Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS
Abstract
:1. Introduction
2. Materials and Methods
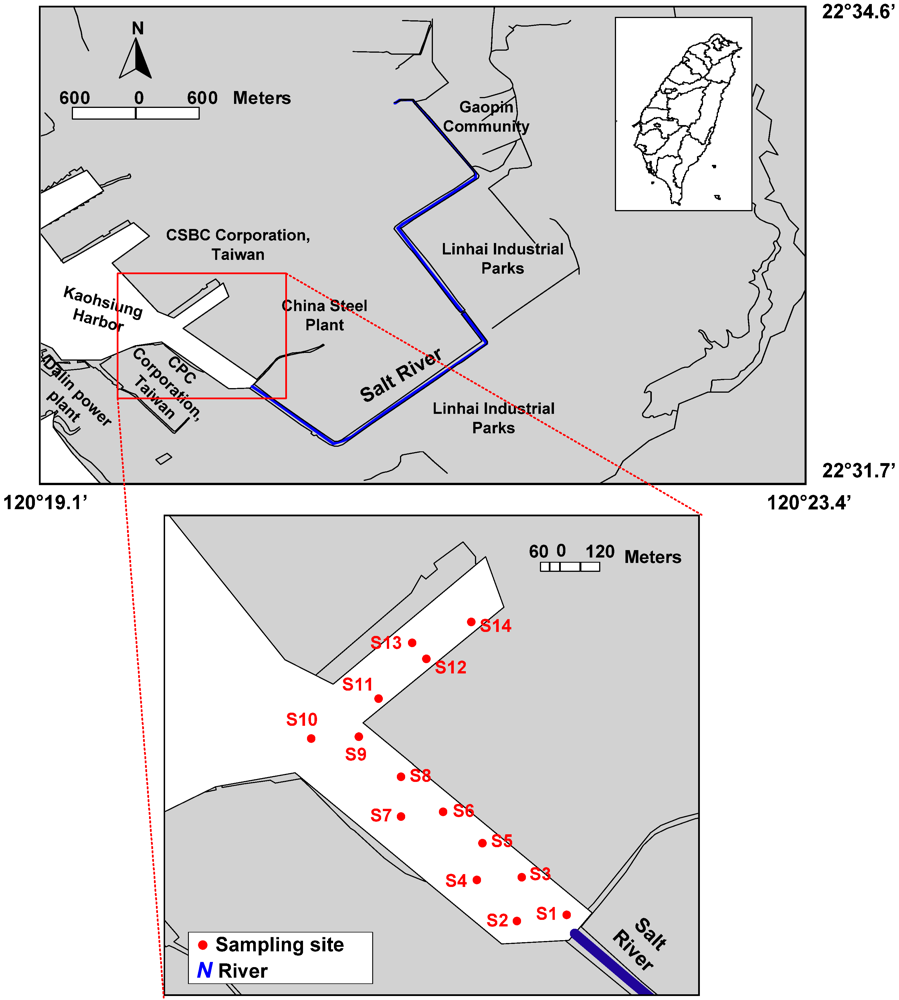
2.1. Sampling
2.2. Chemicals
2.3. Sample Preparation
2.4. GC-MS Instrumentation and Conditions
| Time window (min) | Compound | No. of rings | Retention time (min) | Molecular mass | Ions | m/z window |
|---|---|---|---|---|---|---|
| 4.00–9.45 | Naphthalene-d8 (IS1) | 2 | 7.021 | 136 | 136 | 127,128,129,136,172 |
| Naphthalene | 2 | 7.052 | 128 | 128, 129,127 | ||
| 2-Fluorobiphenyl (SS1) | 2 | 8.297 | 172 | 172 | ||
| 9.45–13.50 | Acenaphthylene | 3 | 10.128 | 152 | 152, 151,153 | 151,152,153,154,164,166,167 |
| Acenaphthene-d10 (IS2) | 3 | 10.495 | 164 | 164 | ||
| Acenaphthene | 3 | 10.577 | 154 | 154, 153,152 | ||
| Fluorene | 3 | 12.049 | 166 | 166, 165,167 | ||
| 13.50–21.50 | Phenanthrene-d10 (IS3) | 3 | 15.250 | 188 | 188 | 101,176,178,179,188,200,202,203 |
| Phenanthrene | 3 | 15.334 | 178 | 178, 179,176 | ||
| Anthracene | 3 | 15.526 | 178 | 178, 176,179 | ||
| Fluoranthene | 4 | 20.224 | 202 | 202, 101,203 | ||
| Pyrene | 4 | 21.164 | 202 | 202, 200,203 | ||
| 21.50–29.00 | 4-Terphenyl-d14 (SS2) | 4 | 22.179 | 244 | 244 | 226,228,229,240,244 |
| Benzo[a]anthracene | 4 | 26.660 | 228 | 228, 229,226 | ||
| Chrysene-d12 (IS4) | 4 | 26.699 | 240 | 240 | ||
| Chrysene | 4 | 26.813 | 228 | 228, 226,229 | ||
| 29.00–51.20 | Benzo[b]fluoranthene | 5 | 31.321 | 252 | 252, 253,125 | 125,138,139,252,253,276,277 |
| Benzo[k]fluoranthene | 5 | 31.431 | 252 | 252, 253,125 | ||
| Benzo[a]pyrene | 5 | 32.587 | 252 | 252, 253,125 | ||
