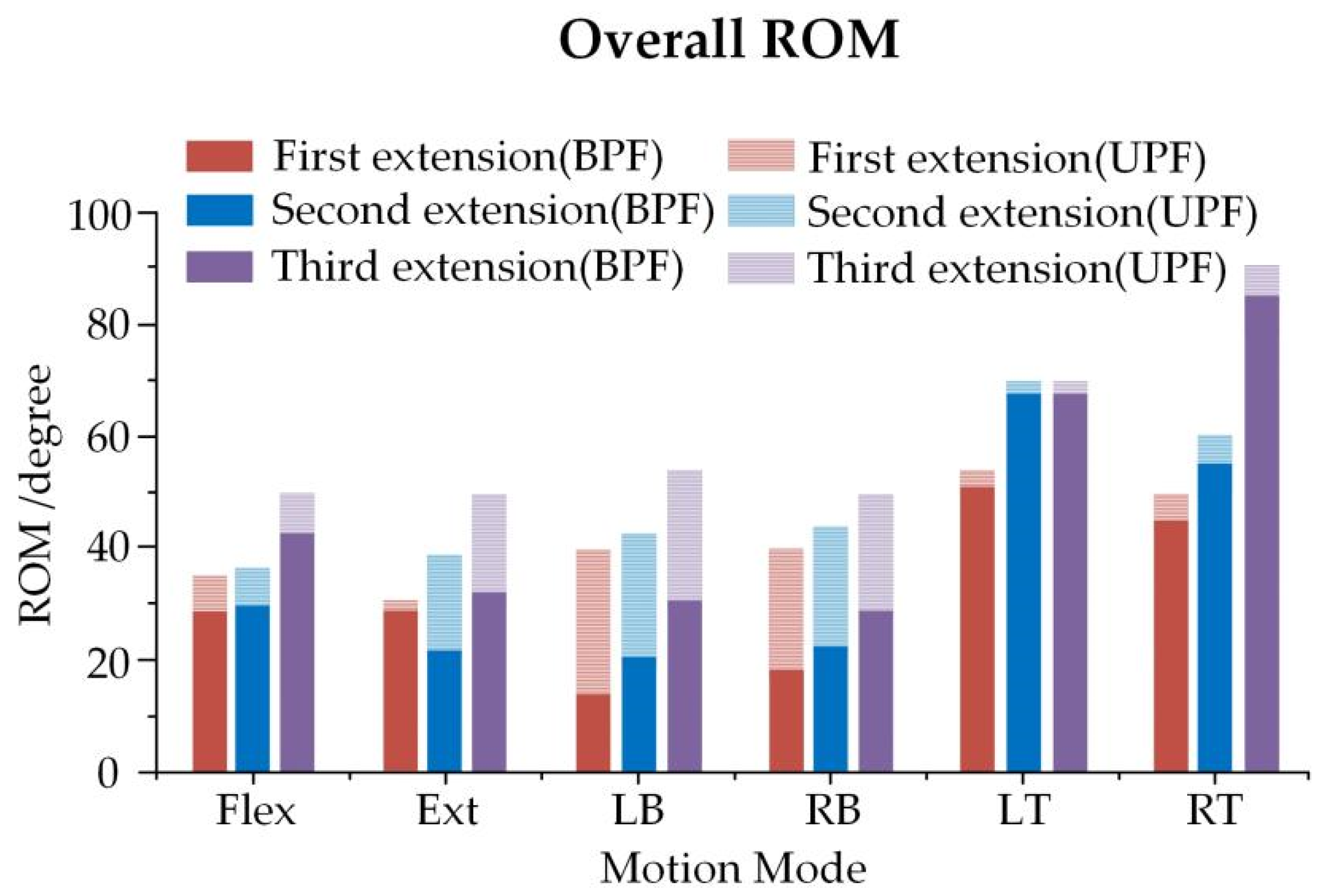
3.3. Overall ROM of Spine and Bracing Force of Growing Rod in Three Growth Phases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Hawary, R.; Akbarnia, B.A. Early onset scoliosis—Time for consensus. Spine Deform. 2015, 3, 105–106. [Google Scholar] [CrossRef]
- Cunin, V. Early-onset scoliosis-current treatment. Orthop. Traumatol. Surg. Res. Otsr. 2015, 101, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Canavese, F.; Dimeglio, A. Normal and abnormal spine and thoracic cage development. World J. Orthop. 2013, 4, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kotwicki, T.; Chowanska, J.; Kinel, E.; Czaprowski, D.; Janusz, P.; Tomaszewski, M. Optimal management of idiopathic scoliosis in adolescence. Adolesc. Health Med. Ther. 2013, 4, 59–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, G.H.; Akbarnia, B.A.; Kostial, P.; Poe-Kochert, C.; Armstrong, D.; Roh, J.; Lowe, R.; Asher, M.A.; Marks, D.S. Comparison of single and dual growing rod techniques followed through definitive surgery: A preliminary study. Spine 2005, 30, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Bowey, A. Growing rod instrumentation in early onset scoliosis. Spine J. 2017, 17, S328. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, G.X.; Wang, Y.P.; Zhang, J.G.; Shen, J.X.; Li, S.G.; Zhao, H.; Jiang, Y.; Li, Y. Comparison of initial efficacy between single and dual growing rods in treatment of early onset scoliosis. Chin. Med. J. 2012, 125, 2862–2866. [Google Scholar] [CrossRef]
- Xu, G.-J.; Fu, X.; Tian, P.; Ma, J.-X.; Ma, X.-L. Comparison of single and dual growing rods in the treatment of early onset scoliosis: A meta-analysis. J. Orthop. Surg. Res. 2016, 11, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driscoll, M.; Aubin, C.-E.; Moreau, A.; Parent, S. Biomechanical comparison of fusionless growing modulation corrective techniques in pediatric scoliosis. Med. Biol. Eng. Comput. 2011, 49, 1437–1445. [Google Scholar] [CrossRef]
- Quick, M.; Grant, C.; Adam, C.; Askin, G.; Labrom, R.; Pearcy, M. A biomechanical investigation of dual growing rods used for fusionless scoliosis correction. Clin. Biomech. 2015, 30, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Aakash, A.; Anand, K.A.; Arvind, J.; Vijay, G. Smaller Interval Distractions May Reduce Chances of Growth Rod Breakage Without Impeding Desired Spinal Growth: A Finite Element Study. Spine Deform. 2014, 2, 430–436. [Google Scholar] [CrossRef]
- Agarwal, A.; Jayaswal, A.; Goel, V.K.; Agarwal, A.K. Patient-specific distraction regimen to avoid growth-rod failure. Spine 2018, 43, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Schmoelz, W.; Huber, J.F.; Nydegger, T.; Claes, L.; Wilke, H.J. Influence of a dynamic stabilization system on load bearing of a bridged disc: An in vitro study of intradiscal pressure. Eur. Spine J. 2006, 15, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A.; McNally, D.S.; Dolan, P. “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J. Bone Jt. Surg. Br. 1996, 78, 965–972. [Google Scholar] [CrossRef]
