1. Introduction
The use of lignocellulose biomass to produce liquid biofuels such as bioethanol can overcome the problem of greenhouse gas (GHG) emissions, reduce reliance on foreign oil, enhance the agricultural economies, create more employment opportunities and ensure the sustainability of the world transportation systems. Agricultural waste such as wheat straw, corn stalks and sugarcane bagasse are abundant and inexpensive natural resources. The worldwide estimate production of lignocellulosic materials is around 1 × 10
10 t annually [
1]. Lignocellulosic biomass is mainly composed of cellulose, hemicellulose, and lignin [
2].
Lignocellulosic materials can be sourced from plant and wood materials. The conversion of lignocellulose materials to biofuels is complex and needs pretreatment and enzymatic hydrolysis steps to enhance the efficiency of biofuel production. The enzymatic hydrolysis of lignocellulosic materials such as sugarcane bagasse is an important process for the production of second generation bioethanol. However, this step is slow, expensive and consumes energy. In a study by Verardi et al. [
3] showed that the yields of glucose, xylose, arabinose and mannose increased by 9% for glucose and were as high as 90% for the other monosaccharides when the stirring rate increased to 300 rpm [
3].
According to the Department of Resources, Energy and Tourism, the Australian production of biofuels in 2006 was 106 million liters (ML) from edible substrates, of which ethanol comprised 62 ML and biodiesel 44 ML. In 2007–2008, production of biofuels reached 199 million liters (ML)/year, of which ethanol comprised 149 ML and biodiesel 50 ML [
4]. Almost any plant-based material can be an ethanol feedstock. All plants contain sugars in different forms, and these sugars can be fermented to make ethanol. There are many processes that convert the plant material to sugar. The conversion process can be done using heat, chemicals and irradiation techniques [
4]. Lignocellulosic materials are non-food based feedstocks such as crop, wood and plant residues. Lignocellulosic feedstocks offer many advantages over starch and sugar-based feedstock because they are waste products with low economical value. However, conversion of this feedstock into ethanol is a challenging process [
5]. The process of converting lignocellulosic materials to ethanol is more tedious than current ethanol production from corn and sugarcane, and the technology of the conversion process is not yet feasible. Corn and sugarcane include high percentages of starch and sugar, respectively. Starch and sugar are easily digested by the yeast (yeast’s favorite food). In contrast, cellulose is a sugar polymer that needs to be broken down to sugar monomers before the yeast can digest it, and it was also shielded from the yeast by lignin and hemicellulose [
4].
The structure of lignocellulosic biomass is very complex in nature and is not suitable to be used directly in a fermentation process. In order to make it more degradable, pretreatment is necessary to increase the efficiency of the fermentation process [
6]. Pretreatment is the most expensive step in the process of converting lignocellulosic biomass to ethanol. Thus, identifying and developing cost-effective pretreatment methods of lignocellulosic biomass is a major challenge [
7].
Currently, the main aim of the pretreatment is to remove the lignin and hemicellulose, reduce cellulose crystalline, increase porosity and increase the internal surface area to alter the visible structure into a microscopic size. This process changes the biomass structure and improves downstream processing. Different pretreatments lead to different chemical reactions and characteristic interaction with the components of lignocellulosic biomass. The feedstocks ranging from grasses to soft and hard woods have a different sugar to lignin ratio. These variations create the need for various pretreatments of the biomass [
8].
During the pretreatment, the cell structure and chemical composition of the bagasse is ruptured. This increases the surface area of the biomass and makes it more biodegradable, which helps to enhance the efficiency of the fermentation process. There are many types of pretreatment configurations that have been studied for the conversion of lignocellulosic biomass to ethanol. Pretreatment methods are broadly classified into physical, chemical and irradiation. Physical pretreatments include comminution (mechanical size reduction), ultrasound, steam explosion and hydro thermolysis [
2]. In chemical pretreatment, acid or base is added to the bagasse to remove certain components. The acid and the alkaline pretreatment processes are aiming to remove lignin and leave the cellulose intact [
9].