| Perylene-d12 (IS5) | 5 | 32.827 | 264 | 264 | ||
| Indeno[1,2,3-cd]pyrene | 6 | 36.683 | 276 | 276, 138,277 | ||
| Dibenz[a,h]anthracene | 5 | 36.820 | 278 | 278, 139,279 | ||
| Benzo[g,h,i]perylene | 6 | 37.616 | 276 | 276, 138,277 |
2.5. Identification and Quantification
3. Results and Discussion
3.1. Analytical Characteristics
| Compound | Response factor (RF) (n = 5) | Detection Limits DL (ng/g) | Check analysis (n = 7) R a (%) | Duplication analysis (n = 7) RPD a (%) | |
|---|---|---|---|---|---|
| Average ± SD a | RSD a (%) | ||||
| Naphthalene | 2.08 ± 0.17 | 8.4 | 2.9 | 122 ± 12 | 8.7 ± 4.6 |
| Acenaphthylene | 1.85 ± 0.11 | 5.9 | 1.4 | 87 ± 6 | 11.8 ± 3.8 |
| Acenaphthene | 1.14 ± 0.05 | 4.4 | 1.9 | 107 ± 9 | 9.2 ± 5.0 |
| Fluorene | 0.86 ± 0.01 | 1.1 | 0.6 | 98 ± 3 | 11.2 ± 5.4 |
| Phenanthrene | 1.09 ± 0.14 | 12.9 | 2.3 | 105 ± 9 | 10.7 ± 3.3 |
| Anthracene | 1.28 ± 0.10 | 7.6 | 2.0 | 89 ± 8 | 7.0 ± 6.0 |
| Fluoranthene | 1.17 ± 0.06 | 4.8 | 1.8 | 90 ± 9 | 8.9 ± 2.4 |
| Pyrene | 1.22 ± 0.07 | 5.9 | 1.7 | 90 ± 8 | 12.3 ± 2.3 |
| Benzo[a]anthracene | 0.97 ± 0.11 | 11.6 | 2.2 | 105 ± 9 | 13.3 ± 3.6 |
| Chrysene | 1.47 ± 0.20 | 13.7 | 2.2 | 105 ± 8 | 11.9 ± 7.2 |
| Benzo[b]fluoranthene | 0.79 ± 0.09 | 11.9 | 3.5 | 120 ± 16 | 12.3 ± 4.3 |
| Benzo[k]fluoranthene | 1.39 ± 0.14 | 10.2 | 3.0 | 107 ± 14 | 10.8 ± 2.1 |
| Benzo[a]pyrene | 0.78 ± 0.03 | 4.2 | 3.5 | 91 ± 16 | 12.3 ± 4.6 |
| Indeno[1,2,3-cd]pyrene | 0.64 ± 0.06 | 9.3 | 4.4 | 103 ± 18 | 12.4 ± 6.4 |
| Dibenz[a,h]anthracene | 0.41 ± 0.06 | 14.1 | 5.4 | 112 ± 14 | 11.2 ± 4.9 |
