The Characteristics of Methane Combustion Suppression by Water Mist and Its Engineering Applications
Abstract
:1. Introduction
2. Analysis of the Process of Methane Combustion and Explosion
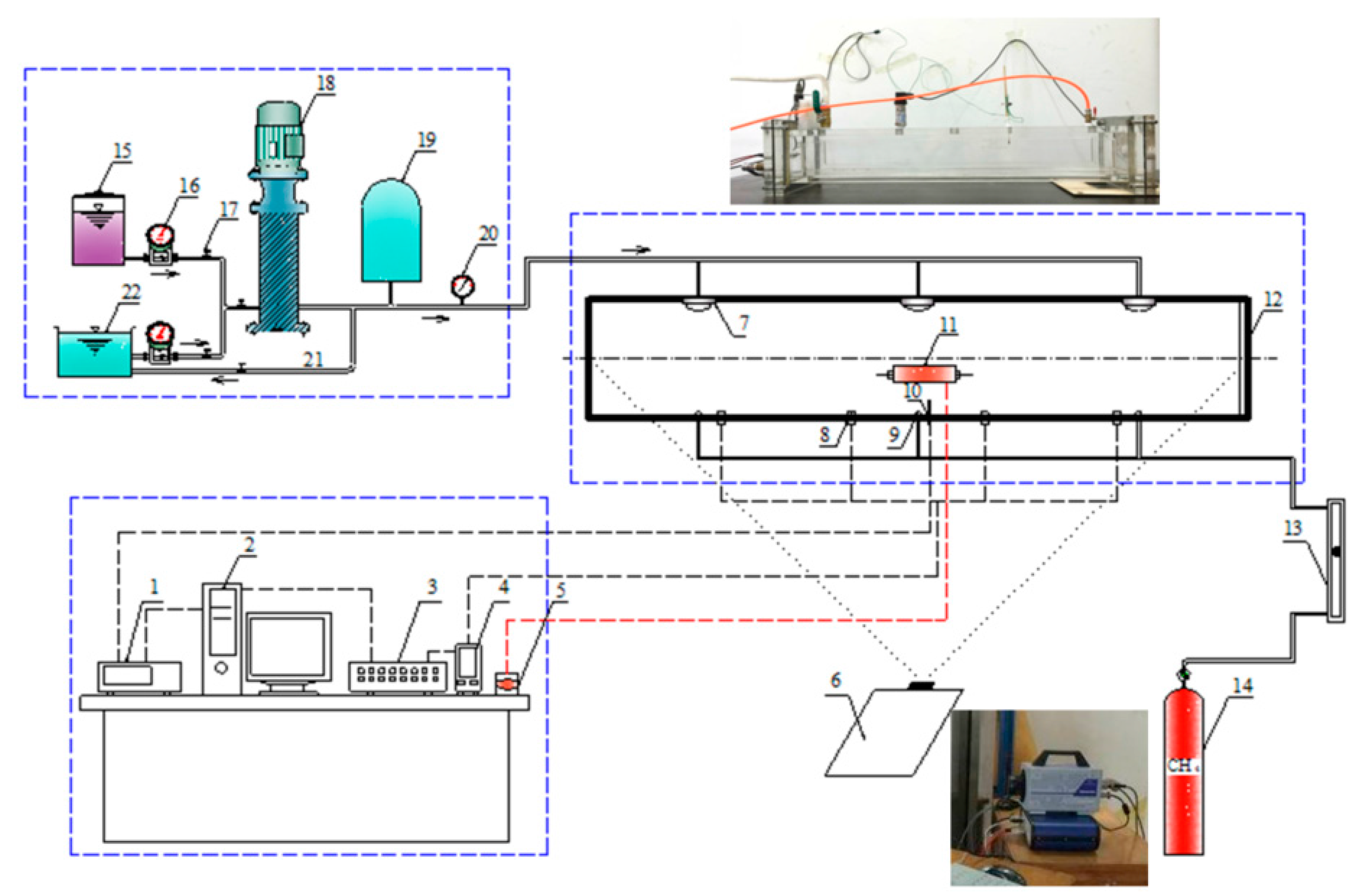
3. Experiment System
3.1. Experimental Equipment
3.2. Experiment Process and Condition
4. Experimental Results and Discussion
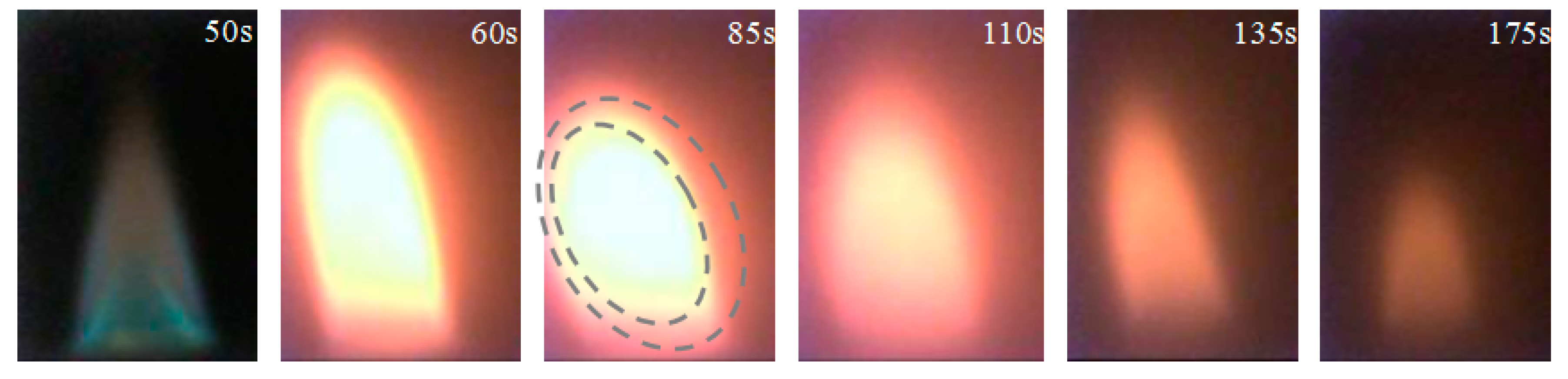
4.1. Analysis of Flame Temperature after Application of Water Mist
4.2. Analysis of the Interaction between the Water Mist and Flame
4.3. The Influence of Methane Gas Explosive Pressure by Water Mist with Additives
5. The Mechanism for Suppressing Methane Combustion Using Water Mist
5.1. The Effect of Endothermic Cooling
5.2. The Effect of Oxygen Replacement
5.3. Changes in the Ignition Energy
5.4. The Effect of a Heterogeneous Chemical Reaction
Fe(OH)2 + 2H+ → Fe2+ + 2H2O;
Cl− + RH → R− + HCl;
HCl + OH− → Cl− + H2O.
6. Engineering Application and Analysis
6.1. Prevention and Control Technology for Methane Gas Combustion in Goaf
6.2. Prevention and Control Measures for Drilling Gas Combustion
6.3. Prevention and Control Measures in the Upper-Corner of the Working Face
7. Discussion
- (1)
- The release of water mist can disturb the flame by rapid decalescence and vaporization, which cools and lowers the flame temperature.
- (2)
- Water mist evaporated and formed a closed spray circle around the outside of the flame area, effectively restricting oxygen around the flame, lowering the oxygen concentration in the combustion zone and suppressing fire spread.
- (3)
- Water mist can effectively reduce the pressure of a methane gas explosion and prevent disasters from further expanding.
- (4)
- For a flame ejection speed of 10 m/s from a borehole, the release of a water mist flow of 1.8 L/min results in the borehole flame being extinguished within 6 s.
