The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Signaling for Glucose Utilization, Myofiber Distribution, and Metabolic Function after Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Interventions
2.2.1. Comb-NMES Intervention
2.2.2. Sham Intervention
2.3. Clinical and Laboratory Procedures
2.3.1. Muscle Biopsy
2.3.2. Myofiber-Type Distribution
2.3.3. Immunoblotting
2.3.4. Determination of Blood Glucose, Insulin, and Lipids
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
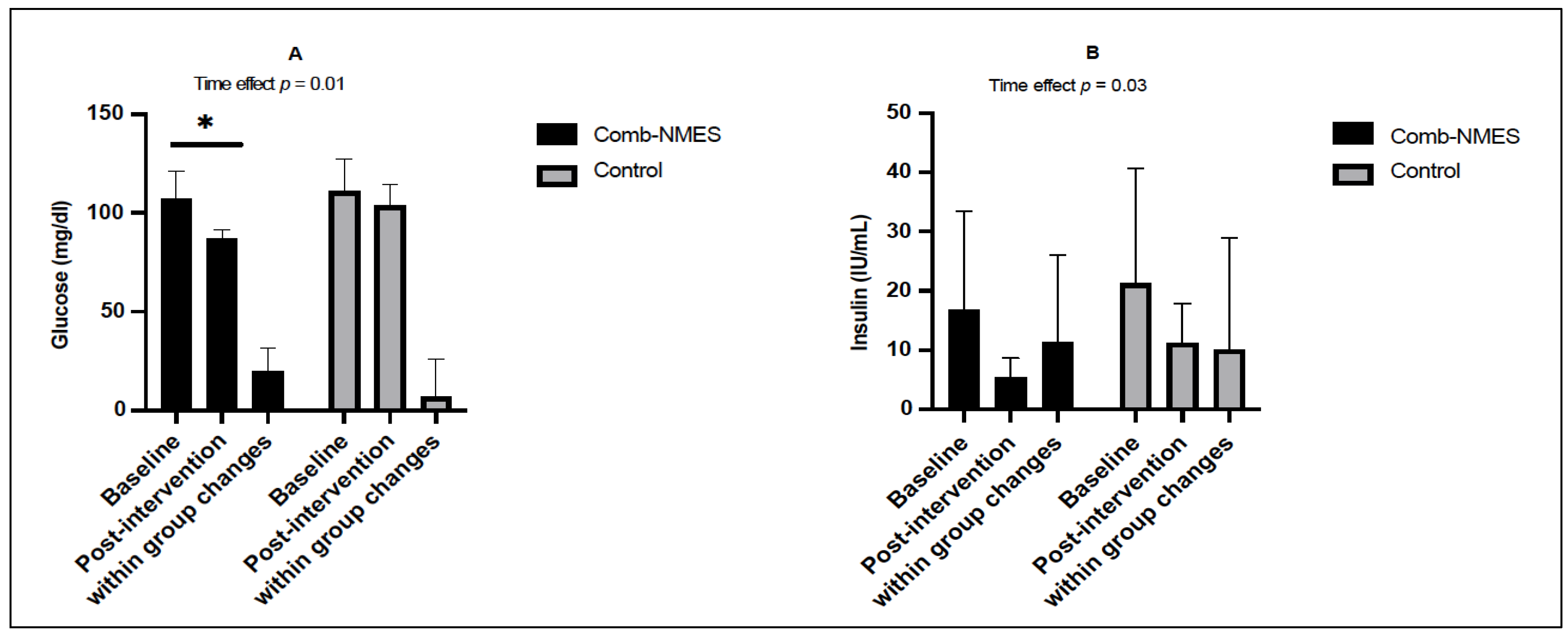
3.2. Glucose Homeostasis (N = 14)
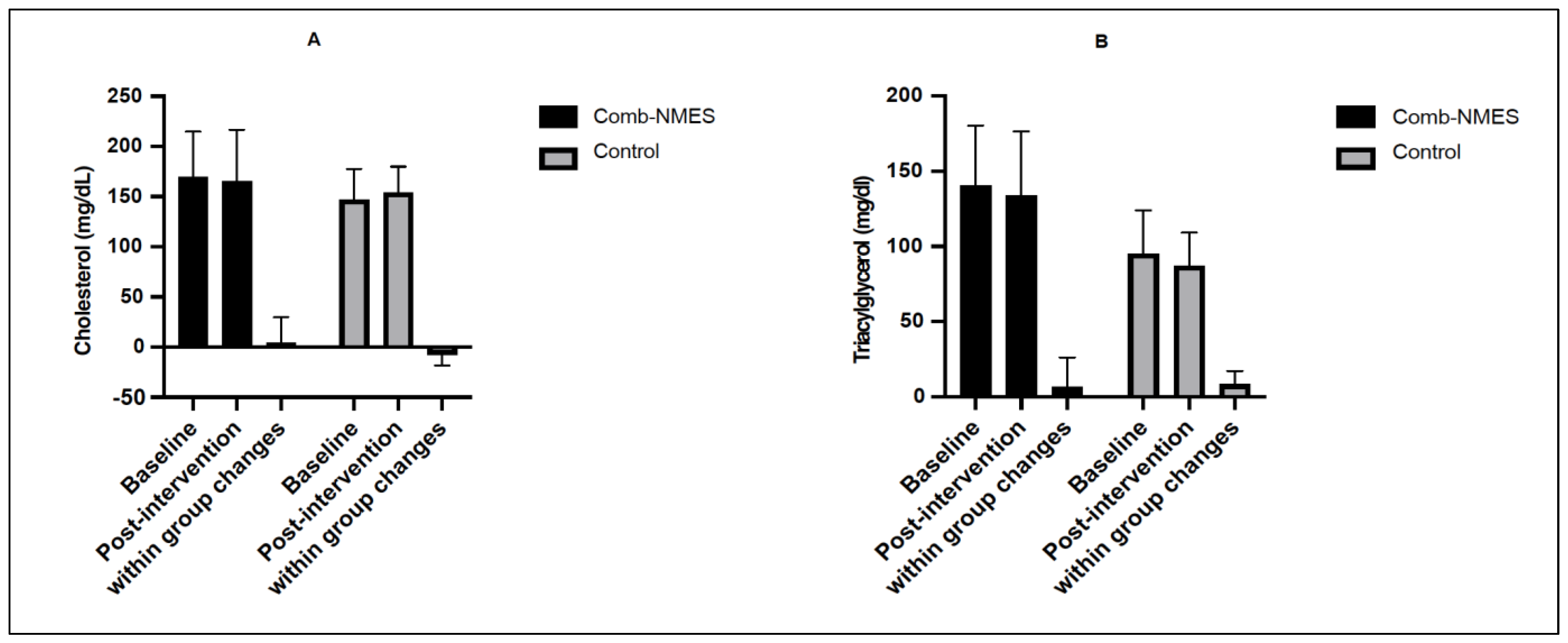
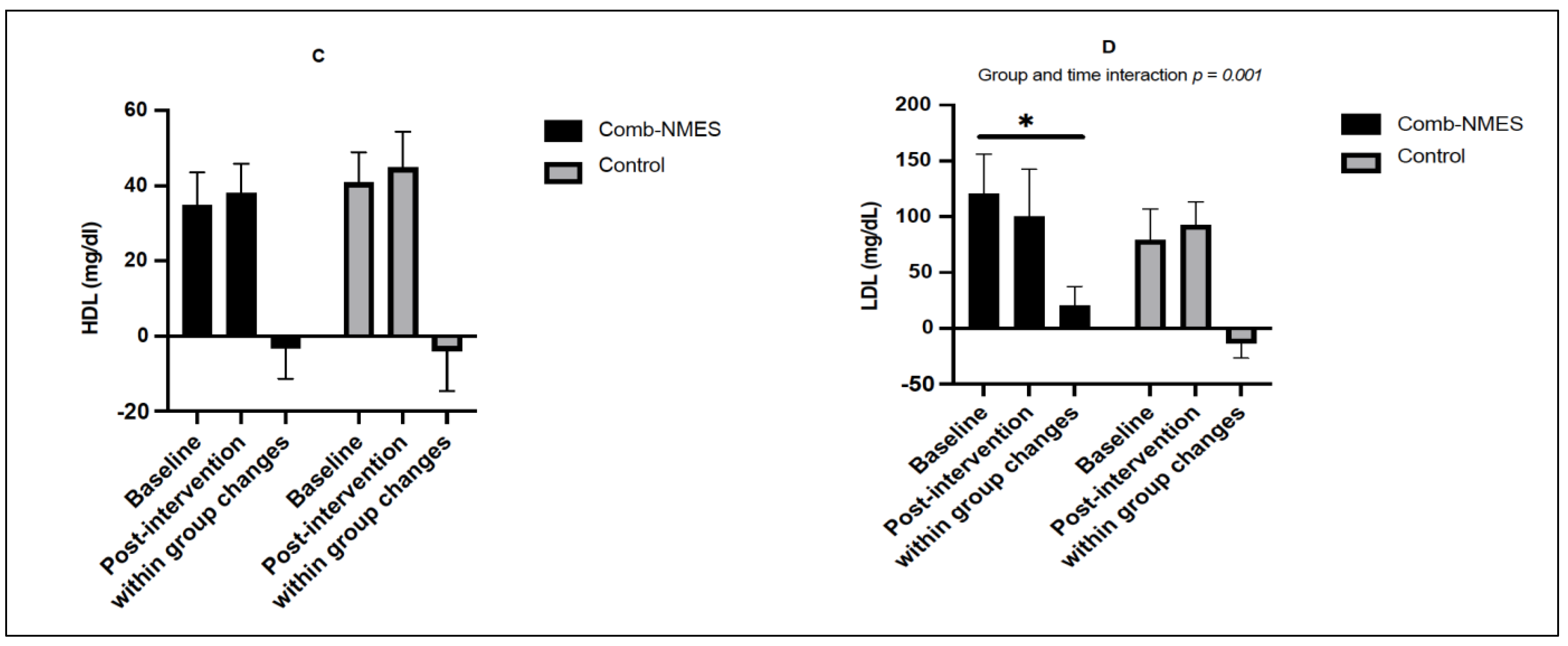
3.3. Lipid Profile (N = 14)
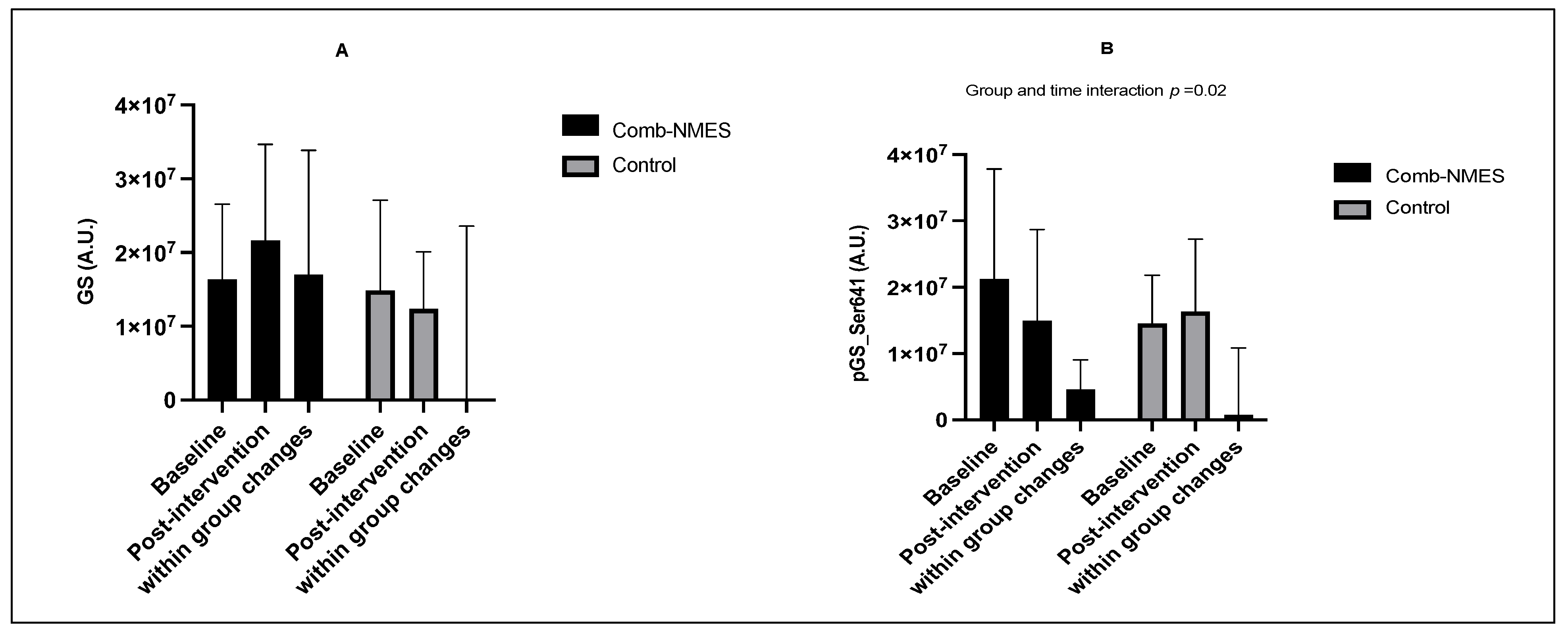
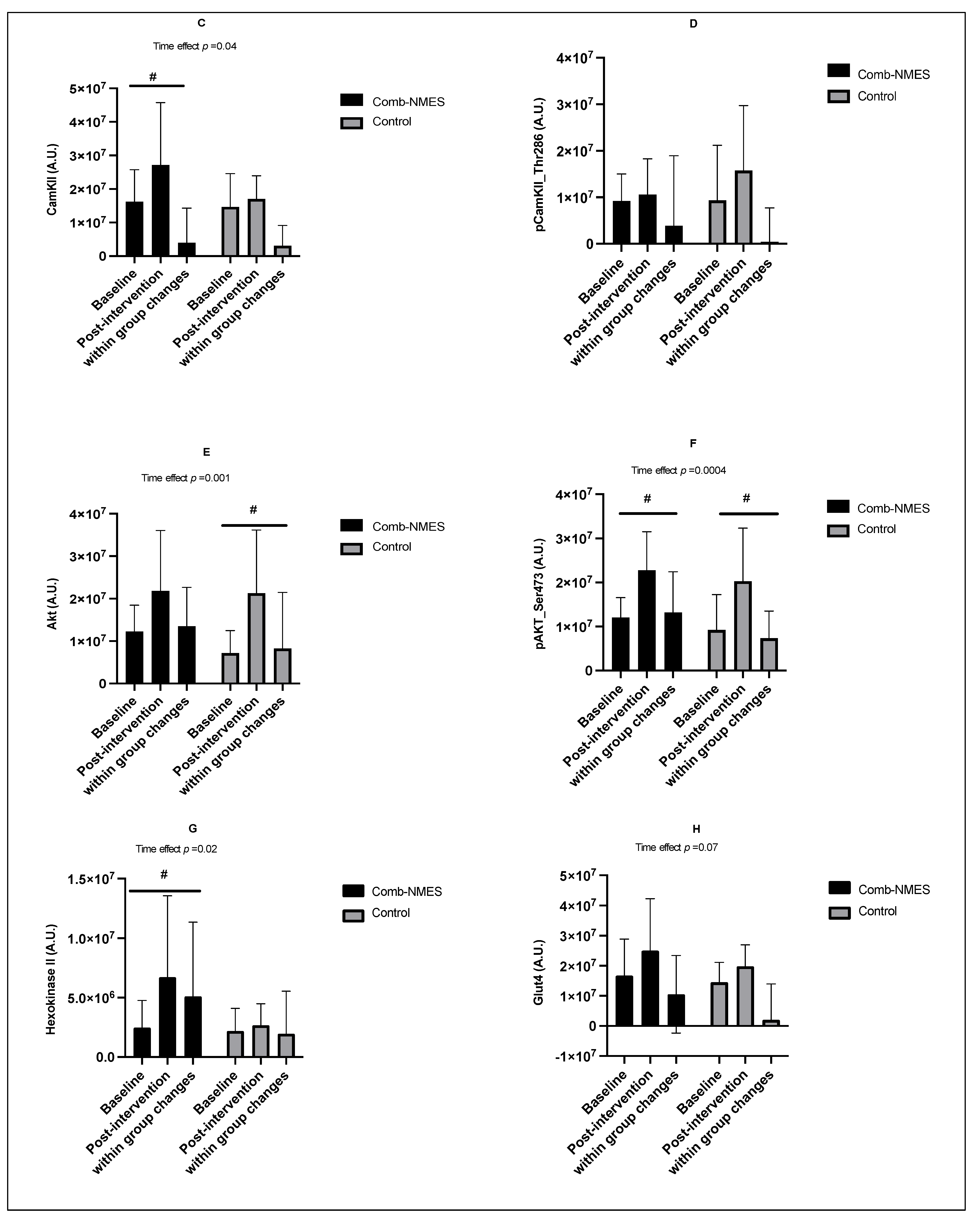
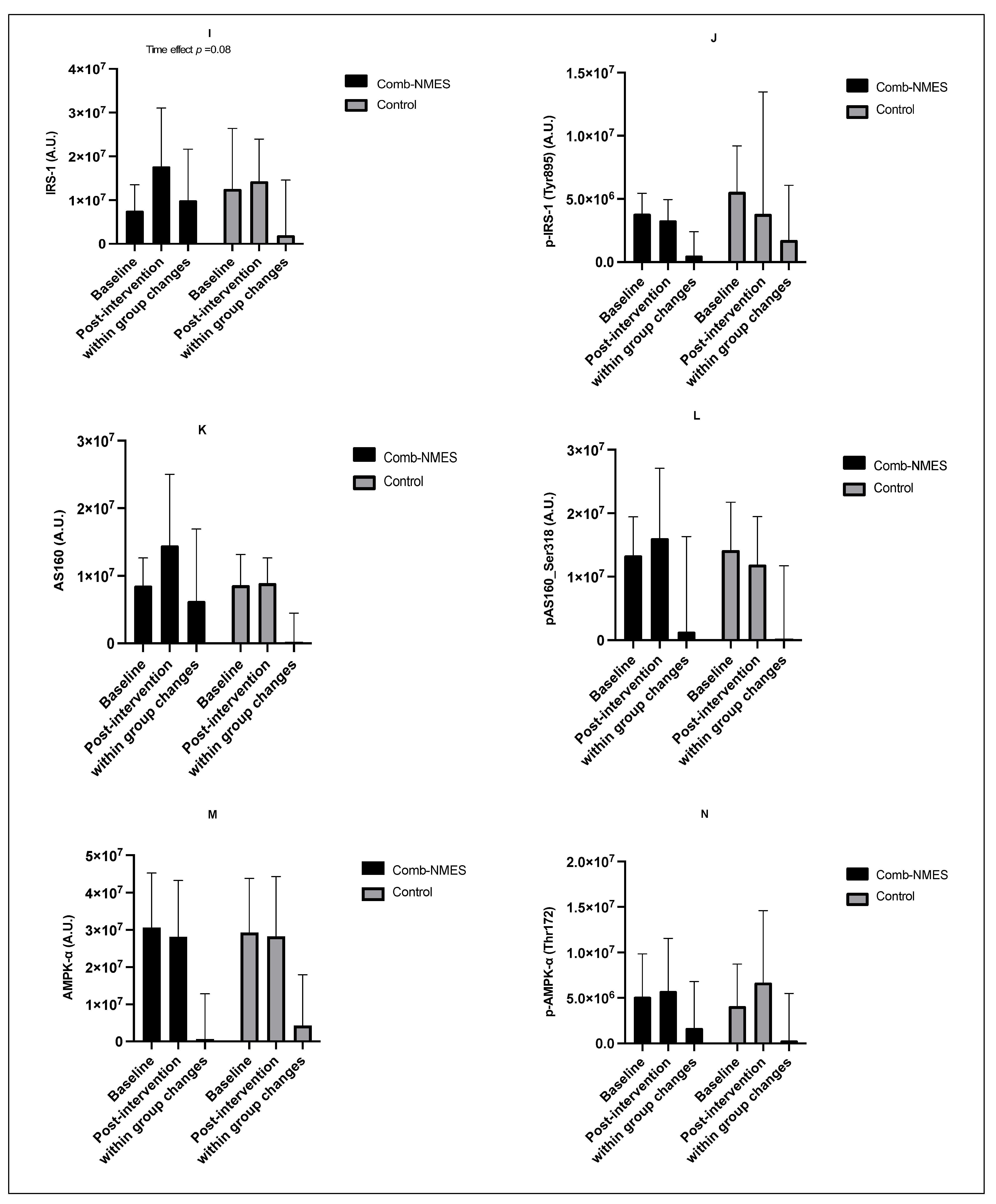
3.4. Skeletal Muscle Intracellular Signaling (N = 15)
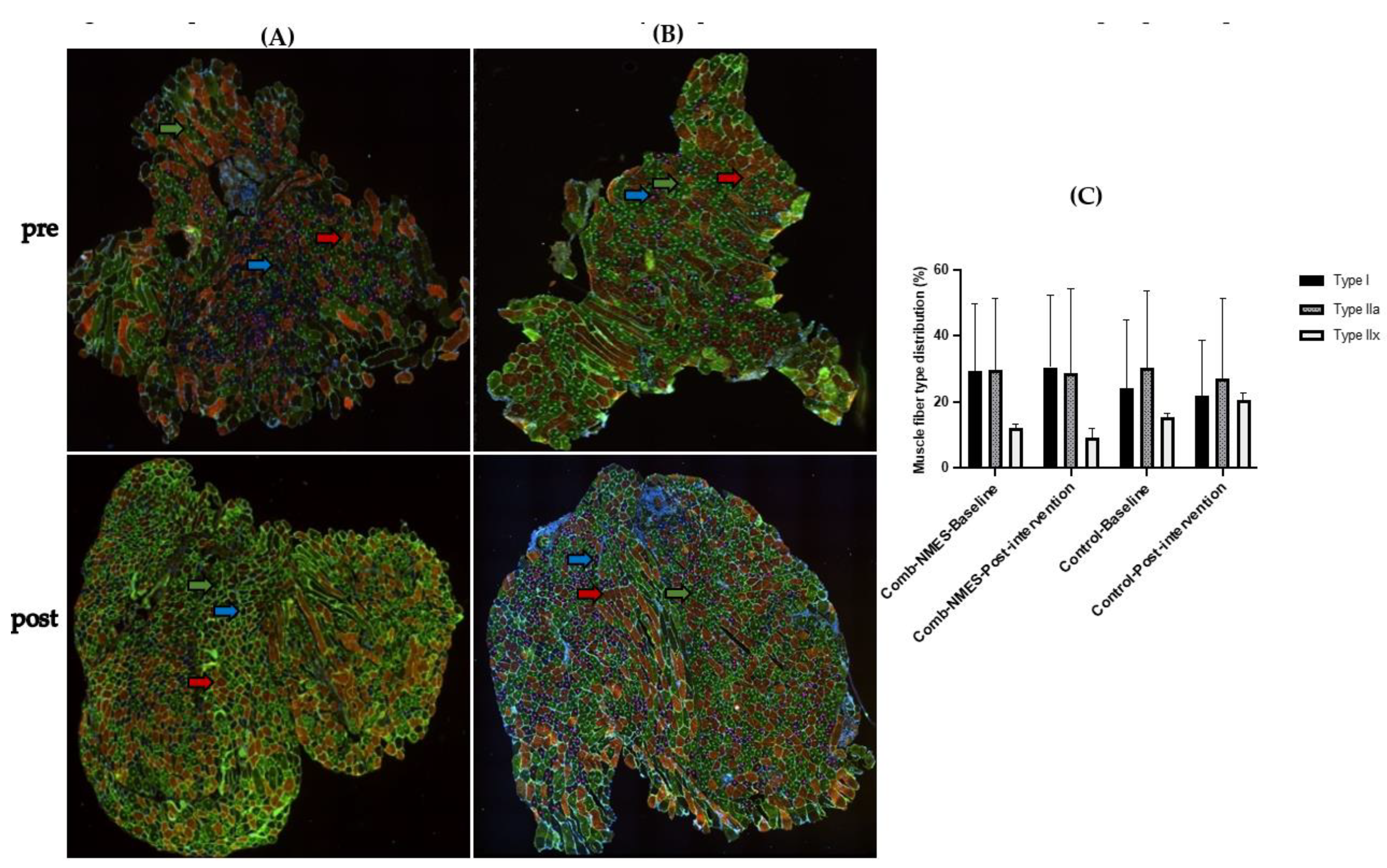
3.5. Myofiber-Type Distribution (N = 15)
4. Discussion
4.1. Glucose, Insulin, and Lipid Profile
4.2. Skeletal Muscle Intracellular Signaling
4.3. Myofiber Type Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauman, W.A.; Spungen, A.M. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: A model of premature aging. Metabolism 1994, 43, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Spungen, A.M. Invited review carbohydrate and lipid metabolism in chronic spinal Cord Injury. J. Spinal Cord Med. 2001, 24, 266–277. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17078. [Google Scholar] [CrossRef]
- Bickel, C.S.; Slade, J.M.; VanHiel, L.R.; Warren, G.L.; Dudley, G.A. Variable-frequency-train stimulation of skeletal muscle after spinal. J. Rehabil. Res. Dev. 2004, 41, 33–40. [Google Scholar] [CrossRef]
- Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Insulin action in skeletal muscle from patients with NIDDM. Insul. Action 1998, 182, 153–160. [Google Scholar]
- Castro, M.J.; Apple, D.F., Jr.; Hillegass, E.A.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Staten, M.A.; Kelley, D.E. Using oral challenge testing to assess insulin action and secretion with mathematical modeling. Diabetes 2014, 63, 1188. [Google Scholar] [CrossRef]
- Bickel, C.S.; Yarar-Fisher, C.; Mahoney, E.T.; McCully, K.K. Neuromuscular electrical stimulation–induced resistance training after SCI: A review of the Dudley protocol. Top. Spinal Cord Inj. Rehabil. 2015, 21, 294–302. [Google Scholar] [CrossRef]