After the pretreatment step of lignocellulosic materials, an expensive enzymatic treatment is required to convert the separated cellulose to its sugar monomers. For example, in a study by Manzoor et al. [
10], bagasse was pretreated with various concentrations of sulfuric acid at different time intervals from 0 to 3 h. Before adding the acid to the bagasse, it was autoclaved at 121 °C for 30 min. Then, 10 g of bagasse was put in a conical flask and different concentrations of sulfuric acid (0.5%–5%) were added. After pretreatment with acid, the enzymatic hydrolysis enhanced and the achieved yield of sugar was around 90%.
The main objective of the acid pretreatment is to degrade the hemicellulose of the bagasse, and this increases the surface area of the materials and will be more suitable for fermentation. In the concentrated acid treatment process, sulfuric acid (H
2SO
4) is used at a concentration greater than 40% at room temperature for 1 h [
8]. The acid pretreatment can be divided into two types: high temperature greator than 160 °C with 5%–10% acid concentration and low temperature less than 160 °C with a sulfuric acid concentration of 10%–40% [
6]. According to Njoku [
11], the dilute acid treatment is not effective in dissolving the lignin, but it can rupture the lignin and increase the digestibility of the cellulose, and 100% of hemicellulose can be removed in this method. It has been observed that hydrochloric acid was less active for the degradation of lignocellulosic materials when compared to sulfuric acid [
12].
The main aim of the alkaline pretreatment is delignification of lignin. The removal of lignin is critical for the enzymatic hydrolysis [
13]. The effects of the alkaline solution depend on the lignin content present in the biomass. In the sugar industry, caustic soda and sodium sulphite are combined in the pretreatment process for the effective removal of lignin [
9]. Alkaline pretreatments require low temperature and pressure conditions compared to the other treatment methods [
6]. It can be carried out at room temperature, and the reaction time can be a few minutes to several days. Adding NaOH to hardwood increases its digestibility from 14% to 55% with the decrease in lignin content up to 55%. For softwoods, the lignin removal rate can be greater than 26% [
6]. In a study by O’Hara [
9], lignin is removed up to 75% through the removal of a
p-hydroxyphenyl lignin using alkaline pretreatment. Alkaline pretreatment is less corrosive in nature, and the cost of the reactor is reduced when comparing it with the acidic treatments [
9].
The effect of passing high-frequency ultrasound waves into the biomass solution causes cavitation, and, as a result, disruption of the lignocellulosic structure. The C-H bonds are also affected by the cavitation [
14]. Several factors should be considered during the sonication process to get an effective treatment of lignocellulose biomass. The duration of the sonication has the greatest impact on the pretreatment. The increase of the duration of the sonication increases the delignification of the biomass. The power level, frequency and amplitude are also important parameters. When the amplitude level is increased correspondingly the power level is also increased, which directly affects the intensity of the cavitation. It has been found that increasing the power level by more than 150 W does not improve the delignification of biomass in practice [
14]. The use of ultrasound pretreatment with various lignocellulose materials has shown an increase in the glucose yield of 21.3% [
8]. Rehman et al. [
14] reported that pretreatment of sugarcane bagasse, by mixing it with distilled water (DI) and treating it with 22 kHz and 100 W power for 5–35 min under 55 °C temperature, has resulted in 90% extraction of hemicellulose and lignin. The efficiency of the pretreatment also depends on the particle size of the lignocellulosic biomass. It is reported that the smaller the particle size, the better separation is achieved [
7].
Table 1 shows a comparison between different methods of pretreatments, possible changes to the structure of the lignocellulosic materials and some advantages/disadvantages of the processes. It is obvious from the table that chemical/physicochemical pretreatments are the most efficient and more favorable for the industry. The only disadvantage is the amount of chemicals required for the process, which can be reduced in case it is combined with some other physical processes.
