Investigation of the Flow Characteristics of Methane Hydrate Slurries with Low Flow Rates
Abstract
:1. Introduction
2. Materials and Experimental Section
2.1. Experimental Flow Loop Description
2.2. Experimental Procedure and Materials
3. Results and Discussion
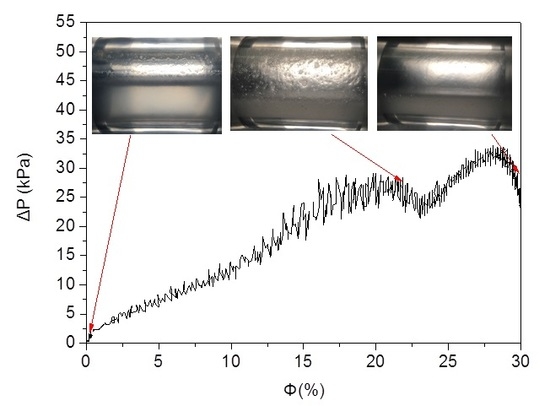
3.1. Typical Experimental Run
3.2. Effect of Initial Subcooling
3.3. Effect of Flow Rate
3.4. Effect of Pressure
3.5. Morphologies of Gas Hydrates in the Pipe
3.6. Pressure Drop Model
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sloan, E.D. Clathrate Hydrates of Natural Gases, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 27–49. [Google Scholar]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, G. Evaluation of Gas Production from Marine Hydrate Deposits at the GMGS2-Site 8, Pearl River Mouth Basin, South China Sea. Energies 2016, 9, 22. [Google Scholar] [CrossRef]
- Warzinski, R.P.; Lynn, R.; Haljasmaa, I.; Leifer, I.; Shaffer, F.; Anderson, B.J.; Levine, J.S. Dynamic morphology of gas hydrate on a methane bubble in water: Observations and new insights for hydrate film models. Geophys. Res. Lett. 2014, 41, 6841–6847. [Google Scholar] [CrossRef]
- Wang, B.; Socolofsky, S.A.; Breier, J.A.; Seewald, J.S. Observations of bubbles in natural seep flares at MC 118 and GC 600 using in situ quantitative imaging. J. Geophys. Res. Oceans 2016, 121, 2203–2230. [Google Scholar] [CrossRef]
- Hammerschmidt, E.G. Formation of gas hydrates in natural gas transmission lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Sun, M.; Firoozabadi, A. Natural gas hydrate particles in oil-free systems with kinetic inhibition and slurry viscosity reduction. Energy Fuels 2014, 28, 1890–1895. [Google Scholar] [CrossRef]
- Kakati, H.; Kar, S.; Mandal, A.; Laik, S. Methane hydrate formation and dissociation in oil-in-water emulsion. Energy Fuels 2014, 28, 4440–4446. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Kim, J.; Shin, K.; Chang, D.; Seo, Y.; Aman, Z.M.; May, E.F. Hydrate plug formation risk with varying watercut and inhibitor concentrations. Chem. Eng. Sci. 2015, 126, 711–718. [Google Scholar] [CrossRef]
- Lovell, D.; Pakulski, M. Hydrate inhibition in gas wells treated with two low dosage hydrate inhibitors (SPE 75668). In Proceedings of the SPE International Symposium on Gas Technology, Calgary, AB, Canada, 30 April–2 May 2002.
- Joshi, S.V.; Grasso, G.A.; Lafond, P.G.; Rao, I.; Webb, E.; Zerpa, L.E.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Experimental flowloop investigations of gas hydrate formation in high water cut systems. Chem. Eng. Sci. 2013, 97, 198–209. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Dybvik, L. Studies on gas hydrate inhibitors (SPE 30420). In Proceedings of the SPE Offshore Europe Conference, Aberdeen, UK, 5–8 September 1995.
