Association of Body Weight and Physical Fitness during the Elementary School Years
Abstract
:1. Introduction
2. Materials and Methods
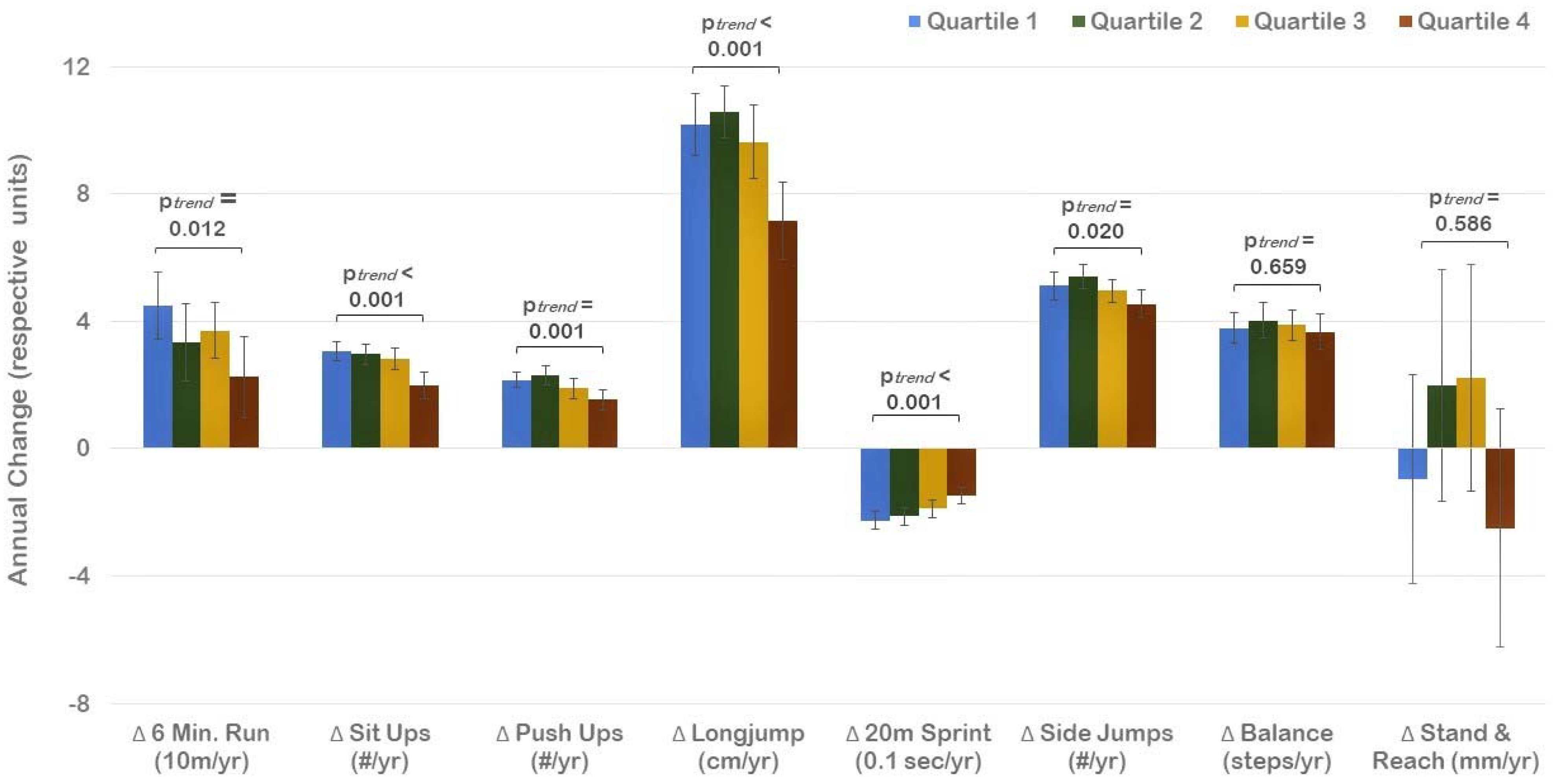
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10 (Suppl. 2), 97S–104S. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed] [Green Version]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayer, J.; Charakida, M.; Deanfield, J.E.; Celermajer, D.S. Lifetime risk: Childhood obesity and cardiovascular risk. Eur. Heart J. 2015, 36, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Lang, J.E.; Bunnell, H.T.; Hossain, M.J.; Wysocki, T.; Lima, J.J.; Finkel, T.H.; Bacharier, L.; Dempsey, A.; Sarzynski, L.; Test, M.; et al. Being Overweight or Obese and the Development of Asthma. Pediatrics 2018, 142, e20182119. [Google Scholar] [CrossRef] [Green Version]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.T.; Wang, Y.; McPherson, K. Child and adolescent obesity: Part of a bigger picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.R.; Castro-Piñero, J.; Artero, E.G.; Ortega, F.B.; Sjöström, M.; Suni, J.; Castillo, M.J. Predictive validity of health-related fitness in youth: A systematic review. Br. J. Sports Med. 2009, 43, 909–923. [Google Scholar] [CrossRef]
- Zaqout, M.; Michels, N.; Bammann, K.; Ahrens, W.; Sprengeler, O.; Molnar, D.; Hadjigeorgiou, C.; Eiben, G.; Konstabel, K.; Russo, P.; et al. Influence of physical fitness on cardio-metabolic risk factors in European children. The IDEFICS study. Int. J. Obes. 2016, 40, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.; Hall, M. Current evidence for theimpact of physical fitness on health outcomes in youth. Am. J. Lifestyle Med. 2015, 9, 388–397. [Google Scholar] [CrossRef]
- Smith, J.J.; Eather, N.; Morgan, P.J.; Plotnikoff, R.C.; Faigenbaum, A.D.; Lubans, D.R. The health benefits of muscular fitness for children and adolescents: A systematic review and meta-analysis. Sports Med. 2014, 44, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Lang, J.J.; Tomkinson, G.R.; Janssen, I.; Ruiz, J.R.; Ortega, F.B.; Léger, L.; Tremblay, M.S. Making a Case for Cardiorespiratory Fitness Surveillance Among Children and Youth. Exerc. Sport Sci. Rev. 2018, 46, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Grøntved, A.; Ried-Larsen, M.; Møller, N.C.; Kristensen, P.L.; Froberg, K.; Brage, S.; Andersen, L.B. Muscle strength in youth and cardiovascular risk in young adulthood (the European Youth Heart Study). Br. J. Sports Med. 2015, 49, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Hermoso, A.; Ramírez-Campillo, R.; Izquierdo, M. Is Muscular Fitness Associated with Future Health Benefits in Children and Adolescents? A Systematic Review and Meta-Analysis of Longitudinal Studies. Sports Med. 2019, 49, 1079–1094. [Google Scholar] [CrossRef]