Almost any organic material including lignocellulosic material, such as bagasse, can be processed with anaerobic digestion to produce biogas, but this does not apply to bioethanol. In recent years, a lot of development has been done in the area of waste to biogas [
16,
17]. Research also showed the feasibility to upgrade the biogas for biomethane production. In many studies [
18,
19,
20], the feasibility of integrating an anaerobic digestion plant with an onsite polymeric membrane purification system to upgrade biogas to biomethane has been shown. Biomethane purity was achieved at 94% for a biogas fed at 50 kg/h with a methane content of about 55%. However, there is still uncertainty in regards to which is more economical to produce biogas or bioethanol from bagasse. Note that both products require pretreatment for the substrate (bagasse) before fermenting in an anaerobic digester.
Sugarcane bagasse was used in this research because it represents almost pure lignocellulosic material after the sugarcane went through crushing and hot water washing. Many studies have been carried out related to pretreatment of bagasse with acidic and alkaline solutions and ultrasound as sole processes [
9,
13,
14]. Some other research combined steam explosion with chemical pretreatment [
8]. To our best knowledge, no study has combined chemical pretreatment with ultrasound in one process. This research is investigating ultrasound in conjunction with chemicals as a pretreatment method to enhance degradability of bagasse. In addition, the aim is to identify suitable levels of ultrasound/chemicals to improve ethanol production. The novelty of this research is combining ultrasound with a chemical pretreatment in one step to enhance the effectiveness of both methods.
3. Results and Discussion
The structure of the bagasse is visualised using an SEM microscope;
Figure 3 shows the structure of bagasse before (
Figure 3a) and after pretreatment with diluted acid (3%) and an ultrasound (
Figure 3b). It is noted that the complex structure of bagasse is destroyed, and the cellulose fibers are now exposed.
The sulfuric acid concentration was chosen between 1% and 5%. Minitab (Version 17, Minitab Inc., State College, PA, USA) was used for experimental design and optimisation purposes. In the first experiment, bagasse underwent a pretreatment with acid, which was followed by an ultrasound separately. The bagasse was treated for 12 h. During the pretreatment, the pH level varied from 2.6 to 4.2 and the temperature ranged from 21 to 49 °C. After 12 h of pretreatment with acid, the bagasse was rinsed with water and then treated with an ultrasound. Continuous ultrasound waves were used and the reaction time was set to 5 min. After pretreatment with the ultrasound, the bagasse was fermented using 3 g of baker’s yeast (Saccharomyces Cerevisiae) for six days. The generated CO2 was collected in upside-down plastic beakers. The final products in each reactor were also analysed with the Shimadzu GCMS, which showed that, apart from the ethanol, there was a small amount of butyric acid, most likely produced by Clostridia bacteria naturally present in the sugar cane bagasse. Additionally, some CO2, acetic acid, valeric acid and other compounds stemming from the metabolism of the yeast and other bacteria in the solution were detected.
