Heart Rate from Progressive Volitional Cycling Test Is Associated with Endothelial Dysfunction Outcomes in Hypertensive Chilean Adults
Abstract
1. Introduction
2. Materials and Methods
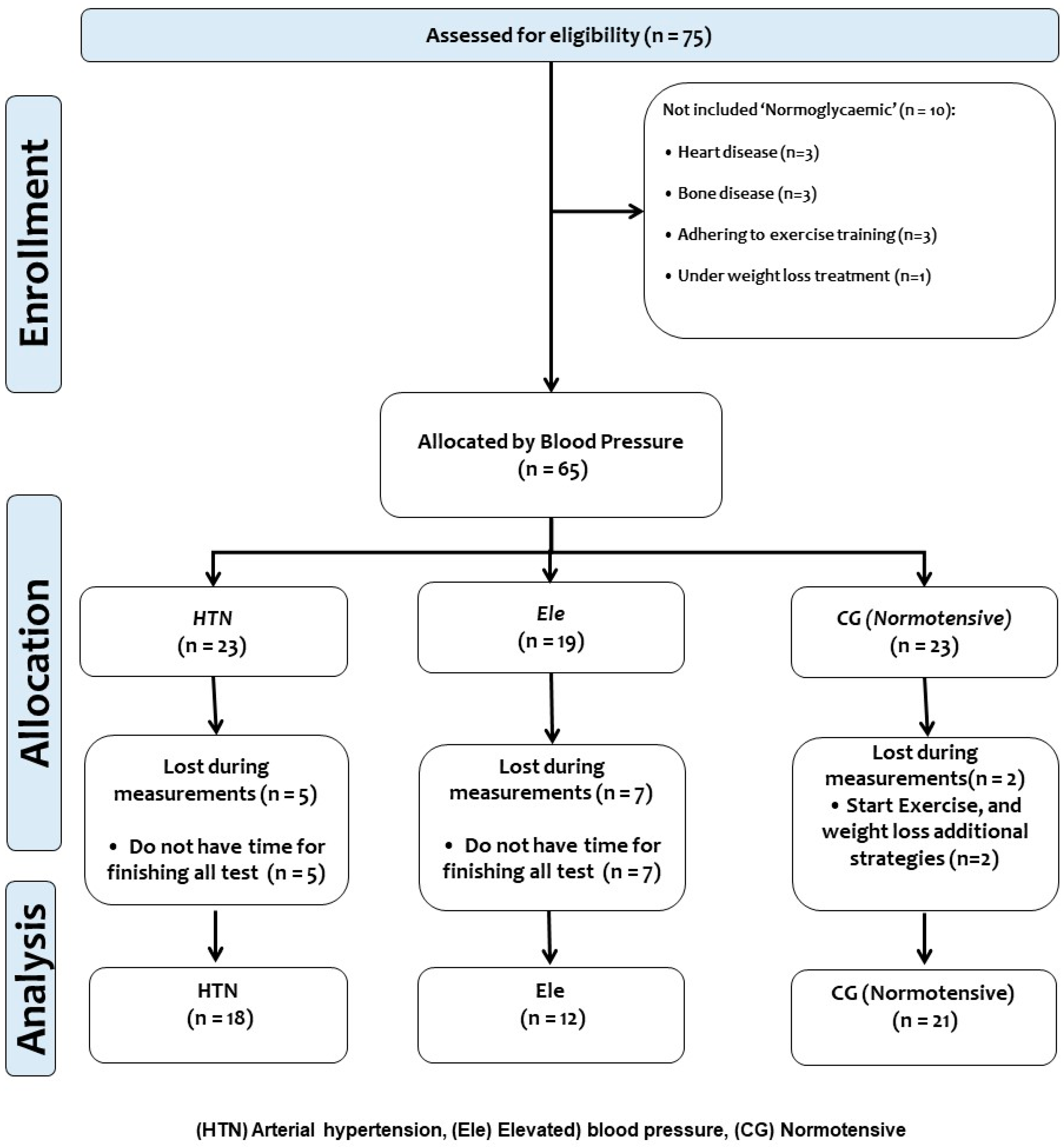
2.1. Participants
2.2. Endothelial Dysfunction Outcomes
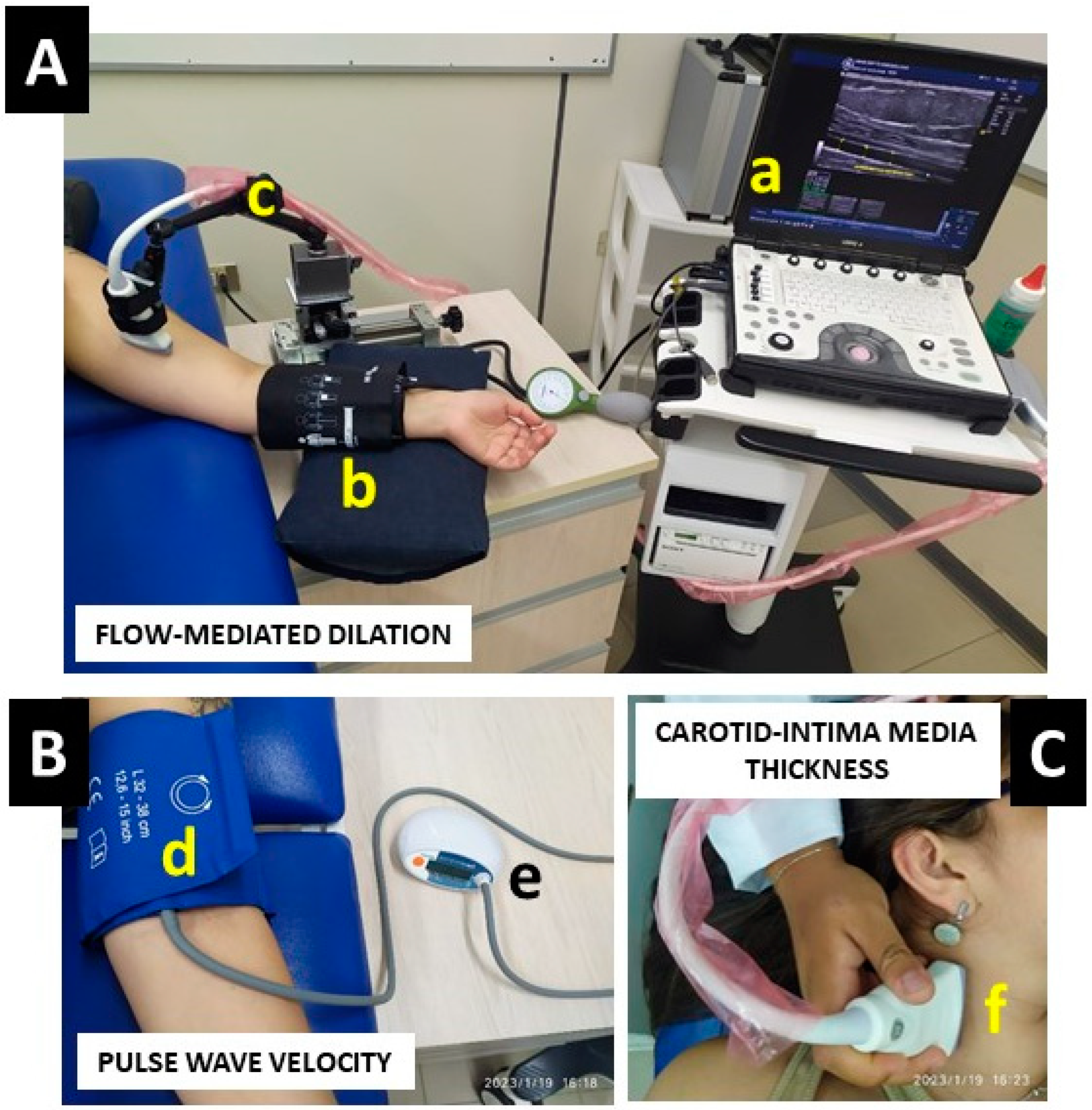
2.2.1. Flow-Mediated Dilation
2.2.2. Carotid Intima-Media Thickness
2.2.3. Pulse Wave Velocity
2.2.4. Blood Pressure and Heart Rate at Rest
2.2.5. Progressive Volitional Cycling Test and Heart Rate during Exercise
2.2.6. Anthropometric and Body Composition (Secondary Outcomes)
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
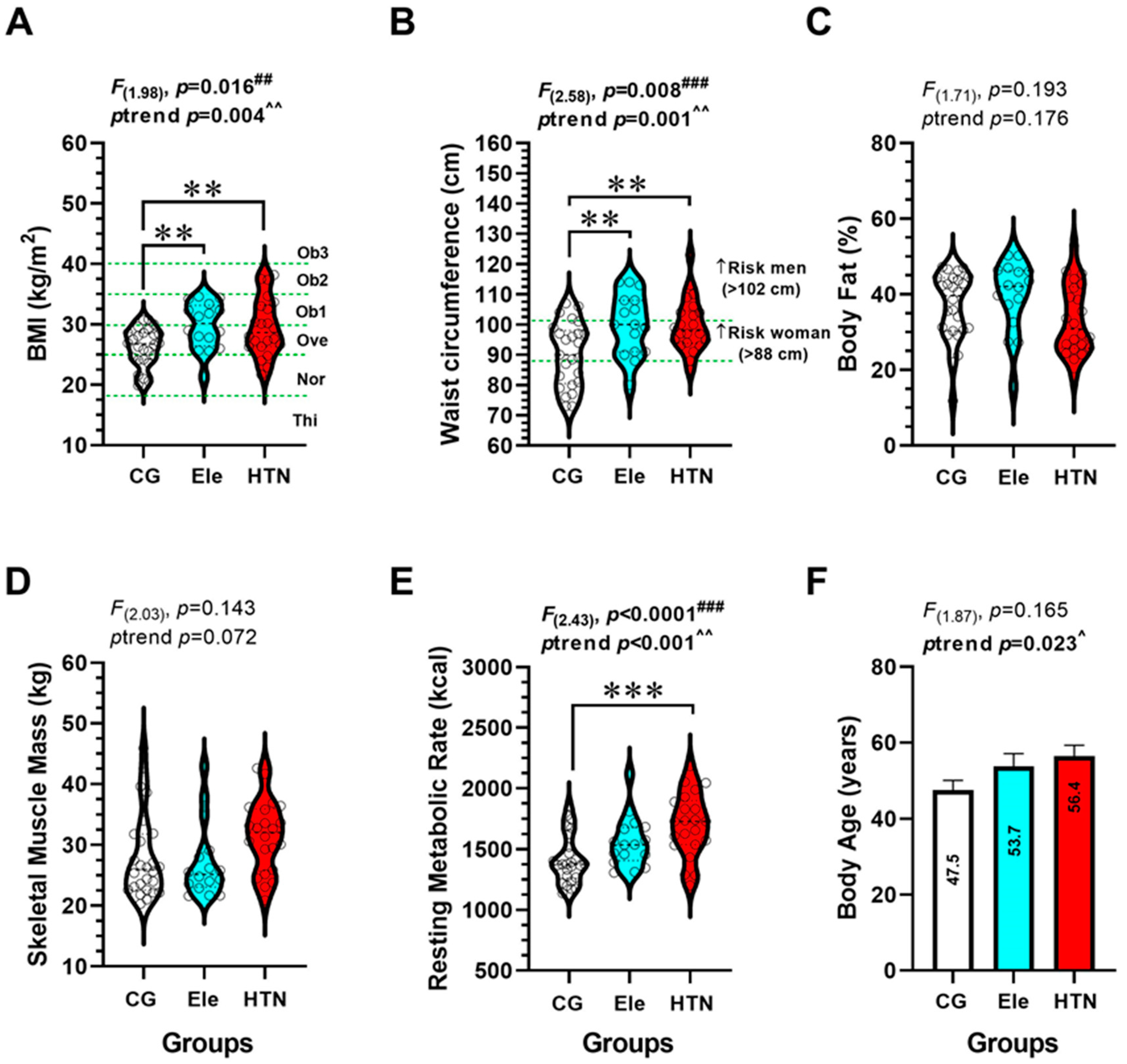
3.2. Anthropometric and Body Composition (Secondary Outcomes)
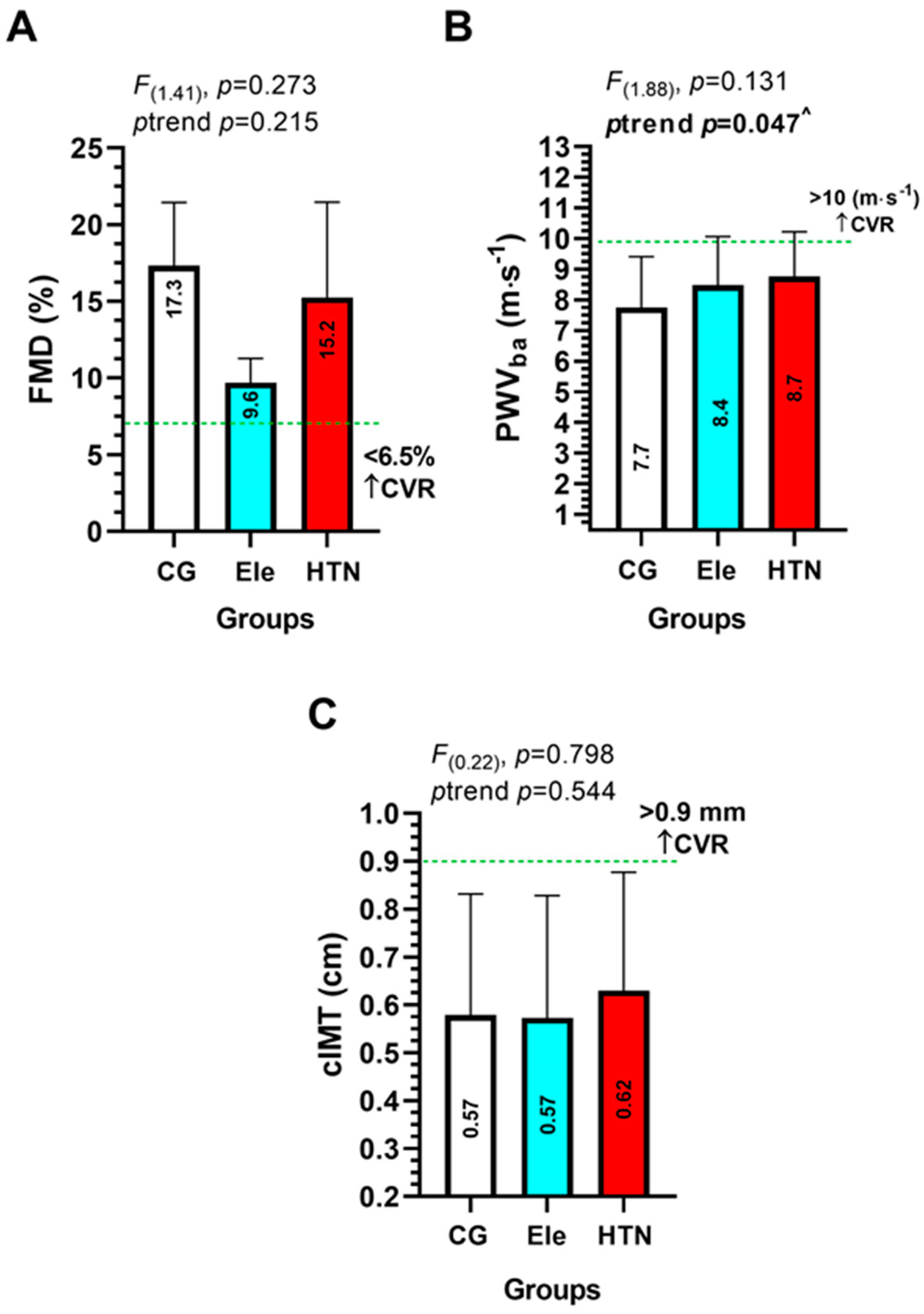
3.3. Endothelial Dysfunction Parameters (Main Outcomes)
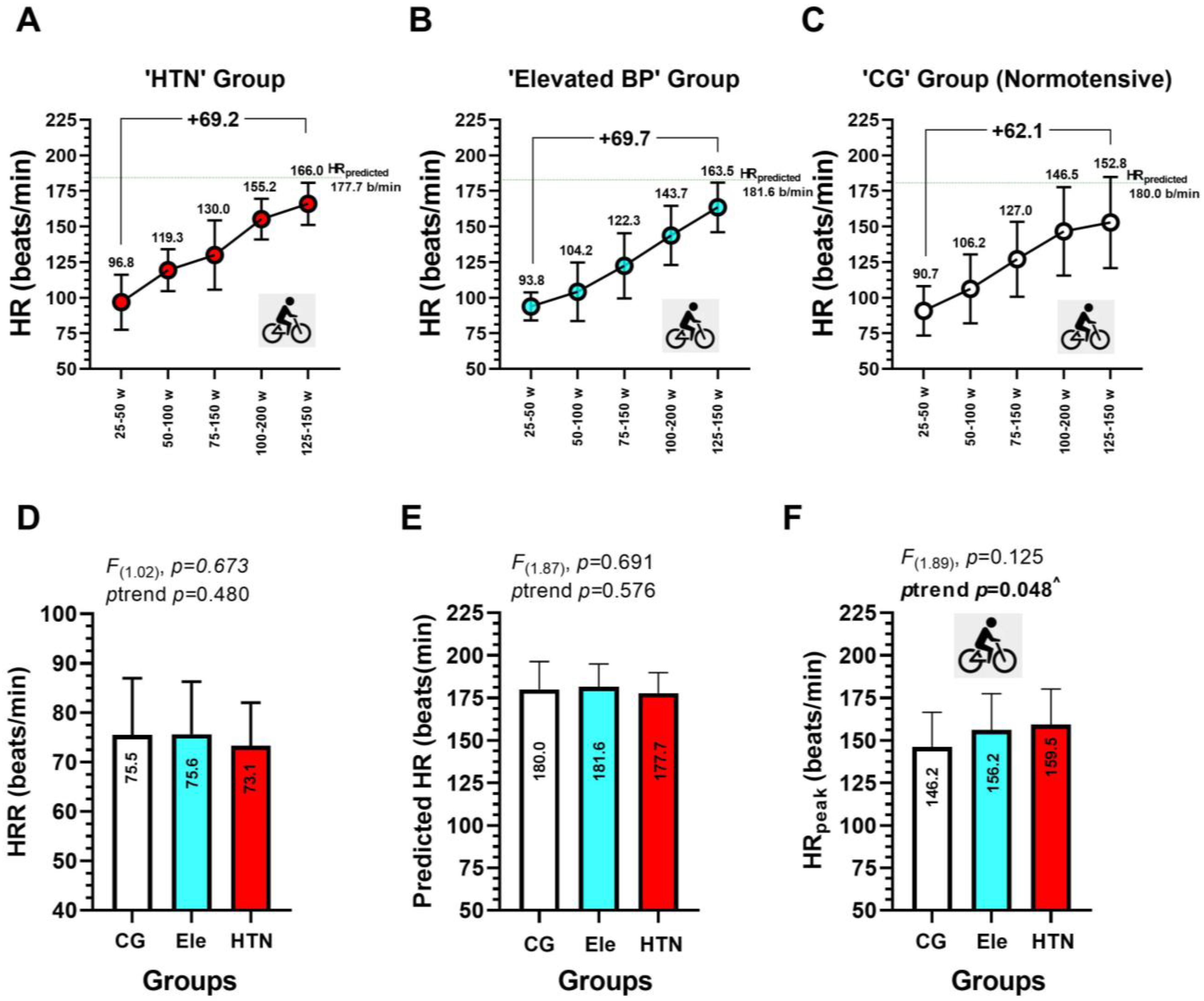
3.4. Heart Rate during Progressive Volitional Cycling Test in the HTN, Ele, and Control Groups
3.5. Association between EDys Outcomes FMD, PWVba, and cIMT with Different Heart Rate during a Progressive Volitional Cycling Test in HTN, Ele, and Control Normotensive Subjects