| Benzo[g,h,i]perylene | 0.72 ± 0.07 | 9.9 | 5.3 | 128 ± 4 | 10.1 ± 7.4 |
| 2-Fluorobiphenyl (SS1) | 1.52 ± 0.09 | 5.86 | - | 102 ± 7 | 7.5 ± 2.5 |
| 4-Terphenyl-d14 (SS2) | 1.41 ± 0.10 | 7.09 | - | 107 ± 18 | 9.2 ± 4.0 |
| Compounds | Measured concentration (ng/g dw) | Certified equate concentration (ng/g dw) | Error a (%) | ||
|---|---|---|---|---|---|
| #1 | #2 | #3 | |||
| Naphthalene | 1,882 | 1,923 | 1,905 | 1,700 | 12.0 ± 1.2 |
| Acenaphthene | 624 | 688 | 645 | 590 | 10.6 ± 5.5 |
| Fluorene | 631 | 623 | 615 | 550 | 13.3 ± 1.5 |
| Phenanthrene | 1,121 | 1,165 | 1,103 | 1,050 | 7.6 ± 3.0 |
| Anthracene | 15 | 17 | 16 | 20 | 20.0 ± 5.0 |
| Fluoranthene | 1,553 | 1,545 | 1,614 | 1,350 | 16.3 ± 2.8 |
| Pyrene | 2,311 | 2,382 | 2,154 | 2,400 | 4.9 ± 4.9 |
| Benzo[a]anthracene | 425 | 410 | 425 | 500 | 16.0 ± 1.7 |
| Chrysene | 1,055 | 1,284 | 1,148 | 1,100 | 8.4 ± 7.2 |
| Benzo[a]pyrene | 176 | 165 | 133 | 150 | 12.9 ± 3.9 |
| Benzo[g,h,i]perylene | 592 | 619 | 612 | 690 | 11.9 ± 2.0 |
| Dibenz[a,h]anthracene | 541 | 537 | 587 | 600 | 7.5 ± 4.6 |
| Indeno[1,2,3-cd]pyrene | 727 | 766 | 773 | 800 | 5.6 ± 3.1 |
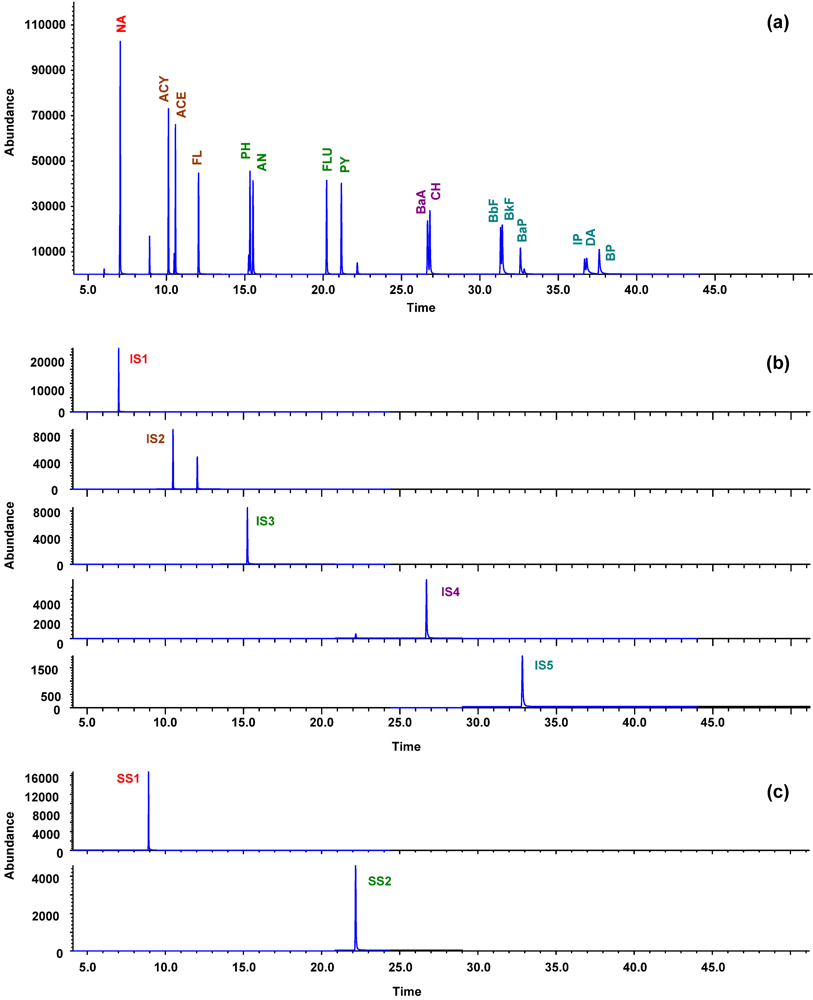
3.2. GC-MS Separation and Identification
| Station | 2-ring | 3-ring | 4-ring | 5-ring | 6-ring | ΣLPAHs a | ΣHPAHs a | ΣPAHs a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA | ACE | AC | FL | PH | AN | FLU | PY | BaA | CH | BbF | BkF | BaP | DA | IP | BP | ||||
| S1 | 1,211 | 292 | 1,412 | 1,370 | 3,181 | 206 | 2,475 | 1,795 | 1,925 | 1,273 | 1,688 | 965 | 957 | 37 | 186 | 354 | 7,671 | 11,654 | 19,325 |
