Research on Exergy Flow Composition and Exergy Loss Mechanisms for Waxy Crude Oil Pipeline Transport Processes
Abstract
:1. Introduction
2. Physical and Chemical Exergy in the Process of a Waxy Crude Oil Pipeline Transport Process
2.1. The Physical Exergy of Waxy Crude Oil Pipeline Transportation
2.1.1. The Calculation of Thermal Exergy
- When the temperature is lower than 22 °C:
- When the temperature is between 22~48 °C:
- When the temperature is greater than 48 °C:
2.1.2. The Calculation of Pressure Exergy
- In the certain environmental state of (T0, P0), the thermal exergy ext and pressure exergy exp are all state parameters of the materials, moreover, thermal exergy ext is only a function of temperature and pressure exergy exp is a function of both pressure and temperature;
- The crude oil properties have no effect on pressure exergy exp. Although thermal exergy ext relates to the crude oil properties, it only depends on the constant pressure-specific heat cp. Therefore, this simple relation will bring about convenience for calculating and analyzing the physical exergy of crude oil.
2.2. The Chemical Exergy of Waxy Crude Oil Pipeline Transportation
2.2.1. The Chemical Reaction Exergy of Waxy Crude Oil Transportation
2.2.2. The Chemical Diffusion Exergy of Waxy Crude Oil Transportation
3. Exergy Loss and Exergy Efficiency in the Process of Waxy Crude Pipeline Transportation
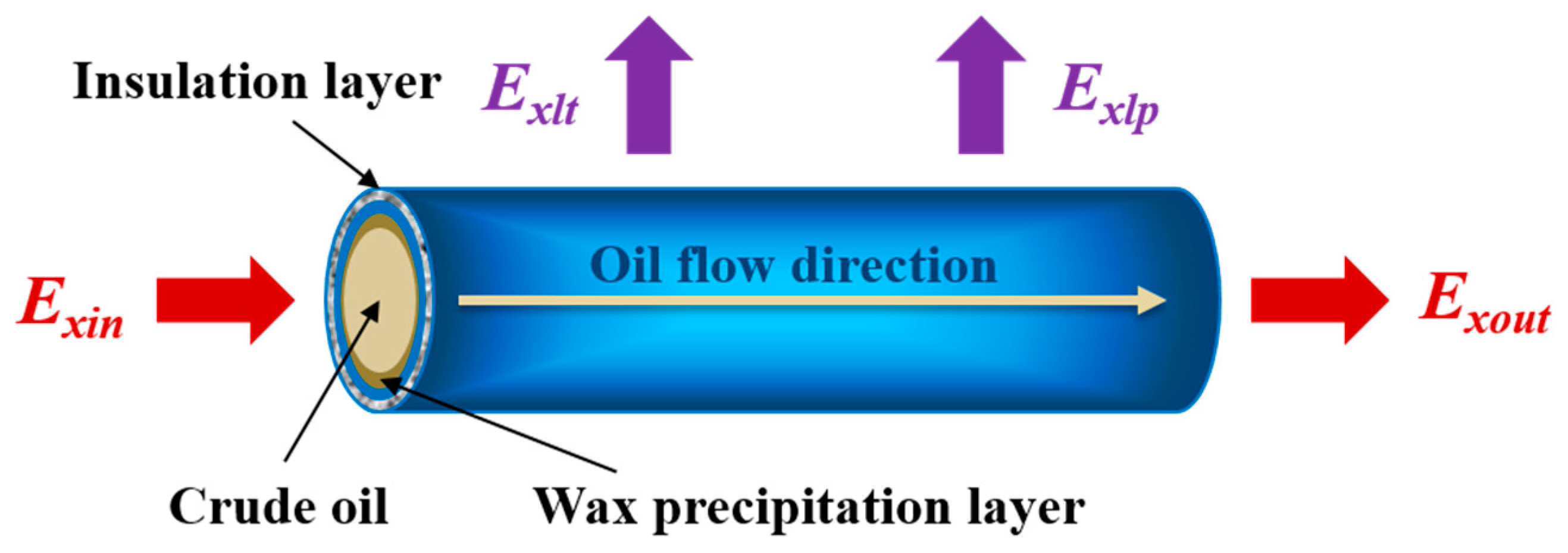
3.1. The Exergy Balance Equation of the Pipeline Transportation
3.2. The Formula for Calculating the Exergy Loss in Pipeline Transportation
3.2.1. The Exergy Flow Loss in the Pipeline Transportation
3.2.2. The Internal and External Exergy Loss in the Pipeline Transportation
3.2.3. The Total Exergy Loss in the Process of Pipeline Transportation
3.3. The Exergy Efficiency in the Process of Transportation
4. Application Example Analysis
4.1. Basic Data