- Nachemson, A.; Morris, J. Lumbar discometry lumbar intradiscal pressure measurements in vivo. Lancet 1963, 7291, 1140–1142. [Google Scholar] [CrossRef]
- Currey, J.D. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J. Biomech. 2004, 37, 549–556. [Google Scholar] [CrossRef]
- Berteau, J.-P.; Baron, C.; Pithioux, M.; Launay, F.; Chabrand, P.; Lasaygues, P. In vitro ultrasonic and mechanic characterization of the modulus of elasticity of children cortical bone. Ultrasonics 2014, 54, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Kopperdahl, D.L.; Keaveny, T.M. Yield strain behavior of trabecular bone. J. Biomech. 1998, 31, 601–608. [Google Scholar] [CrossRef]
- Rohlmann, A.; Zander, T.; Rao, M.; Bergmann, G. Applying a follower load delivers realistic results for simulating standing. J. Biomech. 2009, 42, 1520–1526. [Google Scholar] [CrossRef]
- Kurutz, M.; Oroszváry, L. Finite element analysis of weightbath hydrotraction treatment of degenerated lumbar spine segments in elastic phase. J. Biomech. 2010, 43, 433–441. [Google Scholar] [CrossRef]
- Aakash, A.; Anand, K.A.; Arvind, J.; Vijay, G. Distraction magnitude and frequency affects the outcome in juvenile idiopathic patients with growing rods: Finite element study using a representative scoliotic spine model. Spine J. 2015, 15, 1848–1855. [Google Scholar] [CrossRef]
- Yoganandan, N.; Pintar, F.A.; Kumaresan, S.; Gennarelli, T.A. Pediatric and small female neck injury scale factors and tolerance based on 433 human spine biomechanical characteristics. In Proceedings of the International Ircobi Conference on the Biomechanics of Impact, Montpellier, France, 20–22 September 2000. [Google Scholar]
- Patwardhan, A.G.; Meade, K.P.; Lee, B.A. Frontal plane model of the lumbar spine subjected to a follower load: Implications for the role of muscles. J. Biomech. Eng. 2001, 123, 212–217. [Google Scholar] [CrossRef]
- Pasha, S.; Aubin, C.-E.; Parent, S.; Labelle, H.; Mac-Thiong, J.-M. Biomechanical loading of the sacrum in adolescent idiopathic scoliosis. Clin. Biomech. 2014, 29, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdes, F.J.; Lau, S.; Riley, P.; Lamp, J.; Kent, R. The biomechanics of the pediatric and adult human thoracic spine. Ann. Adv. Automot. Med. 2011, 55, 193–206. [Google Scholar] [PubMed]
- Li, Z.; Song, G.; Su, Z.; Wang, G. Development, validation, and application of ligamentous cervical spinal segment C6-C7 of a six-year-old child and an adult—ScienceDirect. Comput. Methods Programs Biomed. 2019, 183, 105080. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.F. The Biomechanics of the Perinatal, Neonatal and Pediatric Cervical Spine: Investigation of the Tensile, Bending and Viscoelastic Response, American; Duke University: Durham, NC, USA, 2012. [Google Scholar]
