An Early Warning System for the Differential Diagnosis of In-Hospital Acute Kidney Injury for Better Patient Outcome: Study of a Quality Improvement Initiative
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Intervention (EWS)
2.2. Definition of AKI and Outcome Measures
2.3. Endpoints
2.4. Statistical Analysis
3. Results
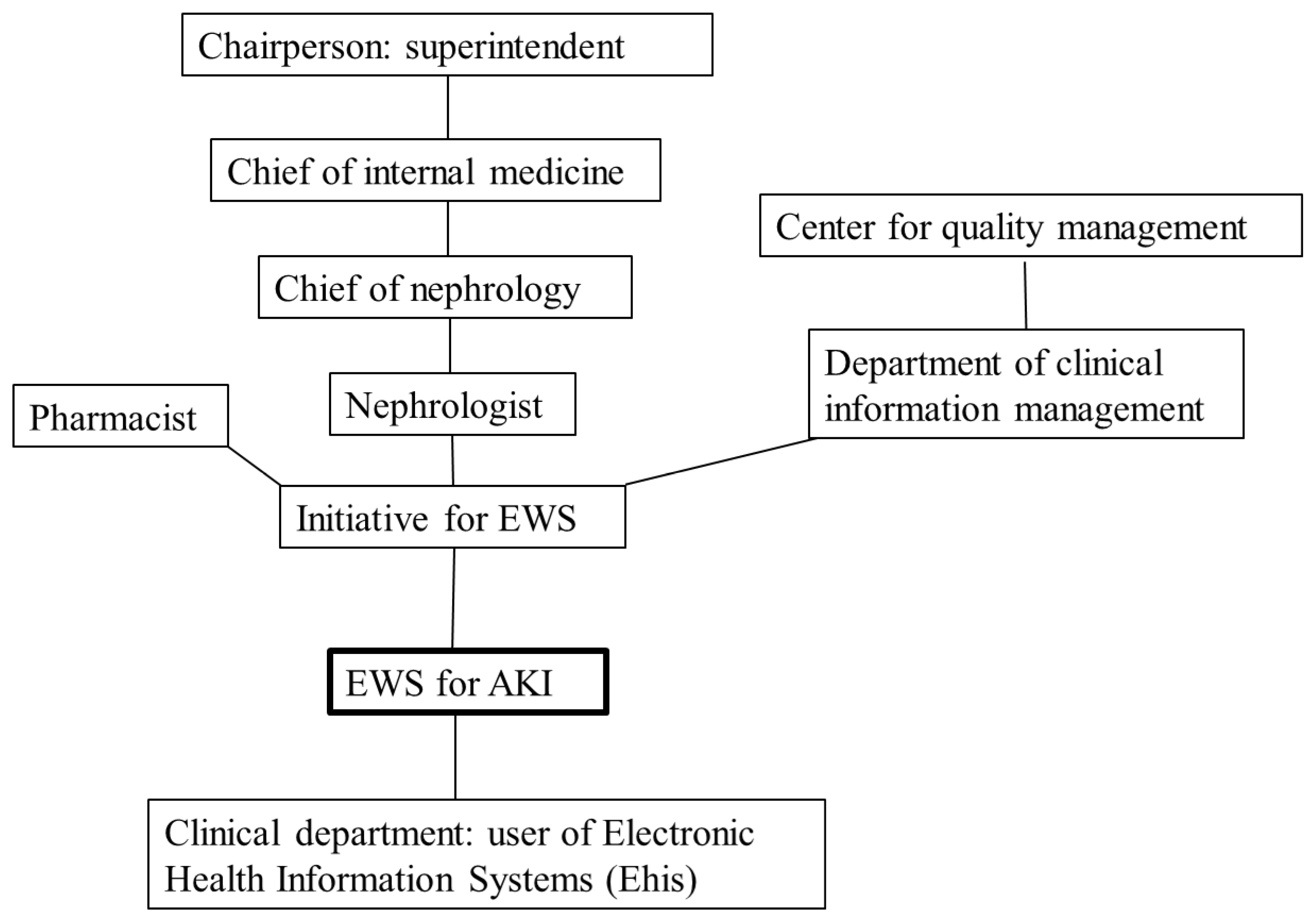
3.1. Enlisting a Stakeholder Mapping
3.2. Action Plan of This Initiative
3.3. Early Warning System (EWS) for Acute Kidney Injury (AKI) in the Electronic Health Information System (EHIS)
3.4. The Daily Incidence of AKI and the Effect of Intervention of AKI-EWS
3.5. Long-Term Outcomes of AKI before and after AKI-EWS
3.6. Cass Numbers of Consultation of Nephrologists
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World incidence of AKI: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- United States Renal Data System. 2014 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2014. [Google Scholar]
- Mehta, R.L.; Cerdá, J.; Burdmann, E.A.; Tonelli, M.; García-García, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Balasubramanian, G.; Al-Aly, Z.; Moiz, A.; Rauchman, M.; Zhang, Z.; Gopalakrishnan, R.; Balasubramanian, S.; El-Achkar, T.M. Early nephrologist involvement in hospital-acquired acute kidney injury: A pilot study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 57, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef] [Green Version]
- Levin, A.; Warnock, D.G.; Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C. Improving outcomes from acute kidney injury: Report of an initiative. Am. J. Kidney Dis. 2007, 50, 1–4. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Levin, A.; Warnock, D.G.; Joannidis, M.; Mehta, R.L.; Kellum, J.A.; Ronco, C.; Shah, S. Improving outcomes from acute kidney injury. J. Am. Soc. Nephrol. 2007, 18, 1992–1994. [Google Scholar] [CrossRef]