- Ajala, O.; Mold, F.; Boughton, C.; Cooke, D.; Whyte, M. Childhood predictors of cardiovascular disease in adulthood. A systematic review and meta-analysis. Obes. Rev. 2017, 18, 1061–1070. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Leblanc, A.G. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Fiori, F.; Bravo, G.; Parpinel, M.; Messina, G.; Malavolta, R.; Lazzer, S. Relationship between body mass index and physical fitness in Italian prepubertal schoolchildren. PLoS ONE 2020, 15, e0233362. [Google Scholar]
- Barnett, L.M.; Lai, S.K.; Veldman, S.L.; Hardy, L.L.; Cliff, D.P.; Morgan, P.J.; Zask, A.; Lubans, D.R.; Shultz, S.P.; Ridgers, N.D.; et al. Correlates of Gross Motor Competence in Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1663–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiros, M.D.; Coates, A.M.; Howe, P.R.; Walkley, J.; Hills, A.P.; Wood, R.E.; Buckley, J.D. Adiposity is related to decrements in cardiorespiratory fitness in obese and normal-weight children. Pediatr. Obes. 2016, 11, 144–150. [Google Scholar] [CrossRef]
- Greier, K.; Drenowatz, C. Bidirectional association between weight status and motor skills in adolescents: A 4-year longitudinal study. Wien. Klin. Wochenschr. 2018, 130, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Ruedl, G.; Franz, D.; Frühauf, A.; Kopp, M.; Niedermeier, M.; Drenowatz, C.; Greier, K. Development of physical fitness in Austrian primary school children: A longitudinal study among overweight and non-overweight children over 2.5 years. Wien. Klin. Wochenschr. 2018, 130, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, C.; Hanssen-Doose, A.; Oriwol, D.; Bös, K.; Worth, A. Beeinflusst ein Veränderung des BMI die Entwicklung der motorischen Leistungsfähigkeit im Kindes- und Jugendalter? Ergebnisse der Motorik-Modul Studie (MoMo). Beweg. und Gesundh. 2016, 32, 168–172. [Google Scholar]
- Lopes, V.; Maia, J.; Rodrigues, L.; Malina, R. Motor coordination, physical activity and fitness as predictors of longitudinal change in adiposity during childhood. Eur. J. Sport Sci. 2012, 12, 384–391. [Google Scholar] [CrossRef]
