Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Aims
2.2. Participants
2.3. Procedures and Data Collection
2.4. Primary and Secondary Outcomes
2.5. Variables and Measurement Instruments
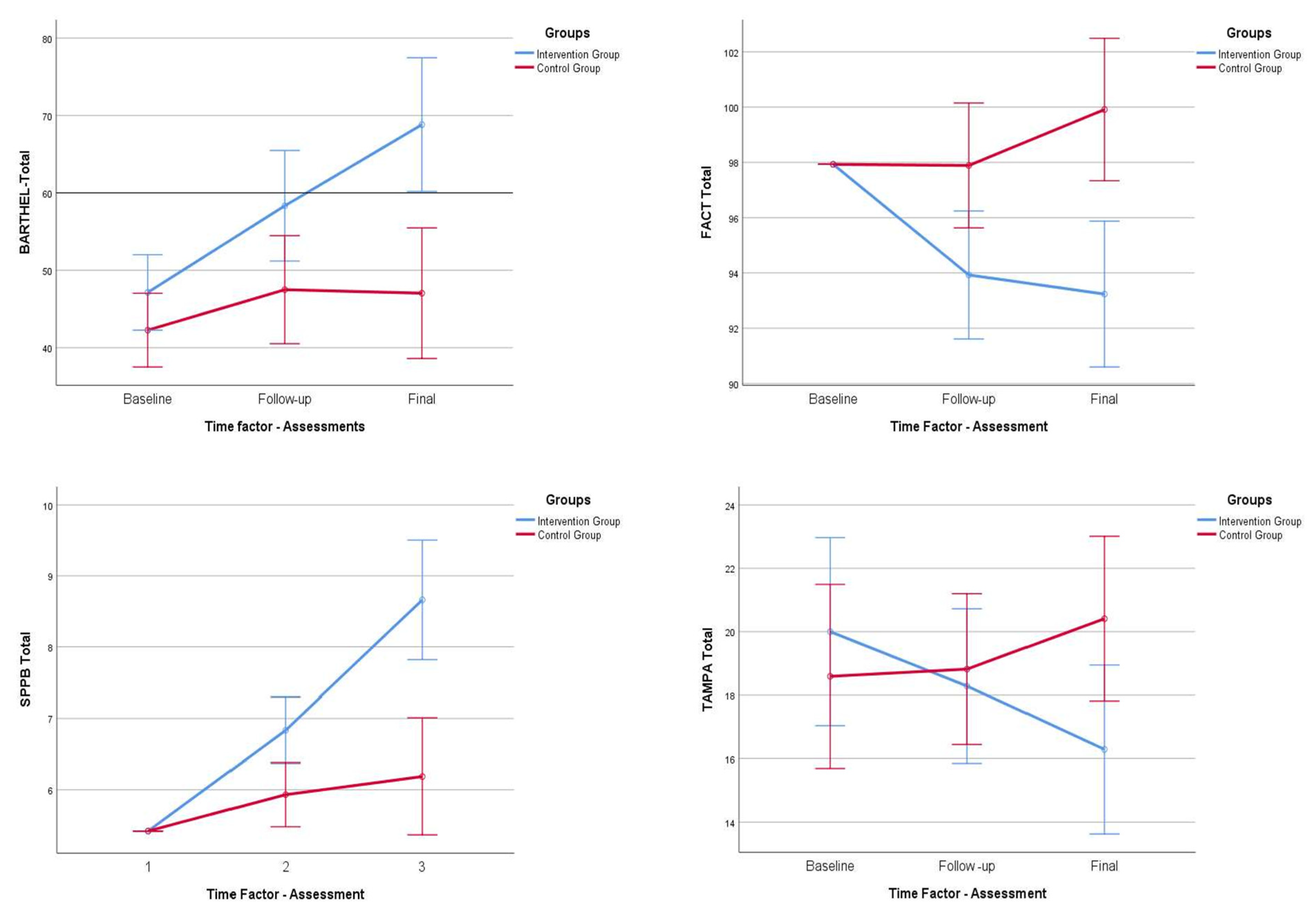
3. Results
Primary and Secondary Outcomes
- −
- For the level of dependence, we found statistically significant differences at the time of follow-up (p = 0.034; p < 0.05) and at the time of the final assessment (p < 0.001), between the experimental and control groups.