- Lachance, P.; Villeneuve, P.M.; Rewa, O.G.; Wilson, F.P.; Selby, N.M.; Featherstone, R.M.; Bagshaw, S.M. Association between e-alert implementation for detection of acute kidney injury and outcomes: A systematic review. Nephrol. Dial. Transplant. 2017, 32, 265–272. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E. Harmonizing acute and chronic kidney disease definition and classification: Report of a kidney disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, J.; Acharya, A.; Cerda, J.; Maccariello, E.R.; Madarasu, R.C.; Tolwani, A.J.; Liang, X.; Fu, P.; Liu, Z.H.; Mehta, R.L. A Prospective International Multicenter Study of AKI in the Intensive Care Unit. Clin. J. Am. Soc. Nephrol. 2015, 10, 1324–1331. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Van Biesen, W.; Vanholder, R.; Lameire, N. Defining acute renal failure: RIFLE and beyond. Clin. J. Am. Soc. Nephrol. 2006, 1, 1314–1319. [Google Scholar] [CrossRef] [Green Version]
- Lewington, A.J.; Cerdá, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.; Lee, J.E.; Yoon, J.Y.; Jang, H.R.; Huh, W.; Kim, Y.G.; Kim, D.J.; Oh, H.Y. Effect of an electronic alert on risk of contrast-induced acute kidney injury in hospitalized patients undergoing computed tomography. Am. J. Kidney Dis. 2012, 60, 74–81. [Google Scholar] [CrossRef]
- Ng, R.R.; Chew, S.T.; Liu, W.; Shen, L.; Ti, L.K. Identification of modifiable risk factors for acute kidney injury after coronary artery bypass graft surgery in an Asian population. J Thorac. Cardiovasc. Surg. 2014, 147, 1356–1361. [Google Scholar] [CrossRef] [Green Version]
- Murakami, R.; Kumita, S.; Hayashi, H.; Sugizaki, K.; Okazaki, E.; Kiriyama, T.; Hakozaki, K.; Tani, H.; Miki, I.; Takeda, M. Anemia and the risk of contrast-induced nephropathy in patients with renal insufficiency undergoing contrast-enhanced MDCT. Eur. J. Radiol. 2013, 82, e521–e524. [Google Scholar] [CrossRef]
- Karkouti, K.; Wijeysundera, D.N.; Yau, T.M.; McCluskey, S.A.; Chan, C.T.; Wong, P.Y.; Beattie, W.S. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology 2011, 115, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, U.A.; Coca, S.G.; Hong, K.; Koyner, J.L.; Garg, A.X.; Passik, C.S.; Swaminathan, M.; Garwood, S.; Patel, U.D.; Hashim, S.; et al. Blood transfusions are associated with urinary biomarkers of kidney injury in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Porter, C.J.; Juurlink, I.; Bisset, L.H.; Bavakunji, R.; Mehta, R.L.; Devonald, M.A. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol. Dial. Transplant. 2014, 29, 1888–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, T.; Lavrentieva, A.; Greenhalgh, D.G. Acute kidney injury in critically ill burn patients. Risk factors, progression and impact on mortality. Burns 2010, 36, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rind, D.M.; Safran, C.; Phillips, R.S.; Wang, Q.; Calkins, D.R.; Delbanco, T.L.; Bleich, H.L.; Slack, W.V. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch. Intern. Med. 1994, 154, 1511–1517. [Google Scholar] [CrossRef]
- Wilson, F.P.; Shashaty, M.; Testani, J.; Aqeel, I.; Borovskiy, Y.; Ellenberg, S.S.; Feldman, H.I.; Fernandez, H.; Gitelman, Y.; Lin, J.; et al. Automated, electronic alerts for acute kidney injury: A single-blind, parallel-group, randomised controlled trial. Lancet 2015, 385, 1966–1974. [Google Scholar] [CrossRef] [Green Version]
- McCoy, A.B.; Waitman, L.R.; Gadd, C.S.; Danciu, I.; Smith, J.P.; Lewis, J.B.; Schildcrout, J.S.; Peterson, J.F. A computerized provider order entry intervention for medication safety during acute kidney injury: A quality improvement report. Am. J. Kidney Dis. 2010, 56, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Selby, N.M. Electronic alerts for acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2013, 22, 637–642. [Google Scholar] [CrossRef]
- Park, S.; Baek, S.H.; Ahn, S.; Lee, K.H.; Hwang, H.; Ryu, J.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.W.; et al. Impact of Electronic Acute Kidney Injury (AKI) Alerts with Automated Nephrologist Consultation on Detection and Severity of AKI: A Quality Improvement Study. Am. J. Kidney Dis. 2018, 71, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Jaber, B.L.; Faubel, S.; Chawla, L.S. AKI transition of care: A potential opportunity to detect and prevent CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]


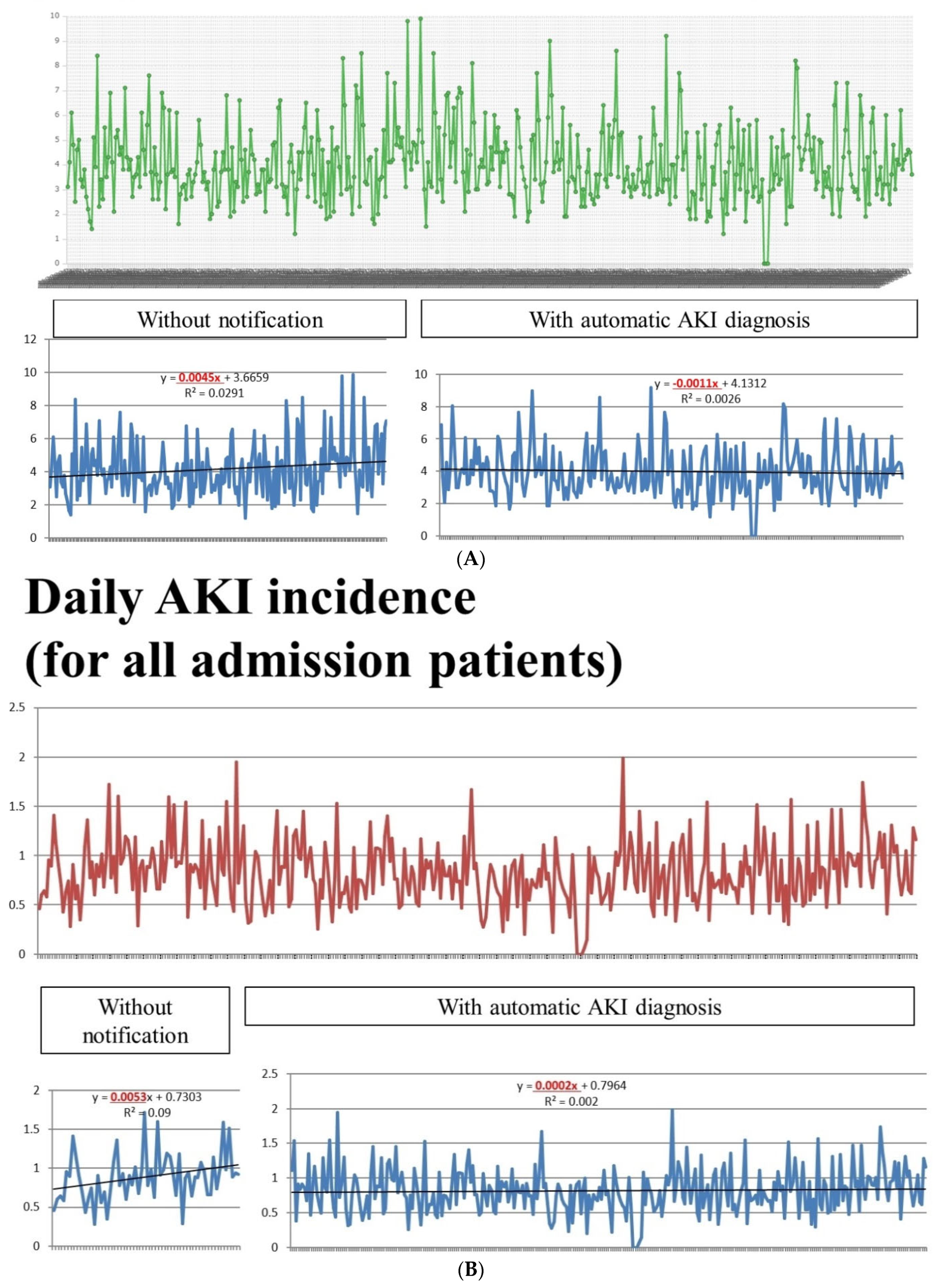


| Before Notification | After Notification | p Value | |
|---|---|---|---|