| S2 | 2,811 | 494 | 6,226 | 2,964 | 6,507 | 2,919 | 7,817 | 6,429 | 2,666 | 964 | 3,891 | 2,196 | 2,327 | 332 | 1,437 | 1,281 | 21,921 | 29,339 | 51,261 |
| S3 | 1,916 | 396 | 4,262 | 2,869 | 5,256 | 2,593 | 6,036 | 4,950 | 3,522 | 2,311 | 2,638 | 1,489 | 1,492 | 240 | 925 | 768 | 17,291 | 24,370 | 41,661 |
| S4 | 1,156 | 159 | 957 | 644 | 2,199 | 134 | 1,636 | 1,284 | 2,101 | 1,472 | 2,245 | 1,277 | 729 | 75 | 603 | 269 | 5,250 | 11,692 | 16,941 |
| S5 | 915 | 5 | 888 | 792 | 2,630 | 154 | 2,035 | 1,550 | 1,344 | 1,245 | 1,495 | 1,102 | 539 | 22 | 197 | 220 | 5,384 | 9,749 | 15,133 |
| S6 | 826 | 14 | 971 | 621 | 1,831 | 1,684 | 987 | 770 | 1,545 | 850 | 1,667 | 941 | 473 | 48 | 460 | 379 | 5,947 | 8,118 | 14,065 |
| S7 | 2,360 | 296 | 2,469 | 1,420 | 3,733 | 2,329 | 6,468 | 4,552 | 1,999 | 2,154 | 3,386 | 1,910 | 217 | 189 | 320 | 324 | 12,608 | 21,519 | 34,127 |
| S8 | 1,836 | 3 | 468 | 859 | 1,827 | 360 | 2,224 | 2,405 | 2,913 | 1,866 | 3,068 | 1,022 | 931 | 198 | 294 | 366 | 5,353 | 15,288 | 20,641 |
| S9 | 973 | 8 | 751 | 723 | 2,223 | 2,092 | 1,447 | 1,189 | 1,359 | 902 | 1,660 | 936 | 1,035 | 54 | 208 | 482 | 6,771 | 9,272 | 16,043 |
| S10 | 934 | 269 | 316 | 274 | 1,390 | 1,442 | 1,585 | 1,465 | 1,046 | 722 | 2,065 | 1,165 | 409 | 151 | 540 | 486 | 4,624 | 9,634 | 14,258 |
| S11 | 440 | 52 | 376 | 184 | 557 | 205 | 460 | 381 | 274 | 149 | 345 | 362 | 154 | 177 | 143 | 167 | 1,814 | 2,611 | 4,425 |
| S12 | 483 | 106 | 315 | 366 | 683 | 550 | 1,068 | 909 | 1,178 | 777 | 944 | 533 | 1,760 | 269 | 512 | 335 | 2,503 | 8,286 | 10,789 |