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abulnaja, K.O.; Kannan, K.; Al-Manzlawi, A.M.K.; Kumosani, T.A.; Qari, M.; Moselhy, S.S. Sensitivity, specificity of biochemical markers for early prediction of endothelial dysfunction in atherosclerotic obese subjects. Afr. Health Sci. 2022, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Collins, R. Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet 2003, 361, 2005–2016. [Google Scholar] [PubMed]
- Restaino, R.M.; Walsh, L.K.; Morishima, T.; Vranish, J.R.; Martinez-Lemus, L.A.; Fadel, P.J.; Padilla, J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H648–H653. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Bruno, R.M.; van Mil, A.C.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Morales, M.S.; Cuffaro, P.E.; Barochiner, J.; Rada, M.A.; Alfie, J.; Aparicio, L.; Marin, M.; Galarza, C.R.; Waisman, G.D. Validation of a new piezo-electronic device for non-invasive measurement of arterial pulse wave velocity according to the artery society guidelines. Artery Res. 2015, 10, 32–37. [Google Scholar] [CrossRef]
- Bender, S.B.; Laughlin, M.H. Modulation of endothelial cell phenotype by physical activity: Impact on obesity-related endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1–H8. [Google Scholar] [CrossRef]
- Petermann, F.; Durán, E.; Labraña, A.M.; Martínez, M.A.; Leiva, A.M.; Garrido-Méndez, A.; Poblete-Valderrama, F.; Díaz-Martínez, X.; Salas, C.; Celis-Morales, C. Factores de riesgo asociados al desarrollo de hipertensión arterial en Chile. Rev. Med. Chile 2017, 145, 996–1004. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Bapir, M.; Skene, S.S.; Sies, H.; Kelm, M. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc. Res. 2022. [Google Scholar] [CrossRef]
- Liu, C.S.; Li, C.I.; Shih, C.M.; Lin, W.Y.; Lin, C.H.; Lai, S.W.; Li, T.C.; Lin, C.C. Arterial stiffness measured as pulse wave velocity is highly correlated with coronary atherosclerosis in asymptomatic patients. J. Atheroscler Thromb 2011, 18, 652–658. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the Management of Arterial Hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiog. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Palatini, P.; Puato, M.; Rattazzi, M.; Pauletto, P. Effect of regular physical activity on carotid intima-media thickness. Results from a 6-year prospective study in the early stage of hypertension. Blood Press. 2011, 20, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lobato, N.S.; Filgueira, F.P.; Akamine, E.H.; Tostes, R.C.; Carvalho, M.H.; Fortes, Z.B. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz. J. Med. Biol. Res. 2012, 45, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tjonna, A.E.; Rognmo, O.; Bye, A.; Stolen, T.O.; Wisloff, U. Time course of endothelial adaptation after acute and chronic exercise in patients with metabolic syndrome. J. Strength Cond. Res. 2011, 25, 2552–2558. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.L.; Johnson, B.T.; Huedo-Medina, T.B.; Larson, K.A.; Ash, G.I.; Pescatello, L.S. The blood pressure response to acute and chronic aerobic exercise: A meta-analysis of candidate gene association studies. J. Sci. Med. Sport 2016, 19, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for hypertension: A prescription update integrating existing recommendations with emerging research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Condliffe, S.; Link, C.R.; Parasuraman, S.; Pollack, M.F. The effects of hypertension and obesity on total health-care expenditures of diabetes patients in the United States. Appl. Econ. Lett. 2013, 20, 649–652. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Correa-Rodríguez, M.; Tordecilla-Sanders, A.; Aya-Aldana, V.; Izquierdo, M.; Correa-Bautista, J.E.; Álvarez, C.; Garcia-Hermoso, A. Exercise and postprandial lipemia: Effects on vascular health in inactive adults. Lipids Health Dis. 2018, 17, 69. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Hernández-Quiñones, P.A.; Tordecilla-Sanders, A.; Álvarez, C.; Ramírez-Campillo, R.; Izquierdo, M.; Correa-Bautista, J.E.; Garcia-Hermoso, A.; Garcia, R.G. Effectiveness of HIIT compared to moderate continuous training in improving vascular parameters in inactive adults. Lipids Health Dis. 2019, 18, 42. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Atkinson, G. Shear rate normalization is not essential for removing the dependency of flow-mediated dilation on baseline artery diameter: Past research revisited. Physiol. Meas. 2014, 35, 1825. [Google Scholar] [CrossRef] [PubMed]
- Coll, B.; Feinstein, S.B. Carotid intima-media thickness measurements: Techniques and clinical relevance. Curr. Atheroscler. Rep. 2008, 10, 444–450. [Google Scholar] [CrossRef]
- Ghardashi-Afousi, A.; Davoodi, M.; Hesamabadi, B.K.; Asvadi-Fard, M.; Bigi, M.A.B.; Izadi, M.R.; Gaeini, A.A. Improved carotid intima-media thickness-induced high-intensity interval training associated with decreased serum levels of Dkk-1 and sclerostin in type 2 diabetes. J. Diabetes Its Complicat. 2020, 34, 107469. [Google Scholar] [CrossRef] [PubMed]
- Ring, M.; Eriksson, M.J.; Zierath, J.R.; Caidahl, K. Arterial stiffness estimation in healthy subjects: A validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques. Hypertens. Res. 2014, 37, 999–1007. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Astrand, P.; Stromme, S. Textbook of Work Physiology: Physiological Bases of Exercise, 4th ed.; Astrand, P.O., Rodahl, K., Dahl, H.A., Stromme, S., Eds.; Human Kinetics: Leeds, UK, 2003; pp. 237–272. [Google Scholar]