- −
- For cancer-related asthenia levels, we found statistically significant differences at follow-up (p = 0.019; p < 0.05) and at the time of the final assessment (p < 0.001), between the experimental and control groups.
- −
- Regarding the levels of health-related quality of life, we found statistically significant differences at follow-up (p = 0.096; p < 0.05) and at the time of the final assessment (p < 0.001), between the experimental and control groups.
- −
- For pain levels, measured with the VAS, we observed that there were no statistically significant differences between the two groups under study, neither at follow-up (p = 0.552; p < 0.05) nor at the final assessment (p = 0.398; p < 0.05).
- −
- In terms of physical performance, we found statistically significant differences at the follow-up (p = 0.009; p < 0.05) and at the final assessment (p < 0.001), between the experimental and control groups.
- −
- Finally, analyzing the levels of kinesiophobia, we observed statistically non-significant differences at the time of follow-up (p = 0.754; p < 0.05), but, on the contrary, we observed statistically significant differences at the time of the final evaluation (p = 0.031; p < 0.05), between the experimental group and the control.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Jones, L.W.; Hornsby, W.; Eves, N.D. Exercise therapy in the management of dyspnea in patients with cancer. Curr. Opin. Support. Palliat. Care 2012, 6, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-Related Fatigue: The Scale of the Problem. Oncol. 2007, 12 (Suppl. 1), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Viswanathan, H.N.; Elkin, E.P.; Traina, S.; Wade, S.; Cella, D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support. Care Cancer 2008, 16, 791–801. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa ESilva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Narayanan, V.; Koshy, C. Fatigue in cancer: A review of literature. Indian J. Palliat. Care 2009, 15, 19–25. [Google Scholar] [CrossRef]
- Hinshaw, D.B.; Carnahan, J.M.; Johnson, D.L. Depression, Anxiety, and Asthenia in Advanced Illness. J. Am. Coll. Surg. 2002, 195, 271–277, discussion 277–278. [Google Scholar] [CrossRef]
- Neefjes, E.C.; Hurk, R.M.V.D.; Blauwhoff-Buskermolen, S.; Van Der Vorst, M.J.; Becker-Commissaris, A.; De Van Der Schueren, M.A.; Buffart, L.M.; Verheul, H.M. Muscle mass as a target to reduce fatigue in patients with advanced cancer. J. Cachex- Sarcopenia Muscle 2017, 8, 623–629. [Google Scholar] [CrossRef]
- Bruera, E.; Sweeney, C. Cachexia and asthenia in cancer patients. Lancet Oncol. 2000, 1, 138–147. [Google Scholar] [CrossRef]
- Gerber, L.H. Cancer-related fatigue: Persistent, pervasive, and problematic. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 65–88. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K. Dissemination and Implementation of Guidelines for Cancer-Related Fatigue. J. Natl. Compr. Cancer Netw. 2016, 14, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, Y.; Duan, Y.; Lai, X.; Cui, S.; Xu, N.; Tang, C.; Lu, L. Nonpharmacological Interventions for Cancer-Related Fatigue: A Systematic Review and Bayesian Network Meta-Analysis. Worldviews Evidence-Based Nurs. 2019, 16, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Barsevick, A.M.; Dudley, W.; Beck, S.; Sweeney, C.; Whitmer, K.; Nail, L. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer 2004, 100, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA: A Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Bluethmann, S.M.; Vernon, S.W.; Gabriel, K.P.; Murphy, C.C.; Bartholomew, L.K. Taking the next step: A systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res. Treat. 2015, 149, 331–342. [Google Scholar] [CrossRef]