| S13 | 873 | 4 | 598 | 344 | 1,245 | 90 | 836 | 663 | 470 | 324 | 1,100 | 621 | 314 | 40 | 292 | 289 | 3,155 | 4,948 | 8,103 |
| S14 | 1,506 | 394 | 2,680 | 1,876 | 4,273 | 262 | 2,170 | 1,754 | 2,908 | 2,048 | 2,660 | 1,501 | 1,548 | 114 | 710 | 216 | 10,991 | 15,628 | 26,619 |
| ERL b | 160 | 44 | 16 | 19 | 240 | 85.3 | 600 | 665 | 261 | 384 | – | – | 430 | 63.4 | – | – | 552 | 1,700 | 4,022 |
| ERM b | 2,100 | 640 | 500 | 540 | 1,500 | 1,100 | 5,100 | 2,600 | 1,600 | 2,800 | – | – | 1,600 | 260 | – | – | 3,160 | 9600 | 44,792 |
3.3. Distribution and Composition of PAHs
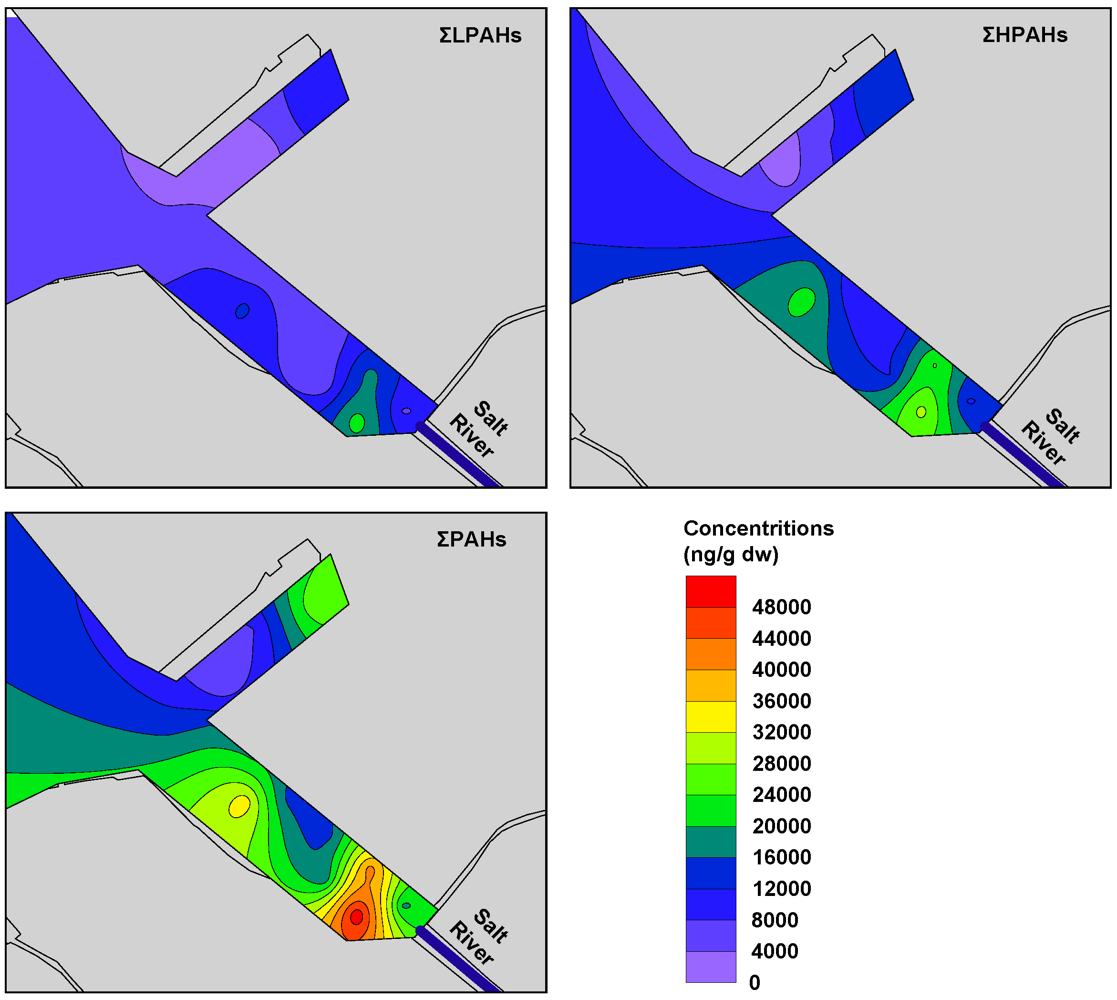
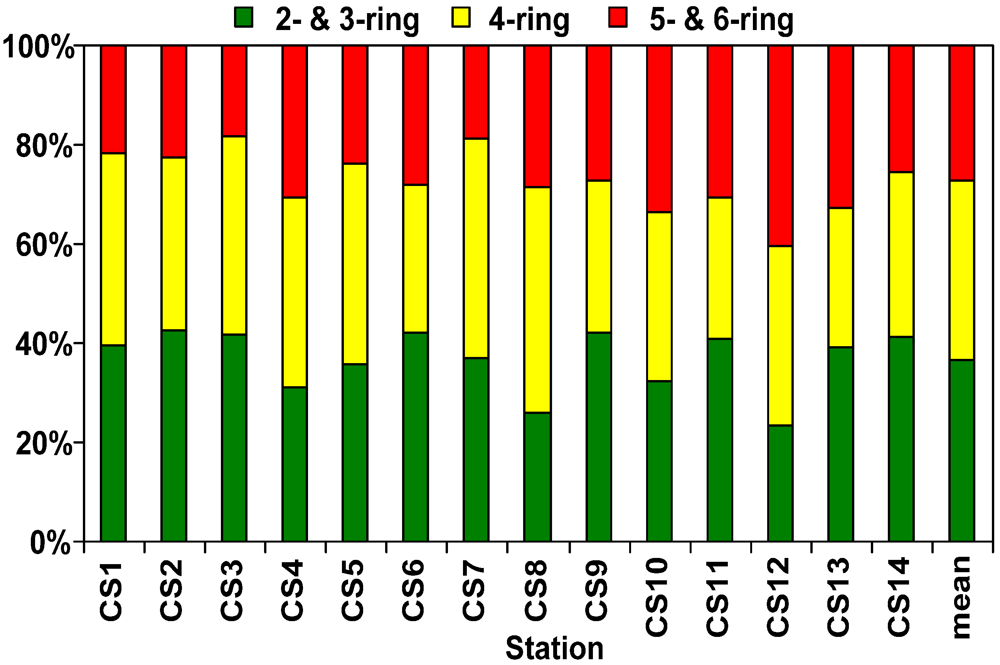

3.4. Sources of PAHs in Sediment
3.5. Sediment Biological Effects Based on PAHs
4. Conclusions
Acknowledgments
References
- Sverdrup, L.E.; Nielsen, T.; Krogh, P.H. Soil ecotoxicity of polycyclic aromatic hydrocarbons in relation to soil sorption, lipophilicity, and water solubility. Environ. Sci. Technol. 2002, 36, 2429–2435. [Google Scholar]
- Qiao, M.; Wang, C.; Huang, S.; Wang, D.; Wang, Z. Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environ. Int. 2006, 32, 28–33. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, G. Characterization and Desorption Kinetics of PAHs from Contaminated Sediment in Houston Ship Channel. In 2003 Annual Report; Environmental Institute of Houston, University of Houston-Clear Lake: Houston, TX, USA, 2003; pp. 39–41. [Google Scholar]