- Gower, B.A.; Chandler-Laney, P.C.; Ovalle, F.; Goree, L.L.; Azziz, R.; Desmond, R.A.; Granger, W.M.; Goss, A.M.; Bates, G. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin. Endocrinol. 2013, 79, 550–557. [Google Scholar] [CrossRef]
- Breda, E.; Cavaghan, M.K.; Toffolo, G.; Polonsky, K.S.; Cobelli, C. Oral glucose tolerance test minimal model indexes of β-cell function and insulin sensitivity. Diabetes 2001, 50, 150–158. [Google Scholar] [CrossRef]
- McCully, K.K.; Mulcahy, T.K.; Ryan, T.E.; Zhao, Q. Skeletal muscle metabolism in individuals with spinal cord injury. J. Appl. Physiol. 2011, 111, 143–148. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Shepherd, C. Skeletal muscle hypertrophy and decreased intramuscular fat after unilateral resistance training in spinal cord injury: Case report. J. Spinal Cord Med. 2010, 33, 90–95. [Google Scholar] [CrossRef]
- Yarar-Fisher, C.; Polston, K.F.; Eraslan, M.; Henley, K.Y.; Polston, K.F.; Eraslan, M.; Henley, K.Y.; Kinikli, G.I.; Bickel, C.S.; Windham, S.T.; et al. Paralytic and nonparalytic muscle adaptations to exercise training versus high-protein diet in individuals with long-standing spinal cord injury. J. App. Phys. 2018, 125, 64–72. [Google Scholar] [CrossRef]
- Dudley, G.; Castro, M.; Rogers, S.; Apple, D., Jr. A simple means of increasing muscle size after spinal cord injury: A pilot study. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 394–396. [Google Scholar] [CrossRef]
- Ryan, T.E.; Brizendine, J.T.; Backus, D.; McCully, K.K. Electrically induced resistance training in individuals with motor complete spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 2166–2173. [Google Scholar] [CrossRef]
- Mahoney, E.T.; Bickel, C.S.; Elder, C.; Black, C.; Slade, J.M.; Apple, D., Jr.; Dudley, G.A. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2005, 86, 1502–1504. [Google Scholar] [CrossRef]
- Ryan, T.E.; Erickson, M.L.; Young, H.-J.; McCully, K.K. Case report: Endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 2559–2561. [Google Scholar] [CrossRef]
