Seasonal and Spatial Variations of Indoor Pollen in a Hospital
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Pollen Sampling Methods
2.2. Statistical Analysis
3. Results
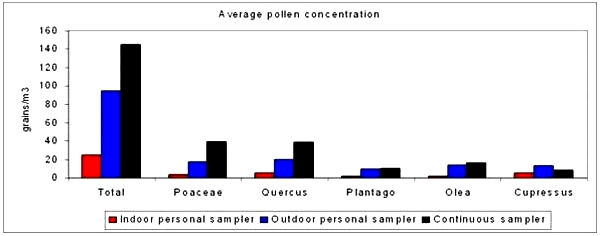
3.1. General Values of Pollen Concentrations
3.2. Temporal Variations in Pollen Concentration
3.3. Comparisons between Sampling Sites
3.4. Correlation with Weather Parameters, People and Vehicles
4. Discussion
5. Conclusions
Acknowledgments
References and Notes
- Höppe, P; Martinac, I. Indoor climate and air quality. Review of current and future topics in the field of ISB study group 10. Int. J. Biometeorol 1998, 42, 1–7. [Google Scholar]
- Lebowitz, MD. Biological responses to indoor air contaminants. Aerobiologia 1991, 7, 10–16. [Google Scholar]
- Sterling, DA; Lewis, RD. Pollen and fungal spores indoor and outdoor of mobile homes. Ann. Allergy Asthma. Immunol 1998, 80, 279–285. [Google Scholar]
- Holmquist, L; Vesterberg, O. Quantification of birch and grass pollen allergens in indoor air. Indoor Air 1999, 9, 85–91. [Google Scholar]
- Bluyssen, PM. Review: Indoor air quality management: a state of the art review and identification of research needs. Indoor Built Environ 1992, 1, 326–334. [Google Scholar]
- Singh, J. Impact of indoor air pollution on health, comfort and productivity of the occupants. Aerobiologia 1996, 12, 121–127. [Google Scholar]
- Pickering, CAC. Immune respiratory disease associated with the inadequate control of indoor air quality. Indoor Built. Environ 1992, 1, 157–161. [Google Scholar]
- Portnoy, JM; Flappan, S; Barnes, CS. A procedure for evaluation of the indoor environment. Aerobiologia 2001, 17, 43–48. [Google Scholar]
- Kleinman, MT; Eisenbud, M; Lippmann, M; Kne, TJ. The use of tracers to identify sources of airborne particles. Environ. Int 1980, 4, 53–62. [Google Scholar]
- Kettrup, A; Schmidt, PR. Methods to reduce allergic effects: elimination of allergens (pollen, mites, indoor dust, bacteria, gases, etc) from indoor air. In Advances in Aerobiology Proceedings 3rd International Conference Aerobiology; Boehm, G, Leuschner, RM, Eds.; Birkhäuser Verlag: Basel, Switzerland, 1987; pp. 415–417. [Google Scholar]
- Billings, CE; Vanderslice, SF. Methods for control of indoor air quality. Environ. Int 1982, 8, 497–504. [Google Scholar]
- Cheng, YS; Chun, JL; Chen, TR. Efficiency of a portable indoor air cleaner in removing pollens and fungal spores. Aerosol Sci. Tech 1998, 29, 92–101. [Google Scholar]
- Enomoto, T; Onishi, S; Sogo, H; Dake, Y; Ikeda, H; Funakoshi, H; Shibano, A; Sakoda, T. Japanese cedar pollen in floating indoor house dust after a pollinating season. Allergol. Internat 2004, 53, 279–285. [Google Scholar]
- Stock, TH; Morandi, MT. A characterization of indoor and outdoor microenvironmental concentrations of pollen and spores in two Houston neighbourhoods. Environ. Internat 1988, 14, 1–9. [Google Scholar]
- Lebowitz, M; O’Rourke, M; Dodge, R; Holberg, G; Hoshaw, R; Pinnas, J; Barbee, R; Sneller, M. The adverse health effects of biologic aerosols, other aerosols, and indoor microclimate on asthmatics and nonasthmatics. Environ. Int 1982, 8, 375–380. [Google Scholar]
- Takahashi, Y; Takano, K; Suzuki, M; Nagai, S; Yokosuka, M; Takeshita, T; Saito, A; Yasueda, H; Enomoto, T. Two routes for pollen entering indoors: ventilation and clothes. J. Investig. Allergol. Clin. Immunol 2009, 18, 382–388. [Google Scholar]
- Sharma, D; Dutta, BK; Singh, AB. Pollen, fungus and house dust mites survey at the residence of 90 allergic patients in Greater Silchar Area of Assam, India. Res. J. Allergy 2009, 1, 1–11. [Google Scholar]
- O’Rourke, MK; Quackenboss, JJ; Lebowitz, MD. An epidemiological approach investigating respiratory disease response in sensitive individuals to indoor and outdoor pollen exposure in Tucson, Arizona. Aerobiologia 1989, 5, 104–110. [Google Scholar]
- O’Rourke, MK; Lebowitz, MD. A comparison of regional atmospheric pollen with pollen collected at and near homes. Grana 1984, 23, 55–64. [Google Scholar]
- Cariñanos, P; Alcázar, P; Galán, C; Navarro, R; Dominguez, E. Aerobiology as a tool to help in episodes of occupational allergy in work places. J. Invest. Allergol. Clin. Immunol 2004, 14, 300–308. [Google Scholar]