- Mancilla, R.; Torres, P.; Álvarez, C.; Schifferli, I.; Sapunar, J.; Díaz, E. Ejercicio físico interválico de alta intensidad mejora el control glicémico y la capacidad aeróbica en pacientes con intolerancia a la glucosa. Rev. Med. Chile 2014, 142, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Liontou, C.; Chrysohoou, C.; Skoumas, J.; Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Chronotropic response during treadmill exercise and subclinical carotid atherosclerosis after adjusting for the calibrated SCORE risk classification: A cross-sectional study. Heart Vessel. 2016, 31, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gianaros, P.J.; Jennings, J.R.; Olafsson, G.B.; Steptoe, A.; Sutton-Tyrrell, K.; Muldoon, M.F.; Manuck, S.B. Greater intima-media thickness in the carotid bulb is associated with reduced baroreflex sensitivity. Am. J. Hypertens. 2002, 15, 486–491. [Google Scholar] [CrossRef]
- Lábrová, R.; Honzíková, N.; Maderová, E.; Vysocanová, P.; Nováková, Z.; Závodná, E.; Fiser, B.; Semrád, B. Age-dependent relationship between the carotid intima-media thickness, baroreflex sensitivity, and the inter-beat interval in normotensive and hypertensive subjects. Physiol. Res. 2005, 54, 593–600. [Google Scholar] [CrossRef]
- Park, W.; Lee, J.; Park, H.-Y.; Park, S.; Park, J. Vascular Function and Frailty in Community-Dwelling Older Individuals. Artery Res. 2022, 28, 31–39. [Google Scholar] [CrossRef]