- Bamman, M.M.; Ragan, R.C.; Kim, J.-S.; Cross, J.M.; Hill, V.J.; Tuggle, S.C.; Allman, R.M. Myogenic protein expression before and after resistance loading in 26-and 64-yr-old men and women. J. Appl. Physiol. 2004, 97, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.K.; Stec, M.J.; Thalacker-Mercer, A.; Windham, S.T.; Cross, J.M.; Shelley, D.P.; Tuggle, S.C.; Kosek, D.J.; Kim, J.-S.; Bamman, M.M. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J. Appl. Physiol. 2013, 115, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Ford, M.P.; Standaert, D.G.; Watts, R.L.; Bickel, C.S.; Moellering, D.R.; Tuggle, S.C.; Williams, J.Y.; Lieb, L.; Windham, S.T. Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J. Appl. Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.L.; Hornberger, T.A.; Lincoln, H.C.; Bamman, M.M. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J. Physiol. 2011, 589, 3023–3037. [Google Scholar] [CrossRef]
- Thalacker-Mercer, A.E.; Dell, L.J.; Cui, X.; Cross, J.M.; Bamman, M.M. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol. Genom. 2010, 40, 141–149. [Google Scholar] [CrossRef]
- Knopfholz, J.; Disserol, C.C.D.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Ledesma, P.M.; Faria-Neto, J.R.; Olandoski, M.; Da Cunha, C.L.P. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol 2014, 2014, 261878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Polston, K.F.L.; Eraslan, M.; Bickel, C.S.; Windham, S.T.; McLain, A.B.; Oster, R.A.; Bamman, M.M.; Yarar-Fisher, C. A high-protein diet or combination exercise training to improve metabolic health in individuals with long-standing spinal cord injury: A pilot randomized study. Physiol. Rep. 2018, 6, e13813. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.; He, L.; Guma, A.; Wahlström, E.O.; Klip, A.; Wallberg-Henriksson, H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 1996, 39, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Gaster, M.; Staehr, P.; Beck-Nielsen, H.; Schrøder, H.D.; Handberg, A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: Is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes 2001, 50, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, J.R.; Nielsen, J.N.; Kristiansen, S.; Andersen, J.L.; Hargreaves, M.; Richter, E.A. Fiber type-specific expression of GLUT4 in human skeletal muscle: Influence of exercise training. Diabetes 2000, 49, 1092–1095. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Holmes, M.V.; Ala-Korpela, M. What is ‘LDL cholesterol’? Nat. Rev. Cardiol. 2019, 16, 197–198. [Google Scholar] [CrossRef]