- Sheeran, P.; Abraham, C.; Jones, K.; Villegas, M.E.; Avishai, A.; Symes, Y.R.; Ellinger, H.; Miles, E.; Gates, K.M.; Wright, C.E.; et al. Promoting physical activity among cancer survivors: Meta-analysis and meta-CART analysis of randomized controlled trials. Heal. Psychol. 2019, 38, 467–482. [Google Scholar] [CrossRef]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; Thaha, A.M.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018, 2018, CD010192. [Google Scholar] [CrossRef]
- Velthuis, M.J.; Bussche, E.V.D.; May, A.M.; Gijsen, B.C.M.; Nijs, S.; Vlaeyen, J.W.S. Fear of movement in cancer survivors: Validation of the Modified Tampa Scale of Kinesiophobia-Fatigue. Psycho-Oncology 2011, 21, 762–770. [Google Scholar] [CrossRef]
- Poier, D.; Büssing, A.; Recchia, D.R.; Beerenbrock, Y.; Reif, M.; Nikolaou, A.; Zerm, R.; Gutenbrunner, C.; Kröz, M. Influence of a Multimodal and Multimodal-Aerobic Therapy Concept on Health-Related Quality of Life in Breast Cancer Survivors. Integr. Cancer Ther. 2018, 18, 1534735418820447. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, E.J.; González-Sánchez, J.; Puente-González, A.S.; Recio-Rodríguez, J.I.; Sánchez-Gómez, C.; Méndez-Sánchez, R.; Cruz-Hernández, J.J.; Rihuete-Galve, M.I. Multimodal physical exercise and functional rehabilitation program in oncological patients with asthenia. study protocol. BMC Nurs. 2021, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Impact of a comprehensive functional rehabilitation programme on the quality of life of the oncological patient with dyspnoea. Med. Clin. 2021, 157, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Cella, D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Semin. Hematol. 1997, 34, 13–19. [Google Scholar] [PubMed]
- Gusi, N.; Badia, X.; Herdman, M.; Olivares, P.R. Traducción y adaptación cultural de la version Española del cuestionario EQ-5D-Y en niños y adolescents. Aten Primar. 2009, 41, 19–23. [Google Scholar] [CrossRef]
- Badia, X.; Schiaffino, A.; Alonso, J.; Herdman, M. Using the EuroQoI 5-D in the Catalan general population: Feasibility and construct validity. Qual. Life Res. 1998, 7, 311–322. [Google Scholar] [CrossRef]
- Ho, K.; Spence, J.; Murphy, M.F. Review of Pain-Measurement Tools. Ann. Emerg. Med. 1996, 27, 427–432. [Google Scholar] [CrossRef]
- Collins, S.; Moore, A.R.; McQuay, H.J. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain 1997, 72, 95–97. [Google Scholar] [CrossRef]
- Martinez-Monje, F.; Cortés-Gálvez, J.M.; Cartagena-Pérez, Y.; Alfonso-Cano, C.; Sánchez-López, M.I.; Leal-Hernández, M. Assessment with Short Physical Performance Battery Scale of Functional Ability in the Elderly over 70 years. Atención Fam. 2017, 24, 145–149. [Google Scholar]
- Nijs, J.; Roussel, N.; Van Oosterwijck, J.; De Kooning, M.; Ickmans, K.; Struyf, F.; Meeus, M.; Lundberg, M. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: State of the art and implications for clinical practice. Clin. Rheumatol. 2013, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Rihuete Galve, M.I.; Fernández Rodríguez, E.J. Effectiveness of educational program on quality of life of fatigue reported by the oncologic disease. Fund. Index 2018, 27. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1132-12962018000100002&lng=es&nrm=iso&tlng=es (accessed on 2 June 2021).