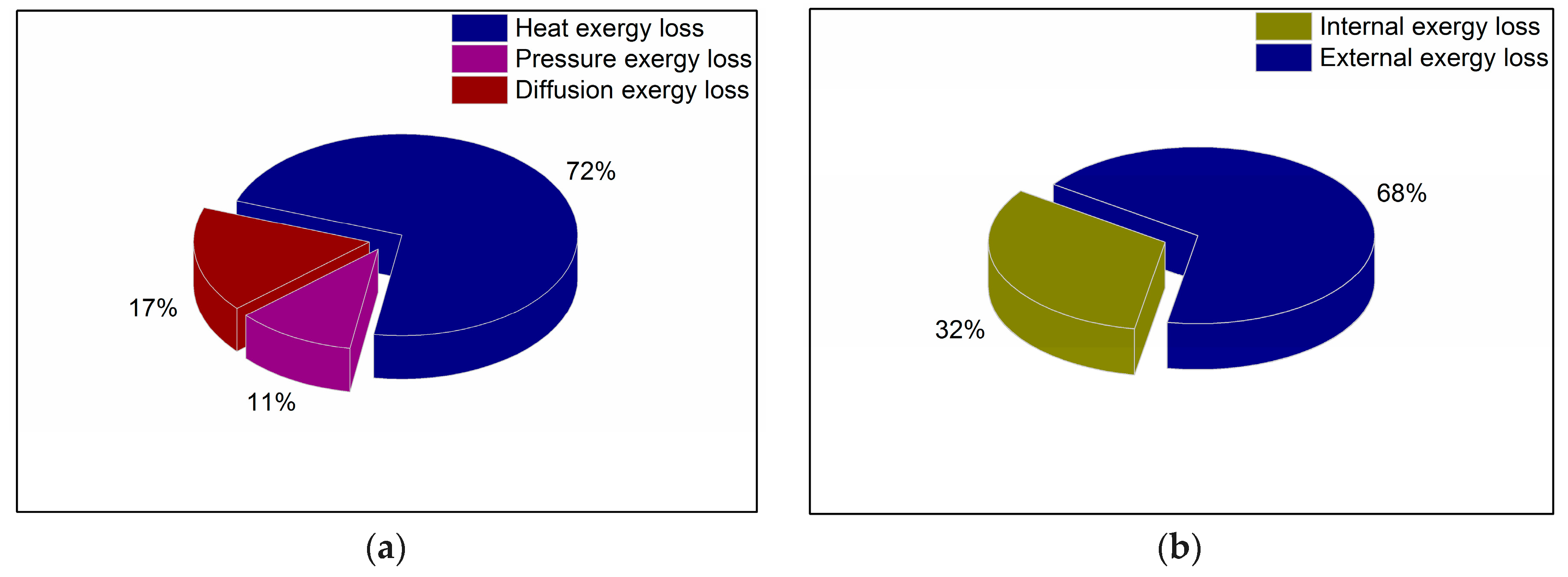
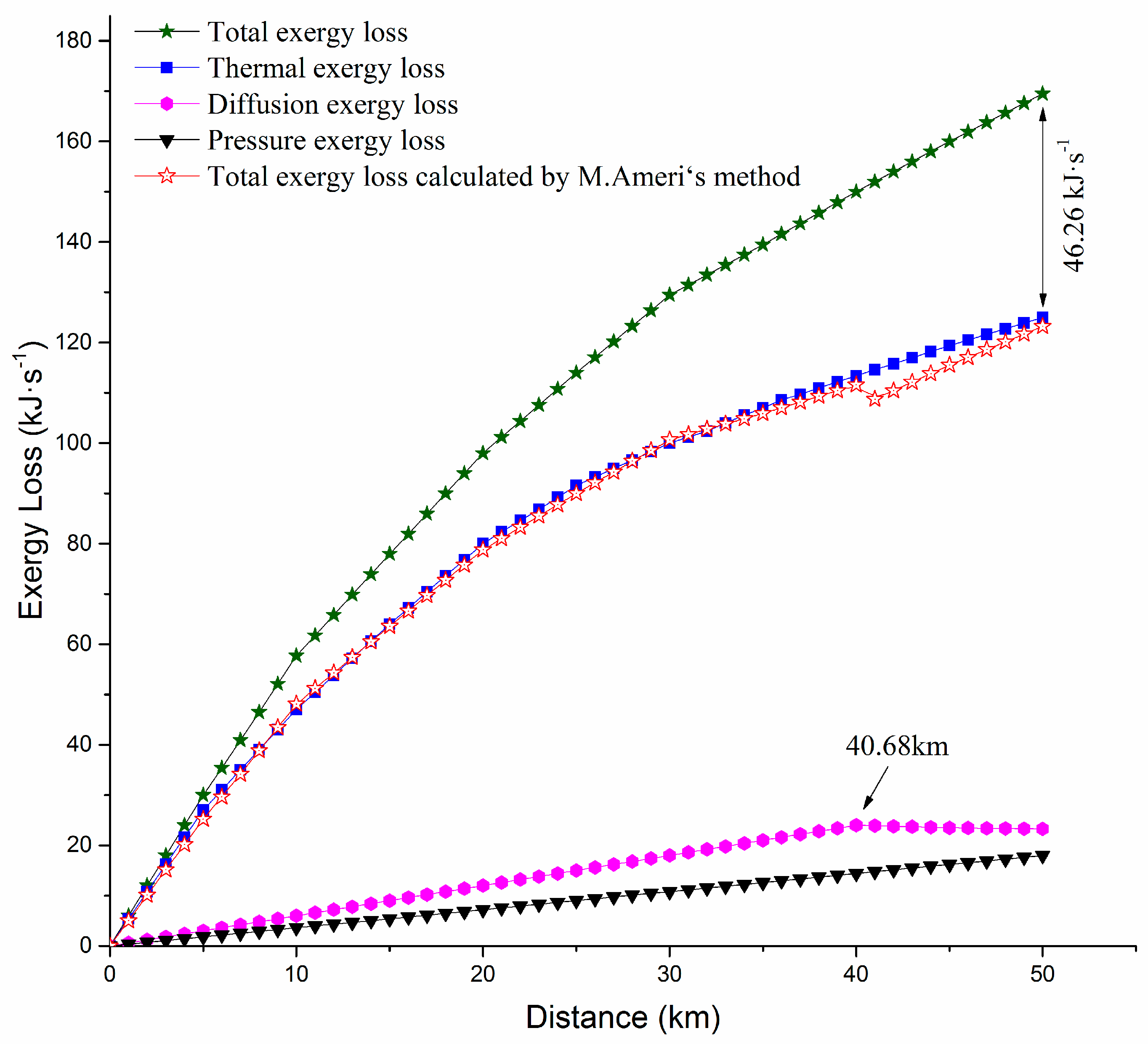
4.2. Analysis of Exergy Loss in the Process of Pipeline Transportation
4.3. The Mechanism Analysis of Exergy Loss in the Pipeline Transportation Process
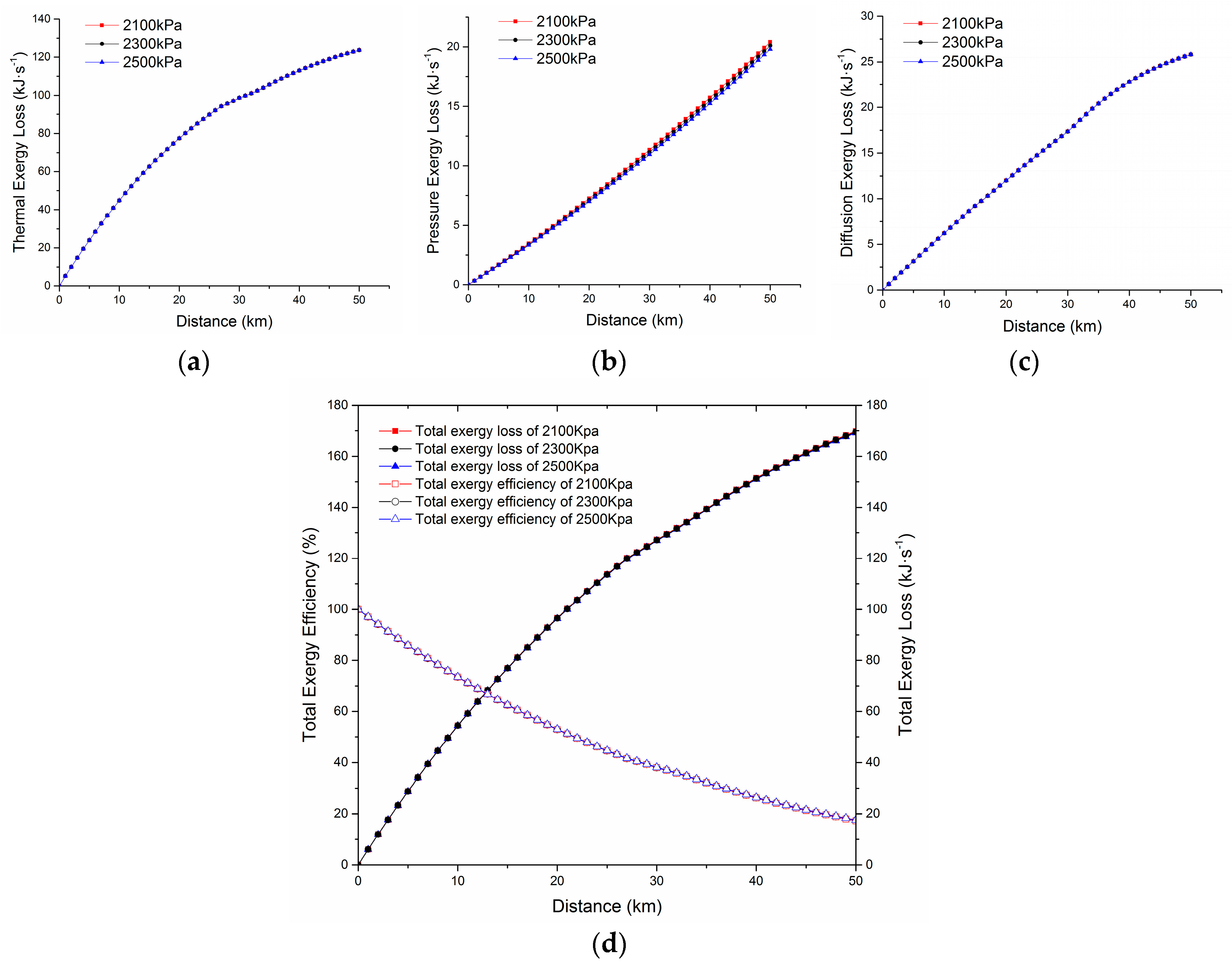
4.4. The Analysis of Exergy Loss under Different Conditions of the Transportation Process
4.4.1. The Axial Distribution of Various Exergy Losses under Different Out-Station Temperatures
4.4.2. The Axial Distribution of Various Exergy Losses under Different Out-Station Pressures
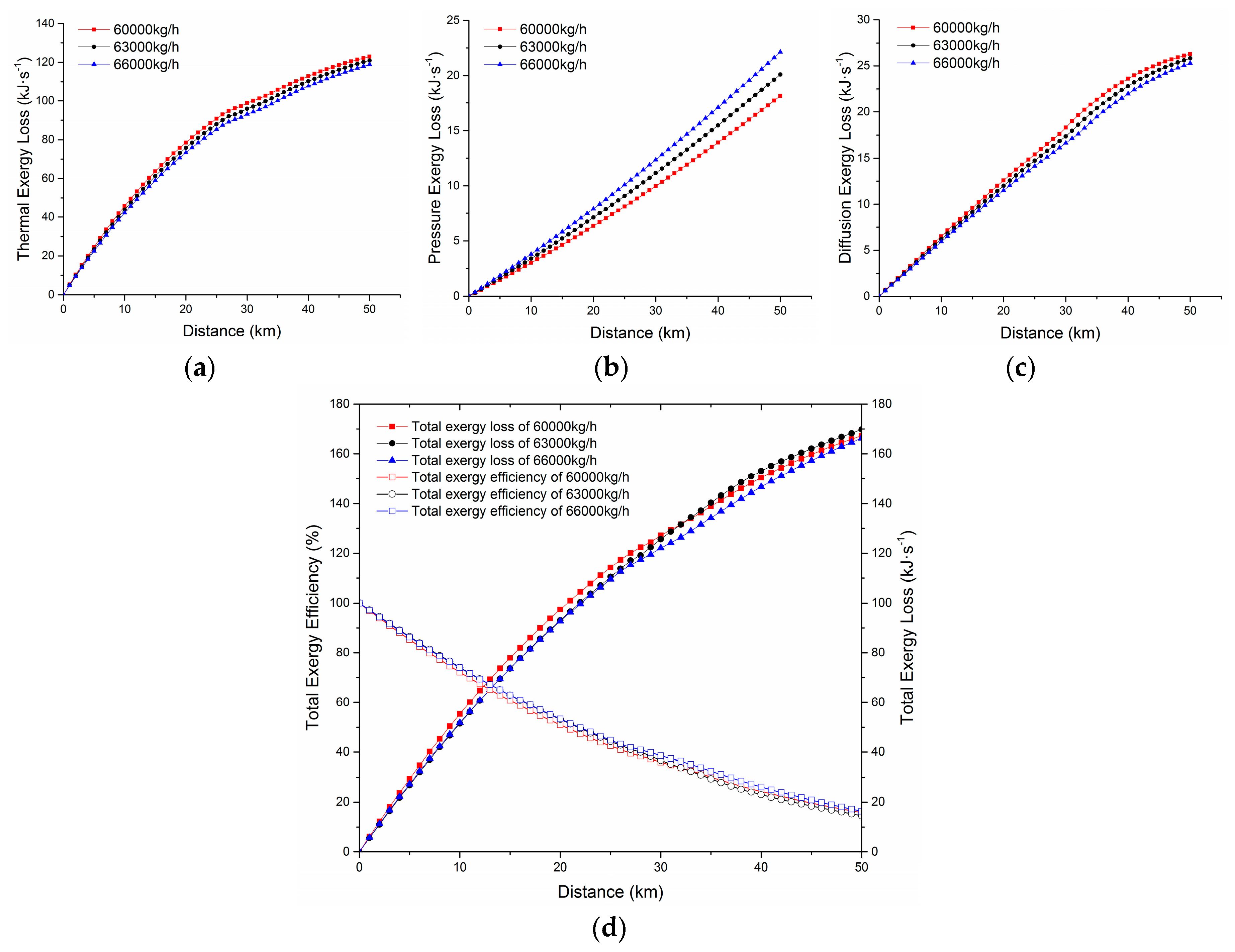
4.4.3. The Axial Distribution of Exergy Loss under Different Flow Rates
4.5. Analysis of the Total Exergy Efficiency of the Orthogonal Test
5. Conclusions
- The physical exergy values for the oil flow in the pipeline transportation process were the state parameters. When the environmental state was given, the thermal exergy was only a function of temperature, but it was associated