- Bochkezanian, V.; Newton, R.U.; Trajano, G.S.; Blazevich, A.J. Effects of neuromuscular electrical stimulation in people with spinal cord injury. Med. Sci. Sports Exerc. 2018, 50, 1733–1739. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Khan, R.; Adler, R.A. Effects of dose de-escalation following testosterone treatment and evoked resistance exercise on body composition, metabolic profile, and neuromuscular parameters in persons with spinal cord injury. Physiol. Rep. 2021, 9, e15089. [Google Scholar] [CrossRef]
- Erickson, M.L.; Ryan, T.E.; Backus, D.; McCully, K.K. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve 2017, 55, 669–675. [Google Scholar] [CrossRef]
- Jessen, N.; Goodyear, L.J. Contraction signaling to glucose transport in skeletal muscle. J. Appl. Physiol. 2005, 99, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O. Exercise-induced increase in muscle insulin sensitivity. J. Appl. Physiol. 2005, 99, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Yarar-Fisher, C.; Bickel, C.S.; Windham, S.T.; McLain, A.B.; Bamman, M.M. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J. Appl. Physiol. 2013, 115, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.W.; Chibalin, A.V.; Zierath, J.R. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol. Scand. 2001, 171, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 2001, 91, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Jensen, T.E.; Cusinato, M.; Garcia, M.; Richter, E.A.; Schiaffino, S. Multiple signalling pathways redundantly control glucose transporter GLUT4 gene transcription in skeletal muscle. J. Physiol. 2009, 587 Pt 17, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Wojtaszewski, J.F. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. 2007, 32, 557–566. [Google Scholar] [CrossRef]
- Mohr, T.; Andersen, J.L.; Biering-Sørensen, F.; Galbo, H.; Bangsbo, J.; Wagner, A.; Kjaer, M. Long term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997, 35, 1–16. [Google Scholar] [CrossRef]
