Spatio-Temporal Bayesian Models for Malaria Risk Using Survey and Health Facility Routine Data in Rwanda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analysis
2.2.1. Binomial Model
2.2.2. Modeling Routine Data Only
2.2.3. Modeling Routine Data with Survey Data
2.3. Bayesian Inference
3. Results
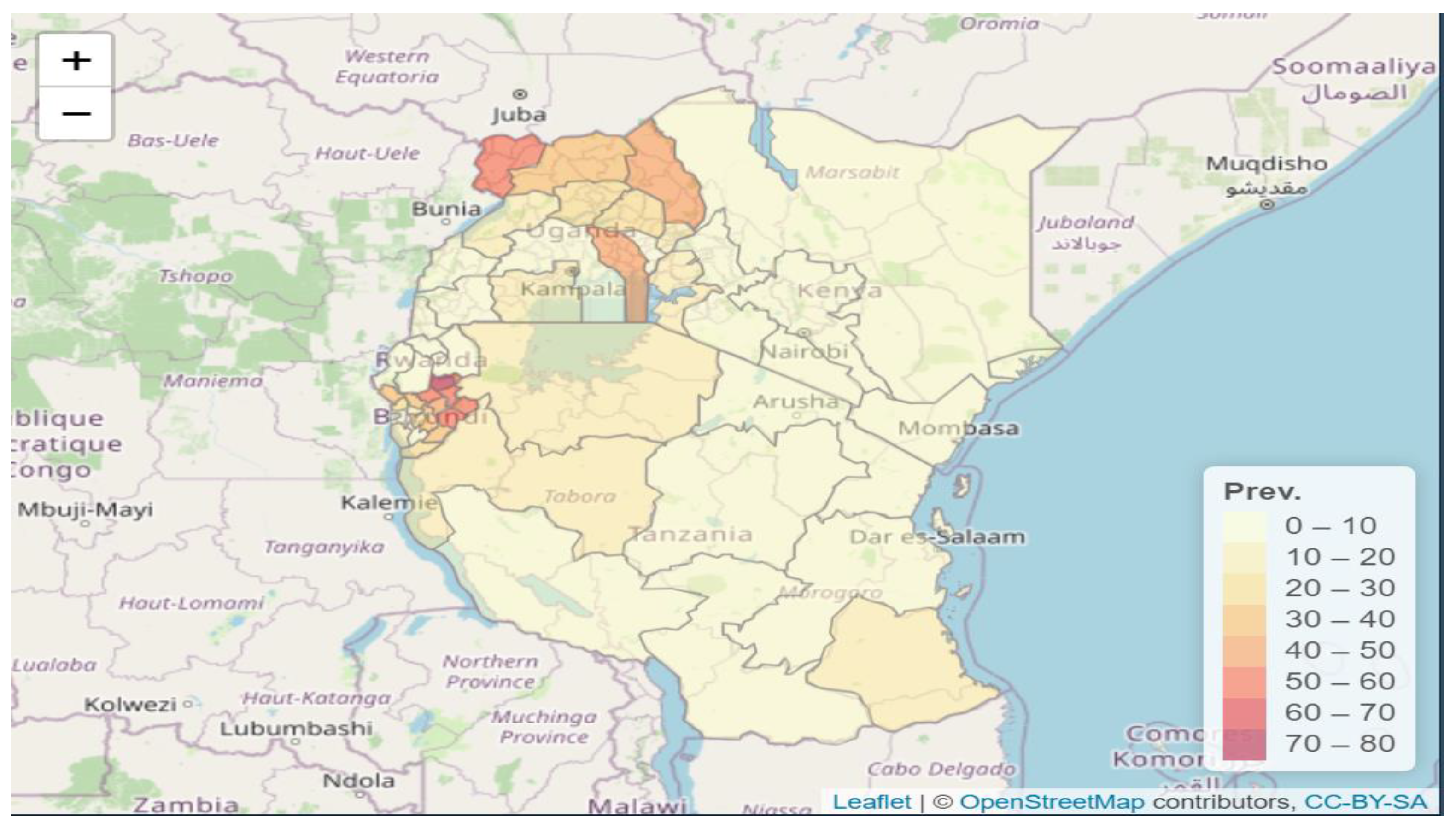
3.1. Data Exploratory Analysis
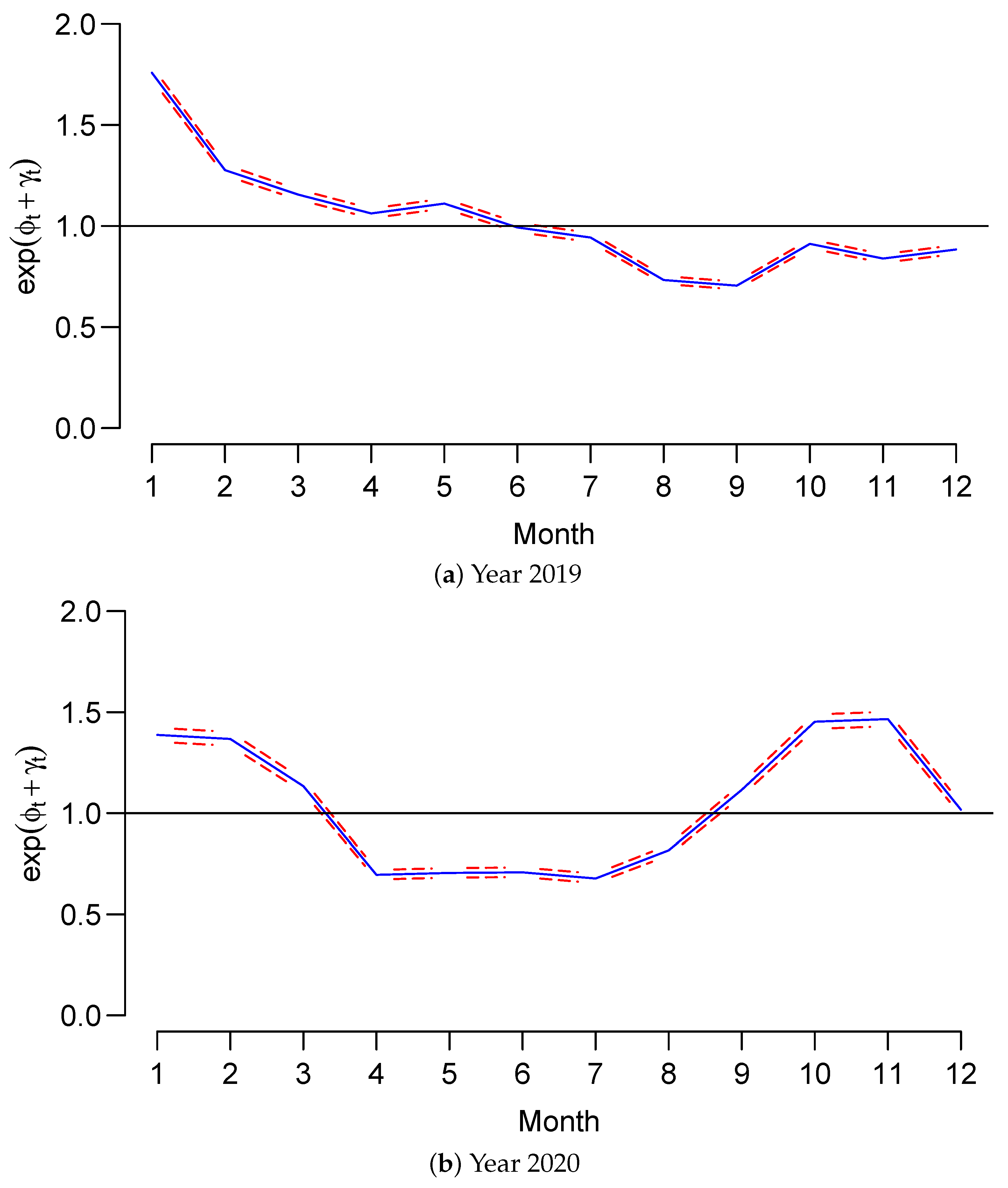
3.2. Main Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021. Global Report. 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 1 May 2022).