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA: Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Echavez, J.F.M.; González-Jiménez, E.; Ramírez-Vélez, R. Supervised exercise reduces cancer-related fatigue: A systematic review. J. Physiother. 2015, 61, 3–9. [Google Scholar] [CrossRef]
- Sweegers, M.G.; Altenburg, T.; Brug, J.; May, A.M.; Van Vulpen, J.K.; Aaronson, N.K.; Arbane, G.; Bohus, M.; Courneya, K.S.; Daley, A.J.; et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: A meta-analysis of individual patient data. Br. J. Sports Med. 2018, 53, 812. [Google Scholar] [CrossRef]
- Nadler, M.; Bainbridge, D.; Tomasone, J.; Cheifetz, O.; Juergens, R.A.; Sussman, J. Oncology care provider perspectives on exercise promotion in people with cancer: An examination of knowledge, practices, barriers, and facilitators. Support. Care Cancer 2017, 25, 2297–2304. [Google Scholar] [CrossRef]
- Mewes, J.C.; Steuten, L.M.; Ijzerman, M.J.; van Harten, W.H. Effectiveness of Multidimensional Cancer Survivor Rehabilitation and Cost-Effectiveness of Cancer Rehabilitation in General: A Systematic Review. Oncologist 2012, 17, 1581–1593. [Google Scholar] [CrossRef]
- Van Vulpen, J.K.; Sweegers, M.G.; Peeters, P.H.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Galvao, A.D.; Chinapaw, M.; Steindorf, K.; et al. Moderators of Exercise Effects on Cancer-related Fatigue: A Meta-analysis of Individual Patient Data. Med. Sci. Sports Exerc. 2019, 52, 303–314. [Google Scholar] [CrossRef]
- Covington, K.R.; Marshall, T.; Campbell, G.; Williams, G.R.; Fu, J.B.; Kendig, T.D.; Howe, N.; Alfano, C.M.; Pergolotti, M. Development of the Exercise in Cancer Evaluation and Decision Support (EXCEEDS) algorithm. Support. Care Cancer 2021, 29, 6469–6480. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.; Gerber, L.H.; Marshall, T.F.; Livinski, A.; Alfano, C.M.; Harrington, S.; Flores, A.M.; Virani, A.; Hu, X.; Mitchell, S.A.; et al. Systematic Review of Functional Outcomes in Cancer Rehabilitation. Arch. Phys. Med. Rehabil. 2022, 103, 1807–1826. [Google Scholar] [CrossRef] [PubMed]
- Covington, K.R.; Hidde, M.C.; Pergolotti, M.; Leach, H.J. Community-based exercise programs for cancer survivors: A scoping review of practice-based evidence. Support. Care Cancer 2019, 27, 4435–4450. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.N.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical activity in cancer prevention and survival: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Wood, K.C.; Pergolotti, M.; Marshall, T.; Leach, H.J.; Sharp, J.L.; Campbell, G.; Williams, G.R.; Fu, J.B.; Kendig, T.D.; Howe, N.; et al. Usability, acceptability, and implementation strategies for the Exercise in Cancer Evaluation and Decision Support (EXCEEDS) algorithm: A Delphi study. Support. Care Cancer 2022, 30, 7407–7418. [Google Scholar] [CrossRef]
- Kröz, M.; Reif, M.; Glinz, A.; Berger, B.; Nikolaou, A.; Zerm, R.; Brinkhaus, B.; Girke, M.; Büssing, A.; Gutenbrunner, C.; et al. Impact of a combined multimodal-aerobic and multimodal intervention compared to standard aerobic treatment in breast cancer survivors with chronic cancer-related fatigue—Results of a three-armed pragmatic trial in a comprehensive cohort design. BMC Cancer 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Moustgaard, H.; Clayton, G.; Jones, H.; Boutron, I.; Jørgensen, L.; Laursen, D.R.T.; Olsen, M.F.; Paludan-Müller, A.; Ravaud, P.; Savović, J.; et al. Impact of blinding on estimated treatment effects in randomised clinical trials: Meta-epidemiological study. BMJ 2020, 368, l6802. [Google Scholar] [CrossRef]