- Ojuka, E.O.; Jones, T.E.; Nolte, L.A.; Chen, M.; Wamhoff, B.R.; Sturek, M.; Holloszy, J.O. Regulation of GLUT4 biogenesis in muscle: Evidence for involvement of AMPK and Ca(2+). Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1008–E1013. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.J.; Kiens, B.; Richter, E.A. Ca2+-calmodulin-dependent protein kinase expression and signalling in skeletal muscle during exercise. J. Physiol. 2006, 574 Pt 3, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.E.; Rose, A.J.; Hellsten, Y.; Wojtaszewski, J.F.; Richter, E.A. Caffeine-induced Ca(2+) release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E286–E292. [Google Scholar] [CrossRef] [PubMed]
- Witczak, C.A.; Fujii, N.; Hirshman, M.F.; Goodyear, L.J. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes 2007, 56, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Witczak, C.A.; Jessen, N.; Warro, D.M.; Toyoda, T.; Fujii, N.; Anderson, M.E.; Hirshman, M.F.; Goodyear, L.J. CaMKII regulates contraction- but not insulin-induced glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1150–E1160. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.H. Insulin, Muscle Glucose Uptake, and Hexokinase: Revisiting the Road Not Taken. Physiology 2022, 37, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Jensen, J.; Printz, R.L.; Granner, D.K.; Ivy, J.L.; Moller, D.E. Overexpression of hexokinase II in transgenic mice. Evidence that increased phosphorylation augments muscle glucose uptake. J. Biol. Chem. 1996, 271, 14834–14839. [Google Scholar] [CrossRef]
- Fueger, P.T.; Lee-Young, R.S.; Shearer, J.; Bracy, D.P.; Heikkinen, S.; Laakso, M.; Rottman, J.N.; Wasserman, D.H. Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: Studies in mice with a 50% reduction of hexokinase II. Diabetes 2007, 56, 2476–2484. [Google Scholar] [CrossRef]
- Yarar-Fisher, C.; Bickel, C.S.; Kelly, N.A.; Stec, M.J.; Windham, S.T.; McLain, A.B.; Oster, R.A.; Bamman, M.M. Heightened TWEAK-NF-κB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E754–E761. [Google Scholar] [CrossRef]
- Duffell, L.D.; Donaldson, N.D.N.; Perkins, T.A.; Rushton, D.N.; Hunt, K.J.; Kakebeeke, T.H.; Newham, D.J. Long-term intensive electrically stimulated cycling by spinal cord–injured people: Effect on muscle properties and their relation to power output. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2008, 38, 1304–1311. [Google Scholar]