- ICF International. Spatial Data Repository. The Demographic and Health Surveys Program. Available online: https://spatialdata.dhsprogram.com (accessed on 16 September 2022).
- Ties Boerma, J.; Sommerfelt, A.E. Demographic and health surveys (DHS: Contributions and limitations. World Health Stat. Q. 1993, 46, 222–226. [Google Scholar]
- Dieudonne, H.; Ntizimira, C.; Mbituyumuremyi, A.; Hakizimana, E.; Mahmoud, H.; Birindabagabo, P.; Musanabaganwa, C.; Gashumba, D. The impact of COVID-19 on malaria services in three high endemic districts in Rwanda: A mixed-method study. Malar. J. 2022, 21, 48. [Google Scholar]
- Wagenaar, B.H.; Hirschhorn, L.R.; Henley, C.; Gremu, A.; Sindano, N.; Chilengi, R. Data-driven quality improvement in low-and middle-income country health systems: Lessons from seven years of implementation experience across Mozambique, Rwanda, and Zambia. BMC Health Serv. Res. 2017, 17, 830. [Google Scholar] [CrossRef] [PubMed]
- WHO. Monitoring and Evaluating Digital Health Interventions: A Practical Guide to Conducting Research and Assessment; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- National Institute of Statistics of Rwanda; Ministry of Health [Rwanda]; ICF International. Rwanda Demographic and Health Survey 2019–2020; NISR: Kigali, Rwanda; MOH: Kigali, Rwanda; ICF International: Reston, VA, USA, 2020.
- Semakula, M.; Niragire, F.; Faes, C. Bayesian spatio-temporal modeling of malaria risk in Rwanda. PLoS ONE 2020, 15, e0238504. [Google Scholar] [CrossRef] [PubMed]
- Birgit, S.; Leonhard, H. Spatio-temporal disease mapping using INLA. Environmetrics 2011, 22, 725–734. [Google Scholar]
- Håvard, R.; Sara, M.; Nicolas, C. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2009, 71, 319–392. [Google Scholar]
- Julian, B.; Peter, G.; David, H.; Kerrie, M. Bayesian computation and stochastic systems. Stat. Sci. 1995, 10, 3–41. [Google Scholar]
- Andrea, R.; Leonhard, H.; Håvard, R.; Matthias, B. Gender-specific differences and the impact of family integration on time trends in age-stratified Swiss suicide rates. J. R. Stat. Soc. Ser. A (Statistics Soc.) 2012, 175, 473–490. [Google Scholar]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; Van Der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2002, 64, 583–639. [Google Scholar] [CrossRef] [Green Version]
- Lesaffre, E.; Lawson, A.B. Bayesian Biostatistics; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pettit, L.I. The conditional predictive ordinate for the normal distribution. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 1990, 52, 175–184. [Google Scholar] [CrossRef]
- Hemingway, J.; Shretta, R.; Wells, T.N.; Bell, D.; Djimdé, A.A.; Achee, N.; Qi, G. Tools and strategies for malaria control and elimination: What do we need to achieve a grand convergence in malaria? PLoS Biol. 2016, 14, e1002380. [Google Scholar] [CrossRef] [PubMed]
- Rwanda Biomedical Centre. Indoor Residual Spraying (IRS) and Long-Lasting Insecticide-Treated Nets (LLINs) Distributed in High Malaria Burden Districts; RBC Report; Rwanda Biomedical Centre: Kigali, Rwanda, 2020.