- Chen, C.W.; Chen, C.F. Distribution, origin and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwa. Mar. Pollut. Bull. 2011, 63, 417–423. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, C.F.; Dong, C.D.; Tu, Y.T. Composition and source apportionment of PAHs in sediments at river mouths and channel in Kaohsiung Harbor, Taiwan. J. Environ. Monit. 2012, 14, 105–115. [Google Scholar] [CrossRef]
- Ke, L.; Yu, K.S.H.; Wong, Y.S.; Tam, N.F.Y. Spatial and vertical distribution of polycyclic aromatic hydrocarbons in mangrove sediments. Sci. Total Environ. 2005, 340, 177–187. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuary sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Garcia-Falcon, M.S.; Soto-Gonzalez, B.; Simal-Gandara, J. Evolution of the concentrations of polycyclic aromatic hydrocarbons in burnt woodland soils. Environ. Sci. Technol. 2006, 40, 759–763. [Google Scholar]
- Baumard, P.; Budzinski, H.; Garrigues, P. Polycyclic aromatic hydrocarbons (PAHs) in sediments and mussels of the western Mediterranean Sea. Environ. Toxicol. Chem. 1998, 17, 765–776. [Google Scholar] [CrossRef]
- Fang, M.D.; Lee, C.L.; Yu, C.S. Distribution and source recognition of polycyclic aromatic hydrocarbons in the sediments of Hsin-Ta Harbour and adjacent coastal areas, Taiwan. Mar. Pollut. Bull. 2003, 46, 941–953. [Google Scholar] [CrossRef]
- Jiang, J.J.; Lee, C.L.; Fang, M.D.; Liu, J.T. Polycyclic aromatic hydrocarbons in coastal sediments of southwest Taiwan: An appraisal of diagnostic ratios in source recognition. Mar. Pollut. Bull. 2009, 58, 752–760. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River Basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, L.Z.; Yuan, D.X.; Chen, M. Distribution and sources of polynuclear aromatic hydrocarbons in mangrove surficial sediments of Deep Bay, China. Mar. Pollut. Bull. 2004, 49, 479–486. [Google Scholar] [CrossRef]