- Gerrits, H.L.; Hopman, M.T.; Sargeant, A.J.; Jones, D.A.; De Haan, A. Effects of training on contractile properties of paralyzed quadriceps muscle. Muscle Nerve 2002, 25, 559–567. [Google Scholar] [CrossRef]
- Martin, T.P.; Stein, R.B.; Hoeppner, P.H.; Reid, D.C. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J. Appl. Physiol. 1992, 72, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.L.; Mohr, T.; Biering-Sorensen, F.; Galbo, H.; Kjaer, M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: Effects of long-term functional electrical stimulation (FES). Pflug. Arch. 1996, 431, 513–518. [Google Scholar] [CrossRef]
- Crameri, R.M.; Weston, A.R.; Rutkowski, S.; Middleton, J.W.; Davis, G.M.; Sutton, J.R. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: A pilot study. Eur. J. Appl. Physiol. 2000, 83, 409–415. [Google Scholar] [CrossRef]
- Salmons, S.; Sreter, F. Significance of impulse activity in the transformation of skeletal muscle type. Nature 1976, 263, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Reichmann, H.; Schröder, M.J. Influence of direct low frequency stimulation on contractile properties of denervated fast-twitch rabbit muscle. Pflüg. Archiv. 1985, 405, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Salmons, S. Adaptive change in electrically stimulated muscle: A framework for the design of clinical protocols. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2009, 40, 918–935. [Google Scholar] [CrossRef]
- Lopez-Guajardo, A.; Sutherland, H.; Jarvis, J.C.; Salmons, S. Induction of a fatigue-resistant phenotype in rabbit fast muscle by small daily amounts of stimulation. J. Appl. Physiol. 2001, 90, 1909–1918. [Google Scholar] [CrossRef]
- Petrie, M.; Suneja, M.; Shields, R.K. Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J. Appl. Physiol. 2015, 118, 723–731. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Bell, G.; Jeon, J.; Weiss, C.B.; Murdoch, G.; MacLean, I.; Ryan, E.; Burnham, R. Functional electrical stimulation exercise increases GLUT-1 and GLUT-4 in paralyzed skeletal muscle. Metabolism 1999, 48, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Crameri, R.M.; Weston, A.; Climstein, M.; Davis, G.M.; Sutton, J.R. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J. Med. Sci. Sports 2002, 12, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Rochester, L.; Barron, M.J.; Chandler, C.S.; Sutton, R.A.; Miller, S.; Johnson, M.A. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia 1995, 33, 514–522. [Google Scholar] [CrossRef] [PubMed]