| Socio-Demographic Variables | Intervention Groups (Mean ± Standard Deviation) | |||
|---|---|---|---|---|
| Intervention Group (n = 24) | Control Group (n = 24) | Group Differences (p-Value) | ||
| Men | n = 14 | n = 16 | ||
| Women | n = 10 | n = 8 | ||
| Age (years) | 61.13 ± 13.33 | 65.79 ± 11.40 | −4.667 | (0.199) |
| Weight (kg) | 65.70 ± 13.41 | 65.61 ± 13.45 | −1.0600 | (0.682) |
| Height (cm) | 169.92 ± 7.76 | 168.83 ± 8.61 | 0.01220 | (0.418) |
| BMI (kg/m2) | 22.58 ± 3.23 | 22.75 ± 3.56 | −0.75189 | (0.459) |
| Number treatment lines | 3.17 ± 1.49 | 3.46 ± 1.71 | −0.292 | (0.533) |
| Number of cohabitants | 2 * | 2 * | −0.417 | (0.236) |
| Charlson comorbidity index | 9.50 ± 4.12 | 9.96 ± 3.18 | −0.458 | (0.669) |
| Estimated survival 10 years | 16.16 ± 32.56 | 9.96 ± 3.18 | 9.725 | (0.213) |
| Variables | Intervention Groups (Mean ± Standard Deviation) | |||
|---|---|---|---|---|
| Intervention Group (n = 24) | Control Group (n = 24) | Group Differences (p-Value) | ||
| Barthel | 47.08 ± 10.47 | 40.63 ± 12.79 | 6.458 | (0.061) |
| FACT-An total | 94.96 ± 11.91 | 102.75 ± 13.00 | −7.792 | (0.036) * |
| FACT physical state | 15.42 ± 4.13 | 16.00 ± 3.98 | −0.583 | (0.621) |
| FACT: social environment | 17.00 ± 4.89 | 16.71 ± 4.00 | 0.292 | (0.822) |
| FACT: emotional state | 12.79 ± 3.32 | 14.71 ± 3.39 | −1.917 | (0.054) |
| FACT: personal functioning | 12.79 ± 3.32 | 10.88 ± 4.48 | 1.917 | (0.125) |
| FACT: other concerns | 37.79 ± 8.62 | 45.21 ± 8.66 | −7.417 | (0.005) * |
| EuroQoL-5D Total | 10.33 ± 1.78 | 10.88 ± 2.19 | −0.542 | (0.353) |
| EuroQoL-5D mobility | 2.04 ± 0.55 | 2.33 ± 0.48 | −0.292 | (0.057) |
| EuroQoL-5D personal care | 2.21 ± 0.50 | 2.38 ± 0.47 | −0.167 | (0.294) |
| EuroQoL-5D daily activities | 2.33 ± 0.48 | 2.54 ± 0.50 | −0.208 | (0.152) |
| EuroQoL-5D pain | 1.96 ± 0.69 | 1.71 ± 0.62 | 0.250 | (0.195) |
| EuroQoL-5D anxiety | 1.79 ± 0.77 | 1.92 ± 0.77 | −0.125 | (0.580) |
| Thermometer EuroQoL-5D | 50.83 ± 16.46 | 41.66 ± 15.01 | 9.16 | (0.050) |
| VAS pain | 4.29 ± 2.21 | 3.58 ± 2.78 | 0.708 | (0.334) |
| VAS fatigue | 15.17 ± 5.30 | 13.79 ± 4.66 | 1.375 | (0.345) |
| SPPB total | 6.21 ± 2.99 | 4.42 ± 3.03 | 1.792 | (0.045) * |
| SPPB balance | 2.58 ± 1.28 | 1.92 ± 1.17 | 0.667 | (0.067) |
| SPPB gait | 1.75 ± 0.94 | 1.17 ± 0.91 | 0.583 | (0.035) |
| SPPB speed get up | 1.88 ± 0.94 | 1.33 ± 1.12 | 0.542 | (0.078) |
| TAMPA total | 20.25 ± 7.07 | 18.71 ± 5.90 | 1.542 | (0.417) |
| TAMPA avoidance | 11.71 ± 4.57 | 10.88 ± 3.26 | 0.833 | (0.471) |
| TAMPA damage | 8.54 ± 3.32 | 7.83 ± 3.18 | 0.455 | (0.708) |
| Variable | Intervention Group (IG) | Control Group (CG) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up (15 Days) | Final (30 Days) | Baseline | Follow-Up (15 Days) | Final (30 Days) | |||||||
| BARTHEL | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 47.14 | ±10.19 | 11.19 | p < 0.001 * | 21.66 | p < 0.001 * | 42.27 | ±11.82 | 5.22 | p = 0.015 * | 4.77 | p = 0.180 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 6.674, 15.707 | 15.363, 27.970 | 0.814, 9.640 | −1.386, 10.931 | |||||||||