- Tomkinson, G.R.; Lang, J.J.; Tremblay, M.S. Temporal trends in the cardiorespiratory fitness of children and adolescents representing 19 high-income and upper middle-income countries between 1981 and 2014. Br. J. Sports Med. 2019, 53, 478–486. [Google Scholar] [CrossRef] [Green Version]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Kornexl, E.; Kastner, H.; Drenowatz, C.; Greier, K. Fitness trend analysis in male Austrian middle and high school students from 1975 to 2010. Curr. Issues Sport Sci. 2021, 6, 007. [Google Scholar] [CrossRef]
- Bolger, L.E.; Bolger, L.A.; O’Neill, C.; Coughlan, E.; O’Brien, W.; Lacey, S.; Burns, C.; Bardidd, F. Global levels of fundamental motor skills in children: A systematic review. J. Sports Sci. 2021, 39, 717–753. [Google Scholar] [CrossRef]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Imo, D.; Jaspers, L.; Colpani, V.; Chaker, L.; van der Lee, S.J.; Mendis, S.; Chowdhury, R.; Bramer, W.M.; Falla, A.; et al. The global impact of non-communicable diseases on healthcare spending and national income: A systematic review. Eur. J. Epidemiol. 2015, 30, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Spieker, E.A.; Pyzocha, N. Economic Impact of Obesity. Prim. Care 2016, 43, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Bös, K.; Schlenker, L.; Büsch, D.; Lämmle, L.; Müller, H.; Oberger, J.; Seidel, I.; Tittelbach, S. Deutscher Motorik-Test 6-18 (DMT6-18) [German Motor Abilities Test 6-18 (DMT6-18)]; Czwalina: Hamburg, Germany, 2009. [Google Scholar]
- Abdelkarim, O.; Fritsch, J.; Jekauc, D.; Bös, K. Examination of Construct Validity and Criterion-Related Validity of the German Motor Test in Egyptian Schoolchildren. Int. J. Environ. Res. Public Health 2021, 18, 8341. [Google Scholar] [CrossRef]
- Ceschia, A.; Giacomini, S.; Santarossa, S.; Rugo, M.; Salvadego, D.; Da Ponte, A.; Driussi, C.; Mihaleje, M.; Poser, S.; Lazzer, S. Deleterious effects of obesity on physical fitness in pre-pubertal children. Eur. J. Sport Sci. 2016, 16, 271–278. [Google Scholar] [CrossRef]
- Musálek, M.; Clark, C.C.T.; Kokštejn, J.; Vokounova, Š.; Hnízdil, J.; Mess, F. Impaired Cardiorespiratory Fitness and Muscle Strength in Children with Normal-Weight Obesity. Int. J. Environ. Res. Public Health 2020, 17, 9198. [Google Scholar] [CrossRef]
- Joensuu, L.; Kujala, U.M.; Kankaanpää, A.; Syväoja, H.J.; Kulmala, J.; Hakonen, H.; Oksanen, H.; Kallio, J.; Tammelin, T.H. Physical fitness development in relation to changes in body composition and physical activity in adolescence. Scand. J. Med. Sci. Sports 2021, 31, 456–464. [Google Scholar] [CrossRef]