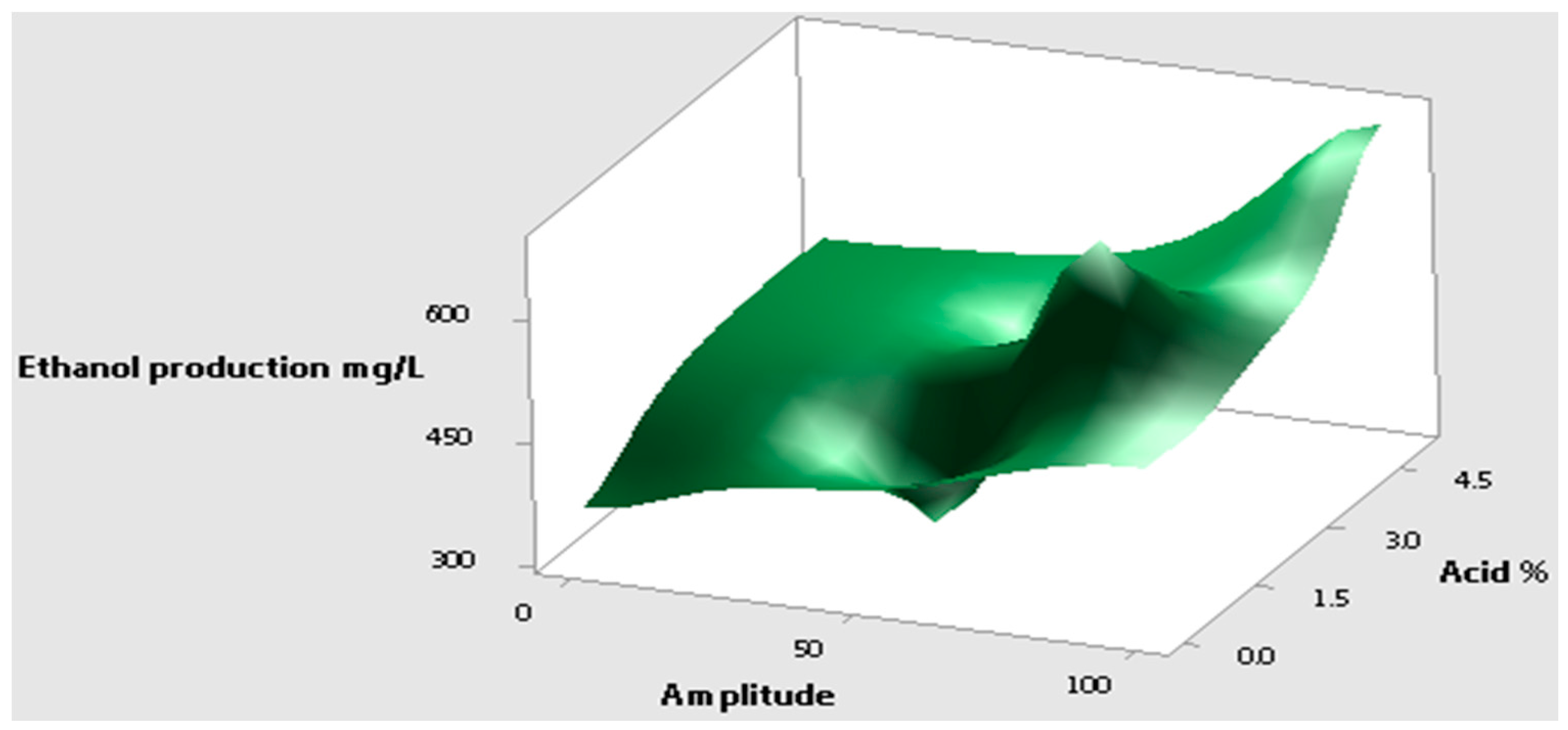
Figure 4 shows a surface plot of ethanol yield against the acid % and amplitude level. The optimum level of the acid % and the amplitude level were identified as 3% and 40–50, respectively. The corresponding ethanol produced is close to 638 mg/L.
Another experiment was carried out where acid and ultrasound pretreatments were combined in one process. After 12 h of acid pretreatment, the bagasse was then directly pre-treated with the contentious ultrasound waves. The acid concentrations used in this experiment were 2.5%, 3% and 3.5% (dilute H2SO4) and the ultrasound amplitude levels were 50, 60 and 70. After the pretreatment stage, the bagasse was rinsed thoroughly with water before fermentation. Yeast was added into the reactors (3 g) and then fermented for six days. The result shows that, as the pretreatment time and amplitude level increases, the ethanol production increases. By combining both pretreatment methods in one process, ethanol production was about 820 mg/L. This result is 22% higher than the results gained from the pretreatment experiment of acid and the ultrasound, which carried out separately.