| Participants | Age | Gender | Race | AIS | LOI | Completeness | Injury Type | Days in Intervention |
|---|---|---|---|---|---|---|---|---|
| Control | ||||||||
| SCI 3 | 29 | F | BLACK | A | T2 | complete | GSW | 10 |
| SCI 4 | 20 | M | BLACK | A | T9 | complete | GSW | 11 |
| SCI 10 | 31 | M | BLACK | B | C7 | incomplete | MVC | 20 |
| SCI 16 | M | BLACK | A | L1 | complete | MVC | 24 | |
| SCI 19 | 21 | F | BLACK | A | T9 | complete | GSW | 16 |
| SCI 20 | 20 | M | WHITE | A | T11 | complete | FALL | 9 |
| SCI 28 | 28 | M | BLACK | A | C6 | complete | GSW | 36 |
| SCI 31 | 26 | M | WHITE | A | T2 | complete | GSW | 36 |
| Summary statistics * | 25 ± 4.28 * | 18 (10.5–30) * | ||||||
| Comb-NMES | ||||||||
| SCI 1 | 22 | M | BLACK | A | C7 | complete | GSW | 17 |
| SCI 2 | 25 | M | WHITE | B | T6 | incomplete | MVC | 15 |
| SCI 6 | 42 | M | WHITE | B | C4 | incomplete | FALL | 20 |
| SCI 11 | 43 | F | WHITE | A | C6 | complete | MVC | 23 |
| SCI 12 | 27 | M | HISPANIC | A | T7 | complete | MVC | 21 |
| SCI 17 | 51 | M | BLACK | C | C3 | incomplete | MVC | 39 |
| SCI 22 | 41 | M | BLACK | A | C4 | complete | MVC | 21 |
| SCI 24 | 45 | M | WHITE | A | T3 | complete | MCC | 28 |
| SCI 29 | 32 | M | BLACK | A | T9 | complete | GSW | 23 |
| SCI 30 | 34 | M | WHITE | A | C7 | complete | GSW | 21 |
| SCI 32 | 32 | M | WHITE | B | C4 | incomplete | MVC | 24 |
| SCI 33 | 24 | M | BLACK | A | T3 | complete | GSW | 17 |
| Summary statistics * | 34.8 ± 9.4 * | 21 (18.5–23.5) * |
| Antibody | Type | Catalog Number | Vendor | Dilution | Host |
|---|---|---|---|---|---|
| MHC I (slow) | Primary antibody | NCL-MHCs | Leica | 1:50 | Rabbit |
| Alexa 594 | Secondary antibody | A-11012 | ThermoFisher Scientific | 1:200 | Goat |
| Anti-Laminin | Primary antibody | L8271-.2ML | Sigma-Aldrich | 1:1000 | mouse |
| Alexa 488 | Secondary antibody | A-11001 | ThermoFisher Scientific | 1:200 | Goat |
| MHC IIa (fast) | Primary antibody | NCL-MHCf | Leica | 1:40 | Rabbit |