- Brunet, M.; Chaput, J.P.; Tremblay, A. The association between low physical fitness and high body mass index or waist circumference is increasing with age in children: The ‘Québec en Forme’ Project. Int. J. Obes. 2007, 31, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Sacchetti, R.; Ceciliani, A.; Garulli, A.; Masotti, A.; Poletti, G.; Beltrami, P.; Leoni, E. Physical fitness of primary school children in relation to overweight prevalence and physical activity habits. J. Sports Sci. 2012, 30, 633–640. [Google Scholar] [CrossRef]
- Santtila, M.; Pihlainen, K.; Koski, H.; Vasankari, T.; Kyröläinen, H. Physical Fitness in Young Men between 1975 and 2015 with a Focus on the Years 2005–2015. Med. Sci. Sports Exerc. 2018, 50, 292–298. [Google Scholar] [CrossRef]
- Armstrong, M.E.G.; Lambert, M.I.; Lambert, E.V. Relationships between different nutritional anthropometric statuses and health-related fitness of South African primary school children. Ann. Hum. Biol. 2017, 44, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Gulías-González, R.; Sánchez-López, M.; Olivas-Bravo, Á.; Solera-Martínez, M.; Martínez-Vizcaíno, V. Physical fitness in Spanish schoolchildren aged 6–12 years: Reference values of the battery EUROFIT and associated cardiovascular risk. J. Sch. Health 2014, 84, 625–635. [Google Scholar] [CrossRef]
- Kakebeeke, T.H.; Lanzi, S.; Zysset, A.E.; Arhab, A.; Messerli-Bürgy, N.; Stuelb, K.; Leeger-Aschmann, C.S.; Schmutz, E.A.; Meyer, A.H.; Kriemler, S.; et al. Association between Body Composition and Motor Performance in Preschool Children. Obes. Facts 2017, 10, 420–431. [Google Scholar] [CrossRef]
- de Andrade Gonçalves, E.C.; Augusto Santos Silva, D.; Gimenes Nunes, H.E. Prevalence and Factors Associated with Low Aerobic Performance Levels in Adolescents: A Systematic Review. Curr. Pediatr. Rev. 2015, 11, 56–70. [Google Scholar] [CrossRef]
- Gallahue, D.; Ozmun, J.; Goodway, J. Understanding Motor Development. Infants, Children, Adolescents, Adults, 7th ed.; McGraw-Hill: Boston, MA, USA, 2012. [Google Scholar]
- Lima, R.A.; Bugge, A.; Ersbøll, A.K.; Stodden, D.F.; Andersen, L.B. The longitudinal relationship between motor competence and measures of fatness and fitness from childhood into adolescence. J. Pediatr. 2019, 95, 482–488. [Google Scholar] [CrossRef]
- Stodden, D.; Goodway, J.; Langendorfer, S.; Roberton, M.; Rudisill, M.; Garcia, C.; Garcia, L.E. A developmental perspective on the role of motor skill competence in physical activity: An emergent relationshihp. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Mateo-Orcajada, A.; González-Gálvez, N.; Abenza-Cano, L.; Vaquero-Cristóbal, R. Differences in Physical Fitness and Body Composition Between Active and Sedentary Adolescents: A Systematic Review and Meta-Analysis. J. Youth Adolesc. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Telama, R.; Yang, X.; Leskinen, E.; Kankaanpää, A.; Hirvensalo, M.; Tammelin, T.; Raitakari, O.T. Tracking of physical activity from early childhood through youth into adulthood. Med. Sci. Sports Exerc. 2014, 46, 955–962. [Google Scholar] [CrossRef]