| FACT | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 94.95 | ±11.89 | −4.00 | p = 0.004 * | −4.69 | p = 0.003 * | 100.77 | ±11.01 | −0.04 | p = 1.000 | 1.98 | 0.383 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −6.865, −1.141 | −7.959, −1.433 | −2.837, 2.752 | −1.203, 5.168 | |||||||||
| FACT Physical condition | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 15.29 | ±4.23 | −2.14 | p < 0.001 * | −3.85 | p < 0.001 * | 15.77 | ±4.02 | −0.63 | p = 0.195 | - | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −3.001, −1.285 | −5.032, −2.683 | −1.474, 0.202 | −1.147, 1.147 | |||||||||
| FACT social environment | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 16.95 | ±5.16 | 0.90 | p < 0.001 * | 1.95 | p < 0.001 * | 16.45 | ±4.09 | 0.00 | p = 1.000 | −0.04 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 0.368, 1.442 | 1.182, 2.722 | −0.524, 0.524 | −0.798, 0.707 | |||||||||
| FACT emotional state | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 12.57 | ±3.32 | −0.57 | p = 0.120 | −1.71 | p = 0.001 * | 14.23 | ±3.31 | 0.40 | p = 0.383 | 1.18 | p = 0.024 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −1.244, 0.101 | −2.796, −0.633 | −0.248, 1.066 | 0.125, 2.238 | |||||||||
| FACT personal functioning | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 13.29 | ±3.79 | 1.28 | p < 0.001 * | 2.85 | p < 0.001 * | 1.64 | ±4.48 | 0.72 | p = 0.013 * | 0.22 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 0.668, 1.903 | 1.734, 3.981 | 0.668, 1.903 | 1.734, 3.981 | |||||||||
| FACT other concerns | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 37.81 | ±7.93 | −2.60 | p < 0.001 * | −4.25 | p < 0.001 * | 44.50 | ±8.44 | −1.65 | p = 0.002 * | −0.80 | p = 0.867 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −3.740, −1.470 | −6.169, −2.337 | −2.757, −0.543 | −2.672, 1.065 | |||||||||
| EuroQoL | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 10.19 | ±1.72 | −1.09 | p < 0.001 * | −2.33 | p < 0.001 * | 10.68 | ±1.721 | −0.40 | p = 0.169 | −0.09 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −0.627, −0.563 | −3.027, −1.640 | −0.929, 0.111 | −0.769, 0.587 | |||||||||
| EuroQoL Thermometer | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 52.61 | ±15.93 | 8.810 | p < 0.001 * | 17.76 | p < 0.001 * | 43.409 | ±14.42 | 3.40 | p = 0.255 | 1.81 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 3.875, 13.744 | 11.106, 24.418 | −1.412, 8.230 | −4.684, 8.321 | |||||||||
| VAS pain | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 4.24 | ±2.18 | −1.23 | p < 0.001 * | −2.19 | p < 0.001 * | 3.41 | ±2.737 | −0.81 | p = 0.021 * | −0.82 | p = 0.112 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −1.975, −0.501 | −3.162, −1.219 | −1.538, −0.098 | −0.649, 0.649 | |||||||||
| VAS scale | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 15.52 | ±5.18 | 2.66 | p < 0.001 * | 5.23 | p < 0.001 * | 14.36 | ±4.414 | 1.18 | p = 0.032 * | 0.95 | p = 0.384 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 1.538, 3.795 | 3.668, 6.808 | 0.079, 2.284 | −0.580, 2.489 | |||||||||
| SPPB | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 6.29 | ±3.05 | 1.41 | p < 0.001 * | 3.24 | p < 0.001 * | 4.59 | ±3.112 | 0.51 | p = 0.082 | 0.76 | p = 0.199 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 0.841, 1.989 | 2.201, 4.285 | −0.047, 1.073 | −0.249, 1.785 | |||||||||