- Li, G.; Xia, X.; Yang, Z.; Wang, R.; Voulvoulis, N. Distribution and sources of polycyclic aromatic hydrocarbons in the middle and lower reaches of the Yellow River, China. Environ. Pollut. 2006, 144, 985–993. [Google Scholar] [CrossRef]
- Magi, E.; Bianco, R.; Ianni, C.; Carro, M.D. Distribution of polycyclic aromatic hydrocarbons in the sediments of the Adriatic Sea. Environ. Pollut. 2002, 119, 91–98. [Google Scholar] [CrossRef]
- Johnson, L.L.; Collier, T.K.; Stein, J.E. An analysis in support of sediment quality thresholds for polycyclic aromatic hydrocarbons (PAHs) to protect estuarine fish. Aquat. Conserv. Mar. Freshw. Ecosyst. 2006, 12, 517–538. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, C.-D.; Chen, C.-F.; Chen, C.-W. Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175-2188. https://doi.org/10.3390/ijerph9062175
Dong C-D, Chen C-F, Chen C-W. Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS. International Journal of Environmental Research and Public Health. 2012; 9(6):2175-2188. https://doi.org/10.3390/ijerph9062175
Chicago/Turabian StyleDong, Cheng-Di, Chih-Feng Chen, and Chiu-Wen Chen. 2012. "Determination of Polycyclic Aromatic Hydrocarbons in Industrial Harbor Sediments by GC-MS" International Journal of Environmental Research and Public Health 9, no. 6: 2175-2188. https://doi.org/10.3390/ijerph9062175