- Li, J.; Siegrist, J. Physical activity and risk of cardiovascular disease-a meta-analysis of prospective cohort studies. Int. J. Environ. Res. Public Health 2012, 9, 391–407. [Google Scholar] [CrossRef]
- Battaglia, G.; Giustino, V.; Tabacchi, G.; Lanza, M.; Schena, F.; Biino, V.; Giuriato, M.; Gallotta, M.C.; Guidetti, L.; Baldari, C.; et al. Interrelationship Between Age, Gender, and Weight Status on Motor Coordination in Italian Children and Early Adolescents Aged 6–13 Years Old. Front. Pediatr. 2021, 9, 738294. [Google Scholar] [CrossRef]
- Battaglia, G.; Giustino, V.; Tabacchi, G.; Alesi, M.; Galassi, C.; Modica, C.; Palma, A.; Bellafiore, M. Effectiveness of a Physical Education Program on the Motor and Pre-literacy Skills of Preschoolers From the Training-To-Health Project: A Focus on Weight Status. Front. Sports Act. Living 2020, 2, 579421. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.A.; Soares, F.C.; Queiroz, D.R.; Aguilar, J.A.; Bezerra, J.; Barros, M.V.G. The importance of body weight status on motor competence development: From preschool to middle childhood. Scand. J. Med. Sci. Sports 2021, 31 (Suppl. 1), 15–22. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Stodden, D.F.; Barnett, L.M.; Lopes, V.P.; Logan, S.W.; Rodrigues, L.P.; D’Hondt, E. Motor Competence and its Effect on Positive Developmental Trajectories of Health. Sports Med. 2015, 45, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Valls, M.R.; Adelantado-Renau, M.; Moliner-Urdiales, D. Reallocating time spent in physical activity intensities: Longitudinal associations with physical fitness (DADOS study). J. Sci. Med. Sport 2020, 23, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Cocca, A.; Carbajal Baca, J.E.; Hernández Cruz, G.; Cocca, M. Does A Multiple-Sport Intervention Based on the TGfU Pedagogical Model for Physical Education Increase Physical Fitness in Primary School Children? Int. J. Environ. Res. Public Health 2020, 17, 5532. [Google Scholar] [CrossRef]
- Kelly, A.S.; Wetzsteon, R.J.; Kaiser, D.R.; Steinberger, J.; Bank, A.J.; Dengel, D.R. Inflammation, insulin, and endothelial function in overweight children and adolescents: The role of exercise. J. Pediatr. 2004, 145, 731–736. [Google Scholar] [CrossRef]