| Outcomes | HTN | Ele | CG | Baseline p-Value |
|---|---|---|---|---|
| (n =) | 18 | 12 | 21 | |
| Anthropometric | ||||
| Age (y) | 42.2 ± 12.2 | 38.3 ± 13.3 | 39.9 ± 16.2 | p = 0.702 |
| Weight (kg) | 84.1 ± 15.1 * | 79.7 ± 12.9 * | 67.3 ± 10.5 | p < 0.0001 |
| Height (m) | 1.70 ± 0.10 * | 1.63 ± 0.07 * | 1.59 ± 0.07 | p < 0.0001 |
| Blood pressure | ||||
| Systolic blood pressure (mmHg) | 143.2 ± 9.1 *** | 124.9 ± 2.6 ** | 110.4 ± 7.0 | p < 0.0001 |
| Diastolic blood pressure (mmHg) | 87.3 ± 10.7 *** | 83.3 ± 7.9 ** | 73.8 ± 7.2 | p < 0.0001 |
| Mean arterial pressure (mmHg) | 105.9 ± 10.2 *** | 97.1 ± 6.1 ** | 86.0 ± 7.1 | p < 0.0001 |
| Pulse pressure (mmHg) | 55.9 ± 1.6 *** | 41.6 ± 5.3 ** | 36.6 ± 2.2 | p < 0.0001 |
| Pharmacologic theatment | ||||
| Angiotensine converting enzyme inhibitors (n = /total) | 2/18 | - | - | |
| Βeta blocker (n = /total) | 2/18 | - | - | |
| Metformin (n = /total) | - | 1/18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, C.; Tuesta, M.; Reyes, Á.; Guede-Rojas, F.; Peñailillo, L.; Cigarroa, I.; Vásquez-Gómez, J.; Cano-Montoya, J.; Durán-Marín, C.; Rojas-Paz, O.; et al. Heart Rate from Progressive Volitional Cycling Test Is Associated with Endothelial Dysfunction Outcomes in Hypertensive Chilean Adults. Int. J. Environ. Res. Public Health 2023, 20, 4236. https://doi.org/10.3390/ijerph20054236