| GLUT-4 | Primary antibody | PA5-23052 | Fisher Scientific | 1:1000 | Rabbit |
| AMPK-α | Primary antibody | 2532S | Cell Signaling | 1:1000 | Rabbit |
| Phospho (Thr172)-AMPK-α | Primary antibody | 2535S | Cell Signaling | 1:1000 | Rabbit |
| CaMKII | Primary antibody | 4436S | Cell Signaling | 1:1000 | Rabbit |
| Phospho (Thr286)-CaMKII | Primary antibody | 12716 | Cell Signaling | 1:1000 | Rabbit |
| Akt | Primary antibody | 9272S | Cell Signaling | 1:1000 | Rabbit |
| Phospho (Ser473)-Akt | Primary antibody | 9271S | Cell Signaling | 1:1000 | Rabbit |
| AS160 | Primary antibody | 2447S | Cell Signaling | 1:1000 | Rabbit |
| Phospho (Thr642)-AS160 | Primary antibody | 4288S | Cell Signaling | 1:1000 | Rabbit |
| Hexokinase II | Primary antibody | 2106S | Cell Signaling | 1:1000 | Rabbit |
| Glycogen Synthase (GS) | Primary antibody | 3893S | Cell Signaling | 1:1000 | Rabbit |
| Phosphor (Ser641)-GS | Primary antibody | 3891S | Cell Signaling | 1:1000 | Rabbit |
| IRS-1 | Primary antibody | 2390S | Cell Signaling | 1:1000 | Rabbit |
| Phospho (Tyr895)-IRS-1 | Primary antibody | 3070S | Cell Signaling | 1:1000 | Rabbit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, A.; Li, J.; Womack, E.; Farrow, M.; Yarar-Fisher, C. The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Signaling for Glucose Utilization, Myofiber Distribution, and Metabolic Function after Spinal Cord Injury. Int. J. Environ. Res. Public Health 2023, 20, 6958. https://doi.org/10.3390/ijerph20206958
Alharbi A, Li J, Womack E, Farrow M, Yarar-Fisher C. The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Signaling for Glucose Utilization, Myofiber Distribution, and Metabolic Function after Spinal Cord Injury. International Journal of Environmental Research and Public Health. 2023; 20(20):6958. https://doi.org/10.3390/ijerph20206958
Chicago/Turabian StyleAlharbi, Amal, Jia Li, Erika Womack, Matthew Farrow, and Ceren Yarar-Fisher. 2023. "The Effect of Lower Limb Combined Neuromuscular Electrical Stimulation on Skeletal Muscle Signaling for Glucose Utilization, Myofiber Distribution, and Metabolic Function after Spinal Cord Injury" International Journal of Environmental Research and Public Health 20, no. 20: 6958. https://doi.org/10.3390/ijerph20206958