- Janz, K.F.; Dawson, J.D.; Mahoney, L.T. Increases in physical fitness during childhood improve cardiovascular health during adolescence: The Muscatine Study. Int. J. Sports Med. 2002, 23 (Suppl. 1), S15–S21. [Google Scholar] [CrossRef]
- Jarnig, G.; Jaunig, J.; van Poppel, M.N.M. Association of COVID-19 Mitigation Measures with Changes in Cardiorespiratory Fitness and Body Mass Index Among Children Aged 7 to 10 Years in Austria. JAMA Netw. Open 2021, 4, e2121675. [Google Scholar] [CrossRef]
- Jurak, G.; Kovač, M.; Sember, V.; Starc, G. 30 years of SLOfit: Its legacy and perspective. Turk. J. Sports Med. 2019, 54, 23–27. [Google Scholar] [CrossRef]
- Strel, J. Analysis of the Program Healthy Lifestyle for the Years 2010/11 and 2011/12; Institute for Sport Planica: Ljubljana, Slovenia, 2013. [Google Scholar]
- Coppinger, T.; Milton, K.; Murtagh, E.; Harrington, D.; Johansen, D.; Seghers, J.; Skovgaard, T.; HEPA Europe Children & Youth Working Group; Chalkley, A. Global Matrix 3.0 physical activity report card for children and youth: A comparison across Europe. Public Health 2020, 187, 150–156. [Google Scholar] [CrossRef]
- Freedman, D.S.; Katzmarzyk, P.T.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: The Bogalusa Heart Study. Am. J. Clin. Nutr. 2009, 90, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, K.; Wong, M.; Khalechelvam, P.; Tam, W. Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: A meta-analysis. Obes. Rev. 2016, 17, 1258–1275. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Grummer-Strawn, L.M.; Pietrobelli, A.; Goulding, A.; Goran, M.I.; Dietz, W.H. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am. J. Clin. Nutr. 2002, 75, 978–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
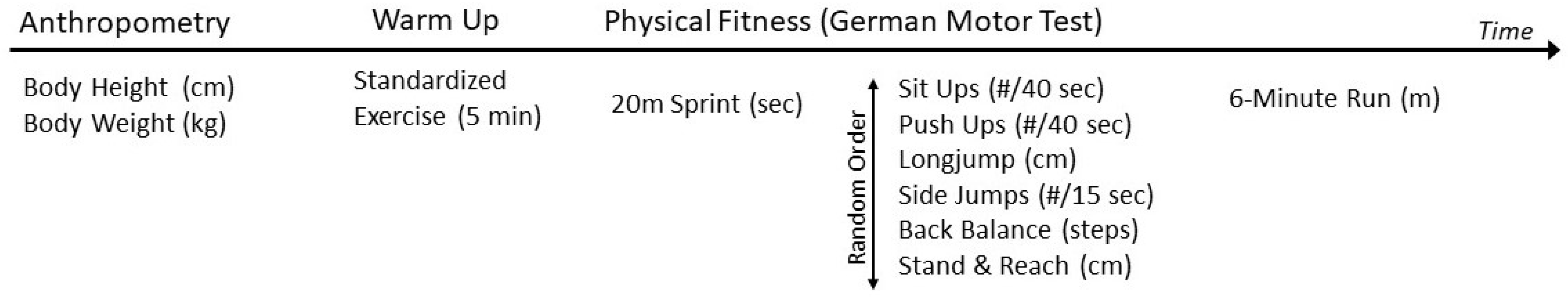

| Total Sample (n = 303) | Girls (n = 135) | Boys (n = 166) | |
|---|---|---|---|
| Age (years) | 6.9 ± 0.5 | 6.9 ± 0.5 | 6.9 ± 0.5 |
| Height (cm) ** | 122.4 ± 5.7 | 121.2 ± 5.3 | 123.4 ± 5.9 |
| Weight (kg) * | 24.3 ± 4.4 | 23.6 ± 4.2 | 24.8 ± 4.5 |
| BMI percentile | 51.8 ± 27.3 | 50.2 ± 26.4 | 53.0 ± 28.1 |
| 6 min run (m) ** | 854 ± 140 | 823 ± 130 | 879 ± 143 |
| Sit ups (# in 40 s) ** | 15.1 ± 5.6 | 14.0 + 6.0 | 16.0 ± 5.2 |
| Push ups (# in 40 s) | 11.6 ± 3.7 | 11.5 ± 3.7 | 11.7 ± 3.6 |
| Long jump (cm) ** | 113.5 ± 17.9 | 106.2 ± 15.8 | 119.4 ± 17.3 |
| 20 m sprint (s) ** | 4.8 ± 0.5 | 5.0 ± 0.6 | 4.6 + 0.4 |
| Side jumps (# in 15 s) | 23.0 ± 5.8 | 22.3 ± 5.6 | 23.5 ± 5.9 |
| Balance (steps) * | 26.7 ± 9.8 | 28.0 ± 10.2 | 25.7 ± 9.5 |
| Stand and reach (cm) 1,** | 0.8 ± 5.6 | 1.9 ± 5.2 | −0.1 ± 5.7 |
| Overall fitness score (Z) | 104.2 ± 5.9 | 103.5 ± 6.2 | 104.7 ± 5.6 |