Alvarez C, Tuesta M, Reyes Á, Guede-Rojas F, Peñailillo L, Cigarroa I, Vásquez-Gómez J, Cano-Montoya J, Durán-Marín C, Rojas-Paz O, et al. Heart Rate from Progressive Volitional Cycling Test Is Associated with Endothelial Dysfunction Outcomes in Hypertensive Chilean Adults. International Journal of Environmental Research and Public Health. 2023; 20(5):4236. https://doi.org/10.3390/ijerph20054236
Chicago/Turabian StyleAlvarez, Cristian, Marcelo Tuesta, Álvaro Reyes, Francisco Guede-Rojas, Luis Peñailillo, Igor Cigarroa, Jaime Vásquez-Gómez, Johnattan Cano-Montoya, Cristóbal Durán-Marín, Oscar Rojas-Paz, and et al. 2023. "Heart Rate from Progressive Volitional Cycling Test Is Associated with Endothelial Dysfunction Outcomes in Hypertensive Chilean Adults" International Journal of Environmental Research and Public Health 20, no. 5: 4236. https://doi.org/10.3390/ijerph20054236
APA StyleAlvarez, C., Tuesta, M., Reyes, Á., Guede-Rojas, F., Peñailillo, L., Cigarroa, I., Vásquez-Gómez, J., Cano-Montoya, J., Durán-Marín, C., Rojas-Paz, O., Márquez, H., Izquierdo, M., & Delgado-Floody, P. (2023). Heart Rate from Progressive Volitional Cycling Test Is Associated with Endothelial Dysfunction Outcomes in Hypertensive Chilean Adults. International Journal of Environmental Research and Public Health, 20(5), 4236. https://doi.org/10.3390/ijerph20054236