- (5)
- After the release of water mist, the gas concentration in the upper-corner of the working face decreased, with the largest drop reaching 37.8%. This process achieved positive results in engineering applications.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, R.K.; Cheng, Y.P.; Yuan, L.; Yu, M.G.; Dong, J. Effect of bedding structural diversity of coal on permeability evolution and gas disasters control with coal mining. Nat. Hazards 2014, 73, 531–546. [Google Scholar] [CrossRef]
- Niu, C.; Shi, L.Q.; Xiao, L.L. Study on accidents classification of coal mine from 2001 to 2013. Saf. Coal Mines 2015, 46, 208–211. [Google Scholar]
- Yu, M.G.; Wan, S.J.; Xu, Y.L.; Zheng, K.; Liang, D. The influence of the charge-to-mass ratio of the charged water mist on a methane explosion. J. Loss Prev. Process Ind. 2016, 41, 68–76. [Google Scholar] [CrossRef]
- Jenft, A.; Collin, A.; Boulet, P.; Pianet, G.; Breton, A.; Muller, A. Experimental and numerical study of pool fire suppression using water mist. Fire Saf. J. 2014, 67, 1–12. [Google Scholar] [CrossRef]
- Yoshida, A.; Kashiwa, K.; Hashizume, S.; Naito, H. Inhibition of counter flow methane/air diffusion flame by water mist with varying mist diameter. Fire Saf. J. 2015, 71, 217–225. [Google Scholar] [CrossRef]
- Brewster, M.Q. Evaporation and condensation of water mist/cloud droplets with thermal radiation. Int. J. Heat Mass Transf. 2015, 88, 695–712. [Google Scholar] [CrossRef]
- Yao, B.; Cong, B.H.; Qin, J.; Chow, W.K. Experimental study of suppressing Poly(methyl methacrylate) fires using water mists. Fire Saf. J. 2012, 47, 32–39. [Google Scholar] [CrossRef]
- Qin, J.; Yao, B.; Chow, W.K. Experimental study of suppressing cooking oil fire with water mist using a cone calorimeter. Int. J. Hosp. Manag. 2004, 23, 545–556. [Google Scholar] [CrossRef]
- Yoshida, A.; Okawa, T.; Ebina, W. Experimental and numerical investigation of flame speed retardation by water mist. Combust. Flame 2015, 162, 1772–1777. [Google Scholar] [CrossRef]
- Yao, B.; Fan, W.; Liao, G. Interaction of water mists with a diffusion flame in a confined space. Fire Saf. J. 1999, 33, 129–139. [Google Scholar] [CrossRef]
- Adiga, K.C.; Hatcher, R.F.; Sheinson, R.S.; Williams, F.W.; Ayers, S. A computational and experimental study of ultra water mist as a total flooding agent. Fire Saf. J. 2007, 3, 150–161. [Google Scholar] [CrossRef]
- Liu, J.H.; Cong, B.H. Experimental study on critical fire concentration of water mist containing additives. J. China Univ. Min. Technol. 2011, 40, 116–119. [Google Scholar]
- Rumminger, M.D.; Reinelt, D.; Babushok, V.; Linteris, G.T. Numerical study of the inhibition of premixed and diffusion flames by iron pentacarbonyl. Combust. Flame 2002, 128, 145–164. [Google Scholar] [CrossRef]
- Almerinda, D.B.; Francesco, C.; Valeria, D.S.; Ernesto, C.; Gennaro, R. Anomalous behavior during explosions of CH4 in oxygen-enriched air. Combust. Flame 2011, 158, 2214–2219. [Google Scholar]
- Almerinda, D.B.; Francesco, C.; Valeria, D.S.; Ernesto, C.; Gennaro, R. Reconsidering the flammability diagram for CH4/O2/N2 and CH4/O2/CO2 mixtures in light of combustion-induced rapid phase transition. Chem. Eng. Sci. 2012, 84, 142–147. [Google Scholar]
- Ernesto, S.; Anna, B.; Francesco, C.; Valeria, D.S.; Almerinda, D.B. Explosions of syngas/CO2 mixtures in oxygen-enriched air. Ind. Eng. Chem. Res. 2012, 51, 7671–7678. [Google Scholar]