| 6 Min Run (m) | Sit Ups (Reps) | Push Ups (Reps) | Long-Jump (cm) | 20 m Sprint (sec) | Side Jumps (Reps) | Balance (Steps) | Stand and Reach (cm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Age: 6.9 years | BMI PCT | −0.21 ** | 0.02 | −0.02 | −0.02 | −0.06 | 0.03 | −0.06 | 0.12 |
| Follow-Up Age: 10.4 years | BMI PCT | −0.36 ** | −0.24 ** | −0.27 ** | −0.32 ** | 0.30 ** | −0.22 ** | −0.29 ** | −0.01 |
| Change (Δ 4 years) | BMI PCT | −0.15 ** | −0.16 ** | −0.11 | −0.17 ** | 0.17 ** | −0.13 * | −0.13 * | −0.11 |
| BL Fitness (β) | BL BMIPCT (β) | Δ BMIPCT (β) | R2 | |
|---|---|---|---|---|
| Total Sample | 0.525 ** | −0.309 ** | −0.252 ** | 0.456 |
| Low BMIPCT | 0.490 ** | −0.015 | −0.008 | 0.241 |
| <avg. BMIPCT | 0.596 ** | 0.042 | −0.238 * | 0.416 |
| >avg. BMIPCT | 0.516 ** | −0.126 | −0.427 ** | 0.530 |
| High BMIPCT | 0.560 ** | −0.054 | −0.427 ** | 0.523 |
| Low BL Fitness | 0.444 ** | −0.502 ** | −0.211 * | 0.508 |
| Avg. BL Fitness | 0.222 * | −0.287 ** | −0.359 ** | 0.268 |
| Above avg. BL Fitness | 0.198 | −0.302 ** | −0.150 | 0.179 |
| High BL Fitness | −0.006 | 0.244 * | −0.485 ** | 0.269 |
| BL Fitness (β) | BL BMIPCT (β) | Δ Fitness (β) | R2 | |
|---|---|---|---|---|
| Total Sample | −0.102 ** | 0.742 ** | −0.203 ** | 0.686 |
| Low BMIPCT | −0.144 | 0.235 * | −0.006 | 0.075 |
| <avg. BMIPCT | −0.068 | 0.227 * | −0.326 ** | 0.155 |
| >avg. BMIPCT | −0.289 ** | 0.342 ** | −0.541 ** | 0.495 |
| High BMIPCT | −0.104 | 0.372 ** | −0.387 ** | 0.291 |
| Low BL Fitness | 0.020 | 0.644 ** | −0.239 ** | 0.631 |
| Avg. BL Fitness | −0.110 | 0.761 ** | −0.238 ** | 0.740 |
| Above avg. BL Fitness | −0.044 | 0.813 ** | −0.101 | 0.733 |
| High BL Fitness | −0.105 | 0.746 ** | −0.261 ** | 0.671 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drenowatz, C.; Chen, S.-T.; Cocca, A.; Ferrari, G.; Ruedl, G.; Greier, K. Association of Body Weight and Physical Fitness during the Elementary School Years. Int. J. Environ. Res. Public Health 2022, 19, 3441. https://doi.org/10.3390/ijerph19063441
Drenowatz C, Chen S-T, Cocca A, Ferrari G, Ruedl G, Greier K. Association of Body Weight and Physical Fitness during the Elementary School Years. International Journal of Environmental Research and Public Health. 2022; 19(6):3441. https://doi.org/10.3390/ijerph19063441
Chicago/Turabian StyleDrenowatz, Clemens, Si-Tong Chen, Armando Cocca, Gerson Ferrari, Gerhard Ruedl, and Klaus Greier. 2022. "Association of Body Weight and Physical Fitness during the Elementary School Years" International Journal of Environmental Research and Public Health 19, no. 6: 3441. https://doi.org/10.3390/ijerph19063441