| SPPB balance | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 2.67 | ±1.31 | 0.23 | p = 0.172 | 0.52 | p = 0.014 | 2.00 | ±1.19 | 0.04 | p = 1.000 | 0.04 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −0.066, 0.542 | 0.088, 0.959 | −0.251, 0.342 | −0.380, 0.471 | |||||||||
| SPPB gait | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 1.71 | ±0.95 | 0.58 | p < 0.001 * | 1.38 | p < 0.001 * | 1.23 | ±0.922 | 0.30 | p = 0.022 * | 0.40 | p = 0.036 * | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 0.312, 0.861 | 0.944, 1.732 | 0.036, 0.572 | 0.020, 0.789 | |||||||||
| SPPB speed get up | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 1.90 | ±0.94 | 0.52 | p < 0.001 * | 1.28 | p < 0.001 * | 1.36 | ±1.17 | 0.18 | p = 0.299 | 0.40 | p = 0.088 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| 0.248, 0.800 | 0.823, 1.174 | −0.088, 0.451 | −0.043, 0.861 | |||||||||
| TAMPA | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 20.00 | ±7.34 | −1.71 | p = 0.005 * | −3.714 | p = 0.001 * | 18.59 | ±6.10 | 0.22 | p = 1.000 | 1.81 | p = 0.176 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −2.97, −0.45 | −6.10, −1.32 | −1.00, 1.46 | −0.51, 4.15 | |||||||||
| TAMPA avoidance | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 11.48 | ±4.57 | −0.81 | p = 0.166 | −1.714 | p = 0.046 * | 10.95 | ±3.40 | 0.77 | 0.183 | 1.50 | 0.087 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −1.83, 0.21 | −3.40, −0.02 | −0.22, 1.77 | −0.15, 3.15 | |||||||||
| TAMPA damage | m | SD | MD | p-Value | MD | p-Value | m | SD | MD | p-Value | MD | p-Value |
| 8.52 | ±3.55 | −0.90 | p = 0.006 * | −1.952 | p < 0.001 * | 7.64 | ±3.12 | −0.13 | p = 1.000 | 0.22 | p = 1.000 | |
| 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | 95% CI (Lower-Upper) | |||||||||
| −1.58, −0.22 | −3.05, −0.84 | −0.80, 0.53 | −0.45, 1.18 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Rodriguez, E.J.; Sanchez-Gomez, C.; Mendez-Sanchez, R.; Recio-Rodriguez, J.I.; Puente-Gonzalez, A.S.; Gonzalez-Sanchez, J.; Cruz-Hernandez, J.J.; Rihuete-Galve, M.I. Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2023, 20, 4938. https://doi.org/10.3390/ijerph20064938
Fernandez-Rodriguez EJ, Sanchez-Gomez C, Mendez-Sanchez R, Recio-Rodriguez JI, Puente-Gonzalez AS, Gonzalez-Sanchez J, Cruz-Hernandez JJ, Rihuete-Galve MI. Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2023; 20(6):4938. https://doi.org/10.3390/ijerph20064938
Chicago/Turabian StyleFernandez-Rodriguez, Eduardo J., Celia Sanchez-Gomez, Roberto Mendez-Sanchez, Jose I. Recio-Rodriguez, A. Silvia Puente-Gonzalez, Jesus Gonzalez-Sanchez, Juan J. Cruz-Hernandez, and Maria I. Rihuete-Galve. 2023. "Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial" International Journal of Environmental Research and Public Health 20, no. 6: 4938. https://doi.org/10.3390/ijerph20064938
APA StyleFernandez-Rodriguez, E. J., Sanchez-Gomez, C., Mendez-Sanchez, R., Recio-Rodriguez, J. I., Puente-Gonzalez, A. S., Gonzalez-Sanchez, J., Cruz-Hernandez, J. J., & Rihuete-Galve, M. I. (2023). Multimodal Physical Exercise and Functional Rehabilitation Program in Oncological Patients with Cancer-Related Fatigue—A Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 20(6), 4938. https://doi.org/10.3390/ijerph20064938