Another set of experiments were conducted to investigate alkaline and ultrasound pretreatment separately and combined. Bagasse was first pretreated with the alkaline (NaOH) solution at different concentrations (21%, 22% and 23%). The pretreatment period was 24 h at room temperature. After 24 h of pretreatment with alkaline, the bagasse was thoroughly rinsed with water for 15 min. Then, the bagasse was fermented with 3 g of
Saccharomyces Cerevisiae yeast for six days. The optimum concentration of NaOH was between 22% and 24%.
Figure 5 shows ethanol production at different NaOH concentrations. It is evident from the graph, when the result is compared with the acid pretreated, that NaOH pretreatment results in remarkably less ethanol production. The difference is around 400 mg/L, and it is concluded that acid pretreatment is more efficient than alkaline pretreatment.
When alkaline and an ultrasound are combined to pretreat the bagasse, the bagasse was first treated with the alkaline solution and then treated with the ultrasound directly. After 24 h of alkaline pretreatment, the bagasse was directly treated with the ultrasound. The alkaline concentrations used were 21%, 22% and 23%, and the ultrasound amplitude levels were 50, 60 and 70. After the pretreatment, the bagasse was rinsed thoroughly with water before fermentation. Baker’s yeast
Saccharomyces cerevisiae (3 g) was added into each of the reactors and fermented for six days.
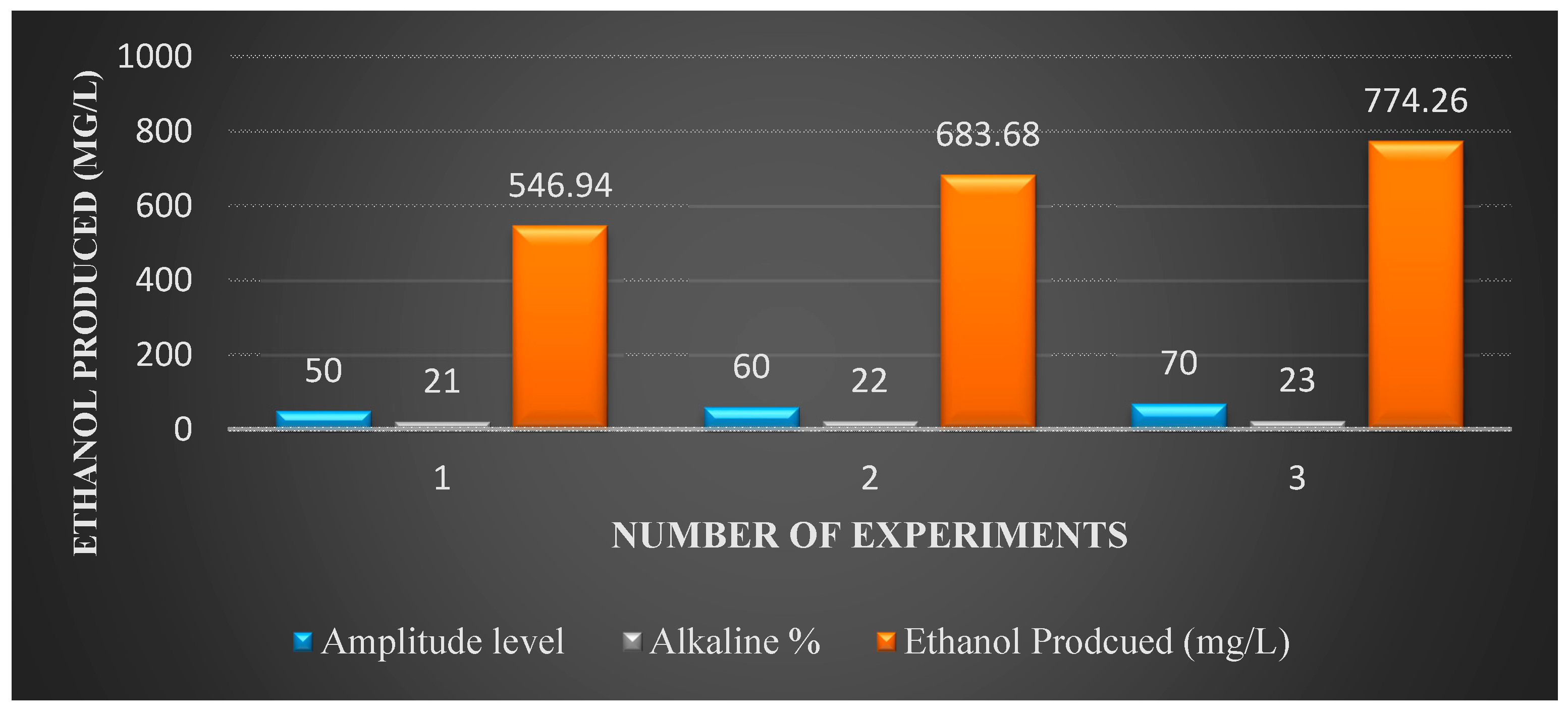