- El-Hawary, R.; Sturm, P.; Cahill, P.; Samdani, A.; Vitale, M.; Gabos, P.; Bodin, N.; D’Amato, C.; Harris, C.; Al Khudairy, A.; et al. What is the risk of developing proximal junctional kyphosis during growth friendly treatments for early-onset scoliosis. J. Pediatr. Orthop. 2015, 37, 86. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Uno, K.; Suzuki, T.; Kawakami, N.; Tsuji, T.; Yanagida, H.; Ito, M.; Hirano, T.; Yamazaki, K.; Minami, S.; et al. Risk factors for proximal junctional kyphosis associated with dual-rod growing-rod surgery for early-onset scoliosis. Clin. Spine Surg. 2016, 29, 428–433. [Google Scholar] [CrossRef]
- Cammarata, M.; Wang, X.; Mac-Thiong, J.M.; Aubin, C. Biomechanical analysis of proximal junctional kyphosis: Preliminary results. Stud. Health Technol. Inform. 2012, 176, 299–302. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Wilke, H.-J.; Liebsch, C.; Villa, T.; Luca, A.; Galbusera, F.; Brayda-Bruno, M. Biomechanical in vitro comparison of anterior column release and pedicle subtraction osteotomy for severe sagittal imbalance correction. Eur. Spine J. 2020, 29, 36–44. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, L.; Brayda-Bruno, M.; Liebsch, C.; Villa, T.; Luca, A.; Galbusera, F.; Wilke, H.-J. Biomechanical advantages of supplemental accessory and satellite rods with and without interbody cages implantation for the stabilization of pedicle subtraction osteotomy. Eur. Spine J. 2018, 27, 2357–2366. [Google Scholar] [CrossRef]
- Luca, A.; Ottardi, C.; Lovi, A.; Brayda-Bruno, M.; Villa, T.; Galbusera, F. Anterior support reduces the stresses on the posterior instrumentation after pedicle subtraction osteotomy: A finite element study. Eur. Spine J. 2017, 26, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Rimnac, C. Biomechanics of immature human cortical bone: A systematic review. J. Mech. Behav. Biomed. Mater. 2022, 125, 104889. [Google Scholar] [CrossRef]
- Berteau, J.-P.; Gineyts, E.; Pithioux, M.; Baron, C.; Boivin, G.; Lasaygues, P.; Chabrand, P.; Follet, H. Ratio between mature and immature enzymatic cross-links correlates with post-yield cortical bone behavior: An insight into greenstick fractures of the child fibula. Bone 2015, 79, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D.; Pond, C.M. Mechanical properties of very young bone in the axis deer (Axis axis) and humans. J. Zool. 1989, 218, 59–67. [Google Scholar] [CrossRef]
- El-Rich, M.; Shirazi-Adl, A. Effect of load position on muscle forces, internal loads and stability of the human spine in upright postures. Comput. Methods Biomech. Biomed. Eng. 2005, 8, 359–368. [Google Scholar] [CrossRef]
- Kamaci, S.; Demirkiran, G.; Ismayilov, V.; Olgun, Z.D.; Yazici, M. The effect of dual growing rod instrumentation on the apical vertebral rotation in early-onset idiopathic scoliosis. J. Pediatr. Orthop. 2014, 34, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Acaroglu, E.; Yazici, M.; Alanay, A.; Surat, A. Three-dimensional evolution of scoliotic curve during instrumentation without fusion in young children. J. Pediatr. Orthop. 2002, 22, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Sponseller, P.D.; Thompson, G.H.; Akbarnia, B.A.; Emans, J.B.; Yazici, M.; Skaggs, D.L.; Shah, S.A.; Salari, P.; Poe-Kochert, C. Growing rod fractures: Risk factors and opportunities for prevention. Spine 2011, 36, 1639. [Google Scholar] [CrossRef]