- Seo, Y.; Kang, S.P. Inhibition of methane hydrate re-formation in offshore pipelines with a kinetic hydrate inhibitor. J. Pet. Sci. Eng. 2012, 88–89, 61–66. [Google Scholar] [CrossRef]
- Naeiji, P.; Arjomandi, A.; Varaminian, F. Amino acids as kinetic inhibitors for tetrahydrofuran hydrate formation: Experimental study and kinetic modeling. J. Nat. Gas Sci. Eng. 2014, 21, 64–70. [Google Scholar] [CrossRef]
- Villano, L.D.; Kelland, M.A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure II gas hydrate. Chem. Eng. Sci. 2010, 65, 5366–5372. [Google Scholar] [CrossRef]
- Nakarit, C.; Kelland, M.A.; Liu, D.J.; Chen, E.Y.-X. Cationic kinetic hydrate inhibitors and the effect on performance of incorporating cationic monomers into N-vinyl lactam copolymers. Chem. Eng. Sci. 2013, 102, 424–431. [Google Scholar] [CrossRef]
- Mohammad, R.T. Experimental investigation of gas consumption for simple gas hydrate formation in a recirculation flow mini-loop apparatus in the presence of modified starch as a kinetic inhibitor. J. Nat. Gas Sci. Eng. 2013, 14, 42–48. [Google Scholar]
- Zhao, H.; Sun, M.; Firoozabadi, A. Anti-agglomeration of natural gas hydrates in liquid condensate and crude oil at constant pressure conditions. Fuel 2016, 180, 187–193. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Akhfash, M.; Boxall, J.A.; Aman, Z.M.; Johns, M.L.; May, E.F. Hydrate formation and particle distributions in gas-water systems. Chem. Eng. Sci. 2013, 104, 177–188. [Google Scholar] [CrossRef]
- Kang, S.P.; Shin, J.Y.; Lim, J.S.; Lee, S. Experimental measurement of the induction time of natural gas hydrate and its prediction with polymeric kinetic inhibitor. Chem. Eng. Sci. 2014, 116, 817–823. [Google Scholar] [CrossRef]
- Andersson, V.; Gudmundsson, J.S. Flow properties of hydrate-in-water slurries. Ann. N. Y. Acad. Sci. 2000, 912, 322–329. [Google Scholar] [CrossRef]
- Zerpa, L.E.; Rao, I.; Aman, Z.M.; Danielson, T.J.; Koh, C.A.; Sloan, E.D.; Sum, A.K. Multiphase flow modeling of gas hydrates with a simple hydrodynamic slug flow model. Chem. Eng. Sci. 2013, 99, 298–304. [Google Scholar] [CrossRef]
- Greaves, D.; Boxall, J.; Mulligan, J.; Sloan, E.D.; Koh, C.A. Hydrate formation from high water content-crude oil emulsions. Chem. Eng. Sci. 2008, 63, 4570–4579. [Google Scholar] [CrossRef]
- Rao, I.; Koh, C.A.; Sloan, E.D.; Sum, A.K. Gas hydrate deposition on a cold surface in water-saturated gas systems. Ind. Eng. Chem. Res. 2013, 52, 6262–6269. [Google Scholar] [CrossRef]
- Daraboina, N.; Pachitsas, S.; Solms, N.V. Natural gas hydrate formation and inhibition in gas/crude oil/aqueous systems. Fuel 2015, 148, 186–190. [Google Scholar] [CrossRef]
- Webb, E.B.; Rensing, P.J.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Liberatore, M.W. High-pressure rheology of hydrate slurries formed from water-in-oil emulsions. Energy Fuels 2012, 26, 3504–3509. [Google Scholar] [CrossRef]
- Sinquin, A.; Palermo, T.; Peysson, Y. Rheological and flow properties of gas hydrate suspensions. Oil Gas Sci. Technol. 2004, 59, 41–57. [Google Scholar] [CrossRef]