| Mean daily case numbers of all inpatients with AKI (n ± standard deviation) | 10.7 ± 3.6 | 11.1 ± 4.4 | 0.286 |
| Definition 1: Daily AKI incidence (%) (for inpatients with creatinine data) | |||
| Mean daily case numbers of all inpatients with creatinine data (n ± standard deviation) | 283.9 ± 117.8 | 291.6 ± 128.0 | 0.552 |
| Mean incidence of AKI | 4.14% | 3.99% | 0.4 |
| Proportion of AKI > 4% | 47.4% | 41.6% | 0.010 |
| Proportion of AKI > 6% | 15.02% | 11.02% | 0.440 |
| Proportion of AKI > 7% | 5.63% | 4.16% | 0.590 |
| Proportion of AKI > 8% | 2.82% | 2.08% | 0.973 |
| Definition 2: Daily AKI incidence (%) (for all inpatients with or without creatinine data) | |||
| Mean daily case numbers of all inpatients with or without creatinine data (n ± standard deviation) | 1268.3 ± 150.8 | 1339.6 ± 101.4 | <0.001 |
| Mean incidence of AKI | 0.89% | 0.82% | 0.085 |
| Proportion of AKI > 0.8% | 58.33% | 49.67% | 0.221 |
| Proportion of AKI > 0.9% | 51.67% | 35.94% | 0.024 |
| Proportion of AKI >1.0% | 25% | 24.51% | 0.936 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-J.; Huang, S.-C.; Chen, C.-H.; Cheng, C.-Y.; Tsai, S.-F. An Early Warning System for the Differential Diagnosis of In-Hospital Acute Kidney Injury for Better Patient Outcome: Study of a Quality Improvement Initiative. Int. J. Environ. Res. Public Health 2022, 19, 3704. https://doi.org/10.3390/ijerph19063704
Wu M-J, Huang S-C, Chen C-H, Cheng C-Y, Tsai S-F. An Early Warning System for the Differential Diagnosis of In-Hospital Acute Kidney Injury for Better Patient Outcome: Study of a Quality Improvement Initiative. International Journal of Environmental Research and Public Health. 2022; 19(6):3704. https://doi.org/10.3390/ijerph19063704
Chicago/Turabian StyleWu, Ming-Ju, Shih-Che Huang, Cheng-Hsu Chen, Ching-Yao Cheng, and Shang-Feng Tsai. 2022. "An Early Warning System for the Differential Diagnosis of In-Hospital Acute Kidney Injury for Better Patient Outcome: Study of a Quality Improvement Initiative" International Journal of Environmental Research and Public Health 19, no. 6: 3704. https://doi.org/10.3390/ijerph19063704