- Oetgen, M.E.; Blakemore, L.C. Growing rods in early-onset scoliosis. Semin. Spine Surg. 2012, 24, 155–163. [Google Scholar] [CrossRef]
- Teli, M. Measurement of forces generated during distraction of growing-rods in early onset scoliosis. World J. Orthop. 2012, 3, 15. [Google Scholar] [CrossRef]




| Element Construction | Element Type | Elasticity Modulus/MPa | Poisson Ratio | Cross-Sectional Area/mm2 | Scale Factors | Reference |
|---|---|---|---|---|---|---|
| Cortical bone | Hexahedron | 1.344 × 104 | 0.30 | — | — | [16,17] |
| Cancellous bone | Quadrilateral | 2.41 × 102 | 0.30 | — | 0.805 (b) | [18] |
| Posterior | Hexahedron | 3.5 × 103 | 0.25 | — | (a) | [19] |
| Endplate cartilage | Quadrilateral | 2.38 × 10 | 0.40 | — | (a) | [20] |
| Nucleus pulposus | Hexahedron | 1.0 | 0.49 | — | (a) | |
| Annulus fibrosus | Hexahedron | 4.2 | 0.45 | — | 0.782 (b) | |
| ALL | Three-dimensional truss | 7.8 | 0.12 | 63.7 | 0.893 (b) | [20] |
| PLL | 1.0 × 10 | 0.11 | 20.0 | |||
| ITL | 1.0 × 10 | 0.18 | 1.80 | |||
| CL | 7.5 | 0.25 | 30.0 | |||
| ISL | 8.0 | 0.14 | 30.0 | |||
| SSL | 1.0 × 10 | 0.20 | 40.0 | |||
| LF | 1.5 × 10 | 0.062 | 40.0 | |||
| Growing rod | Hexahedron | 1.1 × 105 | 0.30 | — | (a) | [21] |
| Vertebral | Percentage of BW (%) | Following Load(N) |
|---|---|---|
| C6 | 6.9 | 20.7 |
| C7 | 1.1 | 3.3 |
| T1 | 1.1 | 3.3 |
| T2 | 1.1 | 3.3 |
| T3 | 1.3 + 4.0 (Superior limbs) | 15.9 |
| T4 | 1.3 + 4.0 (Superior limbs) | 15.9 |
| T5 | 1.3 + 4.0 (Superior limbs) | 15.9 |
| T6 | 1.3 | 3.9 |
| T7 | 1.4 | 4.2 |
| T8 | 1.5 | 4.5 |
| T9 | 1.6 | 4.8 |
| T10 | 2.0 | 6.0 |
| T11 | 2.1 | 6.3 |
| T12 | 2.5 | 7.5 |
| L1 | 2.4 | 7.2 |
| L2 | 2.4 | 7.2 |
| L3 | 2.3 | 6.9 |
| L4 | 2.6 | 7.8 |
| L5 | 2.6 | 7.8 |
| S1 | 2.6 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, B.; Lu, D.; Wu, X.; Xu, Y.; Ma, C.; Wu, S. Effects of Growing Rod Technique with Different Surgical Modes and Growth Phases on the Treatment Outcome of Early Onset Scoliosis: A 3-D Finite Element Analysis. Int. J. Environ. Res. Public Health 2022, 19, 2057. https://doi.org/10.3390/ijerph19042057
Pei B, Lu D, Wu X, Xu Y, Ma C, Wu S. Effects of Growing Rod Technique with Different Surgical Modes and Growth Phases on the Treatment Outcome of Early Onset Scoliosis: A 3-D Finite Element Analysis. International Journal of Environmental Research and Public Health. 2022; 19(4):2057. https://doi.org/10.3390/ijerph19042057
Chicago/Turabian StylePei, Baoqing, Da Lu, Xueqing Wu, Yangyang Xu, Chenghao Ma, and Shuqin Wu. 2022. "Effects of Growing Rod Technique with Different Surgical Modes and Growth Phases on the Treatment Outcome of Early Onset Scoliosis: A 3-D Finite Element Analysis" International Journal of Environmental Research and Public Health 19, no. 4: 2057. https://doi.org/10.3390/ijerph19042057