- Yan, K.L.; Sun, C.Y.; Chen, J.; Chen, L.T.; Shen, D.J.; Liu, B.; Jia, M.L.; Niu, M.; Lv, Y.N.; Li, N.; et al. Flow characteristics and rheological properties of natural gas hydrate slurry in the presence of anti-agglomerant in a flow loop apparatus. Chem. Eng. Sci. 2014, 106, 99–108. [Google Scholar] [CrossRef]
- Fidel-Dufour, A.; Gruy, F.; Herri, J.M. Rheology of methane hydrate slurries during their crystallization in a water in dodecane emulsion under flowing. Chem. Eng. Sci. 2006, 61, 505–515. [Google Scholar] [CrossRef]
- Moradpour, H.; Chapoy, A.; Tohidi, B. Bimodal model for predicting the emulsion-hydrate mixture viscosity in high water cut systems. Fuel 2011, 90, 3343–3351. [Google Scholar] [CrossRef]
- Ding, L.; Shi, B.H.; Lv, X.F.; Liu, Y.; Wu, H.H.; Wang, W.; Gong, J. Investigation of natural gas hydrate slurry flow properties and flow patterns using a high pressure flow loop. Chem. Eng. Sci. 2016, 146, 199–206. [Google Scholar] [CrossRef]
- Lund, A. Comments to some preliminary results from the Exxon Hydrate Flow Loop. Ann. N. Y. Acad. Sci. 1994, 715, 447–449. [Google Scholar] [CrossRef]
- Yan, C.Q. Gas-Liquid Two Phase Flow, 2nd ed.; Harbin Engineering University Press: Harbin, China, 2007; pp. 114–128. [Google Scholar]
- Monteiro, A.C.S.; Bansal, P.K. Pressure drop characteristics and rheological modeling of ice slurry flow in pipes. Int. J. Refrig. 2010, 33, 1523–1532. [Google Scholar] [CrossRef]
- Kitanocski, A.; Vuarnoz, D.; Ata-Caesar, D.; Egolf, P.W.; Hansen, T.M.; Doetsch, C. The fluid dynamics of ice slurry. Int. J. Refrig. 2005, 28, 37–50. [Google Scholar] [CrossRef]
- Kitanovski, A.; Poredoš, A. Concentration distribution and viscosity of ice-slurry in heterogeneous flow. Int. J. Refrig. 2002, 25, 827–835. [Google Scholar] [CrossRef]













| No. | Flow Rate (m/s) | Induction Time (min) |
|---|---|---|
| 1 | 0.88 | 22.5 |
| 2 | 0.66 | 21.0 |
| 3 | 0.33 | 33.8 |
| Initial Condition | A | B | C | K | ||
|---|---|---|---|---|---|---|
| Pressure (MPa) | Subcoolings (°C) | Flow Rate (m/s) | ||||
| 6.3 | 4.5 | 0.33 | 265.75 | 10.05 | 0.368 | 30.6 |
| 8.0 | 4.5 | 0.33 | 165.25 | 10.05 | 3.05 | 35.6 |
| 6.3 | 8.5 | 0.33 | 875.25 | 10.05 | 3.88 | 40.6 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Zhao, X.; Li, D.; He, Y.; Shen, X.; Liang, D. Investigation of the Flow Characteristics of Methane Hydrate Slurries with Low Flow Rates. Energies 2017, 10, 145. https://doi.org/10.3390/en10010145
Tang C, Zhao X, Li D, He Y, Shen X, Liang D. Investigation of the Flow Characteristics of Methane Hydrate Slurries with Low Flow Rates. Energies. 2017; 10(1):145. https://doi.org/10.3390/en10010145
Chicago/Turabian StyleTang, Cuiping, Xiangyong Zhao, Dongliang Li, Yong He, Xiaodong Shen, and Deqing Liang. 2017. "Investigation of the Flow Characteristics of Methane Hydrate Slurries with Low Flow Rates" Energies 10, no. 1: 145. https://doi.org/10.3390/en10010145