- D’Amato, G; Russo, M; Liccardi, G; Saggese, M; Gentili, M; Mistrello, G; D’Amato, M; Falagiani, P. Comparison between outdoor and indoor airborne allergenic activity. Ann. Allergy Asthma Immunol 1996, 77, 147–152. [Google Scholar]
- Comtois, P; Marcoux, N. An indoor air model. Aerobiologia 1999, 15, 115–120. [Google Scholar]
- Muilenberg, ML. Pollen in indoor air: sources, exposures, and health effects. In Indoor Air Quality Handbook; Spengler, JD, Samet, JM, McCarthy, JF, Eds.; McGraw-Hill: Hightstown, NJ, USA, 2001; Chapter 44. [Google Scholar]
- Ishibashi, Y; Ohno, H; Oh-ishi, S; Matsuoka, T; Kizaki, T; Yoshizumi, K. Characterization of pollen dispersion in the neighborhood of Tokyo, Japan in the spring of 2005 and 2006. Int. J. Environ. Res. Public. Health 2008, 5, 76–85. [Google Scholar]
- Hiromi, K; Susumu, Y. Study on the control of indoor pollen exposure Part 1 Intrusion of airborne pollen into indoor environment and exposure dose. J. Architec. Plan. Environ. Engin 2001, 548, 63–68. [Google Scholar]
- Vural, C; İnce, A. Pollen grains in the house dust of Kayseri, Turkey. Internat. J. Nat. Engin. Sci 2008, 2, 41–44. [Google Scholar]
- Reed, CE; Swanson, MC. Indoor allergens: identification and quantification. Environ. Int 1986, 12, 115–120. [Google Scholar]
- Adam, KF; Hyde, HA. Pollen grains and fungal spores indoor and out at Cardiff. J. Palynol 1965, 1, 67–69. [Google Scholar]
- Yankova, R. Outdoor and indoor pollen grains in Sofia. Grana 1991, 30, 171–176. [Google Scholar]
- Loublier, Y. One year indoor pollen and spore and spore deposition: are the contaminations possible? In 1st European Symposium on Aerobiology; Aira, MJ, Jato, V, Iglesias, I, Gálan, C, Eds.; Universidad de Santiago de Compostela: Santiago de Compostela, Spain, 1996; pp. 49–40. [Google Scholar]
- Loublier, Y. Evaluation of indoor passive pollen sedimentation over 1 year: a possible source of contamination? Aerobiologia 1998, 14, 291–298. [Google Scholar]
- Hugg, T; Rantio-Lehtimäki, A. Indoor and outdoor pollen concentrations in private and public spaces during the Betula pollen season. Aerobiologia 2007, 23, 119–129. [Google Scholar]
- Lee, T; Grinshpun, SA; Martuzevicius, D; Adhikari, A; Crawford, CM; Luo, J; Reponen1, T. Relationship between indoor and outdoor bioaerosols collected with a button inhalable aerosol sampler in urban homes. Indoor Air 2006, 16, 37–47. [Google Scholar]
- Tormo, R; Gonzalo, MA; Muñoz, AF; Silva, I. Pollen and spores in the air of a hospital outpatient ward. Allergol. Immunopathol 2002, 30, 232–238. [Google Scholar]
- Hirst, JM. An automatic volumetric spore trap. Ann. Appl. Bio 1952, 39, 257–265. [Google Scholar]
- Roe, FJC. Priorities for research in the field of indoor air pollution. Indoor Built. Environ 1994, 3, 191–199. [Google Scholar]
- Miguel, AF; Aydin, M; Reis, AH. Indoor deposition and forced re-suspension of respirable particles. Indoor Built Environ 2005, 14, 391–396. [Google Scholar]




© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tormo-Molina, R.; Gonzalo-Garijo, Á.; Silva-Palacios, I.; Fernández-Rodríguez, S. Seasonal and Spatial Variations of Indoor Pollen in a Hospital. Int. J. Environ. Res. Public Health 2009, 6, 3169-3178. https://doi.org/10.3390/ijerph6123169
Tormo-Molina R, Gonzalo-Garijo Á, Silva-Palacios I, Fernández-Rodríguez S. Seasonal and Spatial Variations of Indoor Pollen in a Hospital. International Journal of Environmental Research and Public Health. 2009; 6(12):3169-3178. https://doi.org/10.3390/ijerph6123169
Chicago/Turabian StyleTormo-Molina, Rafael, Ángela Gonzalo-Garijo, Inmaculada Silva-Palacios, and Santiago Fernández-Rodríguez. 2009. "Seasonal and Spatial Variations of Indoor Pollen in a Hospital" International Journal of Environmental Research and Public Health 6, no. 12: 3169-3178. https://doi.org/10.3390/ijerph6123169
APA StyleTormo-Molina, R., Gonzalo-Garijo, Á., Silva-Palacios, I., & Fernández-Rodríguez, S. (2009). Seasonal and Spatial Variations of Indoor Pollen in a Hospital. International Journal of Environmental Research and Public Health, 6(12), 3169-3178. https://doi.org/10.3390/ijerph6123169