- Almerinda, D.B.; Francesco, C.; Valeria, D.S.; Ernesto, C.; Gennaro, R. Effect of diluents on rapid phase transition of water induced by combustion. AIChE J. 2012, 58, 2810–2819. [Google Scholar]
- Yu, W.; Miao, X.X.; Mao, X.B. Analysis of the heating—Up mechanism in the course of the rock ram. Chin. J. Rock Mech. Eng. 2005, 24, 1535–1538. [Google Scholar]
- Qin, Y.J.; Jiang, W.Z.; Wang, X.Y. Determination the source of goaf gas explosion (combustion). Saf. Coal Mines 2005, 36, 35–37. [Google Scholar]
- Wu, Y.Y.; Zhou, X.Q.; Zhu, H.Q. Research of environmental factors for gas igniting by sparks induced by high-speed strike. J. China Univ. Min. Technol. 2003, 32, 186–188. [Google Scholar]
- Pan, R.K.; Cheng, Y.P.; Yu, M.G.; Lu, C. Experimental study of new technology for preventing gas combustion in mining face. J. China Coal Soc. 2012, 37, 1854–1858. [Google Scholar]
- Yu, M.G.; Wan, S.J.; Xu, Y.L.; Zheng, K.; Liang, D. Suppressing methane explosion over pressure using a charged water mist containing a NaCl additive. J. Nat. Gas Sci. Eng. 2016, 29, 21–29. [Google Scholar] [CrossRef]
- Birwa, S.K.; Mishra, D.P. Measurements of the visible flame height of a swirl-stabilized kerosene jet diffusion flame. Combust. Explos. Shock Waves 2015, 51, 416–423. [Google Scholar] [CrossRef]
- Rashwan, S.S.; Ibrahim, A.H.; Abou-Arab, T.W.; Nemitallah, M.A.; Habib, M.A. Experimental investigation of partially premixed methane–air and methane–oxygen flames stabilized over a perforated-plate burner. Appl. Energy 2006, 169, 126–137. [Google Scholar] [CrossRef]
- Deilamani, K.S.; Assar, M. A new approach to determine relieving temperature and thermodynamic behavior of trapped single and multi-phase fluid exposed to fire. J. Loss Prev. Process Ind. 2015, 36, 134–145. [Google Scholar] [CrossRef]
- Zheng, L.G.; Lü, X.S.; Zheng, K. Influence of ignition position on overpressure of premixed methane-air deflagration. CIESC J. 2015, 66, 2749–2756. [Google Scholar]
- Yu, M.G.; Li, X.L.; Ma, K.S.; Duan, Y.L.; Hao, Q. Experiments and mechanism of using spray mist with additives to extinguish kerosene pool firet. J. Henan Polytech. Univ. 2006, 25, 433–436. [Google Scholar]
- Kuang, K.S.; Cong, B.H.; Liao, G.X. Experimental study on effectiveness of water mist fire suppression with ferrous chloride additive. Fire Saf. Sci. 2005, 14, 21–27. [Google Scholar]













| Condition | Temperature (°C) | Humidity (%) | Air Leakage (m3) | Gas Concentration (%) |
|---|---|---|---|---|
| No water mist | 21.3 | 71 | 120 | 0.65 |
| Release water mist | 19.6 | 96 | 101 | 0.57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, R.; Xiao, Z.; Yu, M. The Characteristics of Methane Combustion Suppression by Water Mist and Its Engineering Applications. Energies 2017, 10, 1566. https://doi.org/10.3390/en10101566
Pan R, Xiao Z, Yu M. The Characteristics of Methane Combustion Suppression by Water Mist and Its Engineering Applications. Energies. 2017; 10(10):1566. https://doi.org/10.3390/en10101566
Chicago/Turabian StylePan, Rongkun, Zejun Xiao, and Minggao Yu. 2017. "The Characteristics of Methane Combustion Suppression by Water Mist and Its Engineering Applications" Energies 10, no. 10: 1566. https://doi.org/10.3390/en10101566