Figure 6 shows ethanol production from the pretreated bagasse with alkaline solution and ultrasound combined. The figure indicates that, at a higher amplitude level of 70 and at NaOH concentration of 23%, ethanol production is around 774.3 mg/L.
Bagasse was also investigated undergoing three subsequent pretreatments. Bagasse was first pretreated with chemicals: acid (H
2SO
4) for 12 h, then rinsed with water and pretreated with alkaline (NaOH) solution for another 24 h, and then rinsed with water. After the chemical treatments, the bagasse was pre-treated with an ultrasound with amplitude levels of 40, 50 and 60 for five minutes. Finally, the pre-treated bagasse was added to a 500 mL reactor and 400 mL of distilled water was added. The bagasse was then fermented with 3 g of
Saccharomyces Cerevisiae yeast for six days.
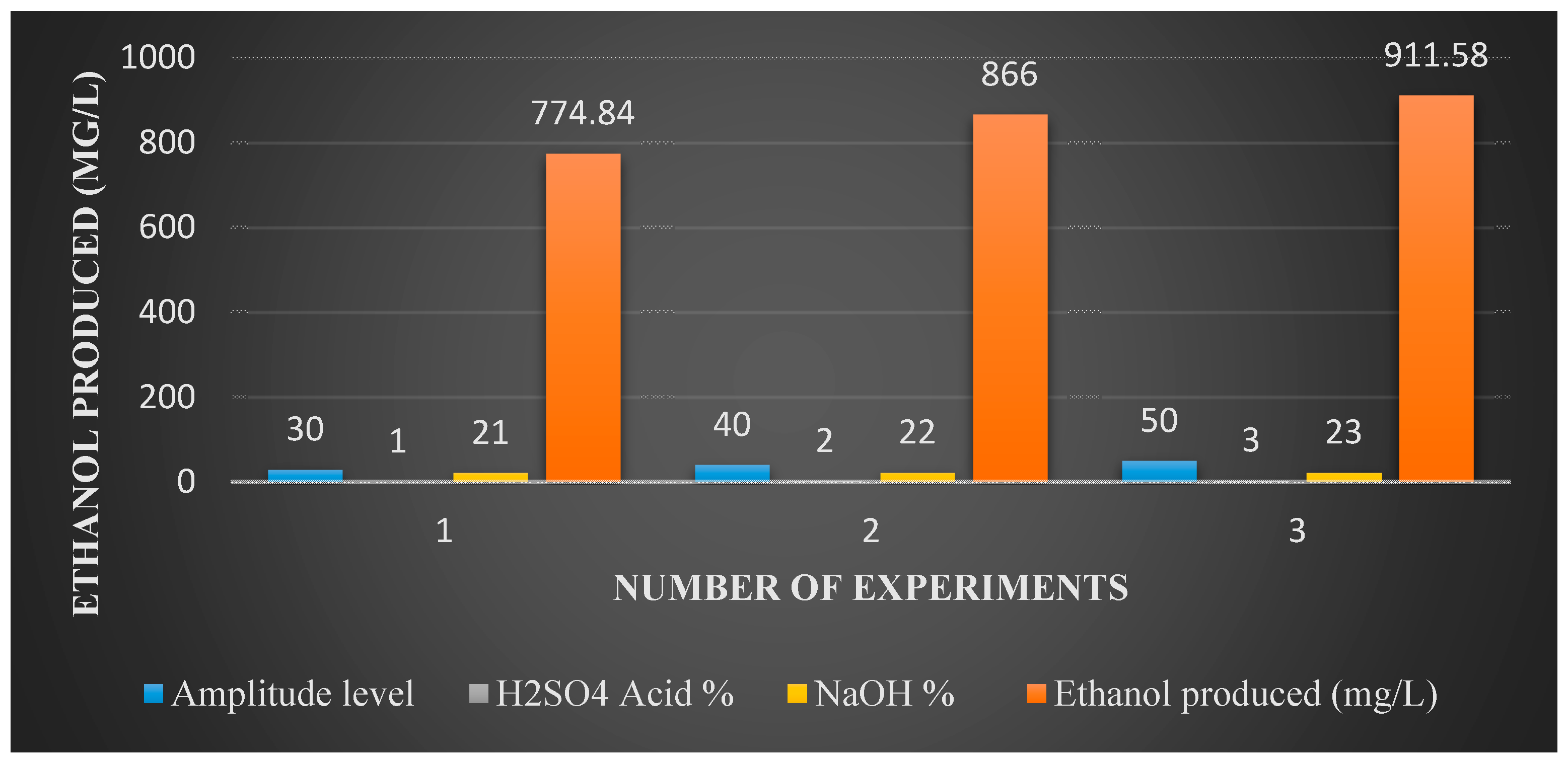
Figure 7 shows ethanol production for various pretreatment methods of acid and alkaline solutions and an ultrasound. By treating the bagasse with all of the methods one after another, 3% sulfuric acid, 23% of NaOH and an amplitude level of 50, and ethanol production reached 911.6 mg/L.
The results of the pretreatment methods carried out in this research were compared with literature results [
13]. Acid pretreatment from literature showed ethanol production of 390 mg/L with a 3% acid pretreatment, whereas for the experiment conducted in this research, by combining both pretreatment methods (Acid + Ultrasound) in one process, ethanol production was about 820 mg/L. For the alkaline pretreatment, the