- Rumisha, S.F.; Lyimo, E.P.; Mremi, I.R.; Tungu, P.K.; Mwingira, V.S.; Mbata, D.; Malekia, S.E.; Joachim, C.; Mboera, L.E.G. Data quality of the routine health management information system at the primary healthcare facility and district levels in Tanzania. BMC Med. Inform. Decis. Mak. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Sabella, M.; Pepela, W.; Soti, D.; Hillary, K.; Benson, D.; Ties, B. Using health-facility data to assess subnational coverage of maternal and child health indicators, Kenya. Bull. World Health Organ. 2017, 95, 683–694. [Google Scholar]
- Agiraembabazi, G.; Ogwal, J.; Tashobya, C.; Kananura, R.M.; Boerma, T.; Waiswa, P. Can routine health facility data be used to monitor subnational coverage of maternal, newborn and child health services in Uganda? BMC Health Serv. Res. 2021, 21, 512. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Hazel, E.; Park, L.; Carter, E.; Moulton, L.H.; Heidkamp, R.; Perin, J. Obtaining district-level health estimates using geographically masked location from Demographic and Health Survey data. Int. J. Health Geogr. 2020, 19, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adane, A.; Adege, T.M.; Ahmed, M.M.; Anteneh, H.A.; Ayalew, E.S.; Berhanu, D.; Berhanu, N.; Beyene, M.G.; Bhattacharya, A.; Bishaw, T.; et al. Routine health management information system data in Ethiopia: Consistency, trends, and challenges. Glob. Health Action 2021, 14, 868–961. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo-Lewis, T.; Keita, Y.; Wilson, E.; Sawadogo, S.; Téréra, I.; Sangho, H.; Munos, M. Can We Use Routine Data for Strategic Decision Making? A Time Trend Comparison Between Survey and Routine Data in Mali. Glob. Health Sci. Pract. 2021, 9, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Thwing, J.; Camara, A.; Candrinho, B.; Zulliger, R.; Colborn, J.; Painter, J.; Plucinski, M.M. A Robust Estimator of Malaria Incidence from Routine Health Facility Data. Am. J. Trop Med. Hyg. 2020, 102, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Nzabakiriraho, J.D.; Gayawan, E. Geostatistical modeling of malaria prevalence among under-five-year old children in Rwanda. BMC Public Health 2021, 21, 369. [Google Scholar] [CrossRef] [PubMed]
- Masimbi, O.; Schurer, J.M.; Rafferty, E.; Ndahimana, J.D.A.; Amuguni, J.H. A cost analysis of the diagnosis and treatment of malaria at public health facilities and communities in three districts in Rwanda. Malar. J. 2022, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Malaria Program; WHO: Geneva, Switzerland, 2022. [Google Scholar]




| Type | Cases | Sample | Domain | Freq | Covariates |
|---|---|---|---|---|---|
| Routine | 39,936 | Sector (416) | Daily/Month | Gender, location | |
| DHS | 99 | 3665 | District (30) | Cross-sectional | Gender, HHD, location |
| Shapefile | Sector (416) | ||||
| Population size | Sector (416) |
| Models | M1 | M3 | ||||
|---|---|---|---|---|---|---|
| Parameters | Est (sd) | LL | UL | Est (sd) | LL | UL |
| Fixed Effect | ||||||
| −2.584 (1.069) | −4.796 | |||||
| −5.892 (0.047) | ||||||
| −5.865 (0.047) | ||||||
| −0.002 (0.001) | ||||||
| Random Effect | ||||||
| −4.62 (0.268) | 0.584 (0.124) | |||||
| 2.69 (0.186) | 2.33 | 3.06 | 1.242 (0.192) | 0.905 | 1.658 | |
| 15.59 (4.72) | 8.025 | 26.420 | ||||
| 3.072 (0.975) | 1.538 | 5.336 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semakula, M.; Niragire, F.; Faes, C. Spatio-Temporal Bayesian Models for Malaria Risk Using Survey and Health Facility Routine Data in Rwanda. Int. J. Environ. Res. Public Health 2023, 20, 4283. https://doi.org/10.3390/ijerph20054283
Semakula M, Niragire F, Faes C. Spatio-Temporal Bayesian Models for Malaria Risk Using Survey and Health Facility Routine Data in Rwanda. International Journal of Environmental Research and Public Health. 2023; 20(5):4283. https://doi.org/10.3390/ijerph20054283
Chicago/Turabian StyleSemakula, Muhammed, François Niragire, and Christel Faes. 2023. "Spatio-Temporal Bayesian Models for Malaria Risk Using Survey and Health Facility Routine Data in Rwanda" International Journal of Environmental Research and Public Health 20, no. 5: 4283. https://doi.org/10.3390/ijerph20054283