literature result is 220 mg/L of ethanol. The ethanol produced in this research is around 774.3 mg/L when combining both alkaline and ultrasound in one process. Pretreating bagasse with acid, alkaline and ultrasound in subsequent processes resulted in 911.6 mg/L of ethanol.
5. Conclusions
In this research, experimental works were conducted to investigate ethanol production through various pretreatment methods. Acid (H2SO4), alkaline (NaOH) and ultrasound were separately and in combinations tested as pretreatments for bagasse. The results from this study were compared with literature to show the impact of an ultrasound when combined with chemical pretreatments. The results from the experiments were processed through Minitab software for optimization purposes.
The optimum level of acid, alkaline and ultrasound amplitude were obtained as the following: the optimum level of acid was shown to be in the range of 2.5%–3.5%, for alkaline solution it was in the range of 22%–25% and, for the ultrasound, the amplitude level range was 45–60 (76–127 W).
The results show higher ethanol production than that reported in literature. The optimum ethanol production value of 820 mg/L was achieved when ultrasound (60 amplitude level or 127 W) and acid (3% H2SO4 concentration) pretreatments were combined. In addition, a value of 911 mg/L was achieved when all the pretreatments were used, and bagasse was treated with acid (3%) and ultrasound (50 or 109 W) and then treated with alkaline (23%) and ultrasound (50 or 109 W).