with the physical properties Cp of the crude oil. The pressure exergy of the oil flow was a function of both temperature and pressure, and it had nothing to do with the properties of crude oil. The pressure exergy decreased with the increase of the temperature, and increased with the increasing of pressure.
- The chemical reaction exergy is generally calculated by approximation algorithms in the process of waxy crude oil transportation. However, this proportion was so small that it can be ignored in actual engineering calculations. The chemical diffusion exergy had an association with the chemical potential of the crude oil components. When the temperature of the oil dropped to the wax precipitation point, the wax molecules in the crude oil precipitated extensively, and with the decrease of temperature, the amount of wax precipitation increased, and the molecular concentration gradient changed intensively, resulting in a great change of chemical diffusion exergy.
- Taking a waxy crude oil transportation pipeline from an eastern Chinese oilfield as an example, the distribution of various types of exergy loss was studied. The results showed that along the waxy crude oil pipeline, all kinds of axial exergy losses were increasing, the thermal exergy loss was far greater than the pressure exergy loss and diffusion exergy loss, and the difference between the diffusion exergy loss and the pressure exergy was small. It was illustrated that the waxy crude oil transmission was greatly affected by the temperature field, the effect of the wax concentration field on the transportation process was in the second place, and the effect of the pressure field was the smallest. Compared with the external exergy loss, the internal exergy loss accounted for a smaller proportion. The internal exergy loss was often caused by an irreversible process, and we can only attempt to reduce, but not eliminate it.
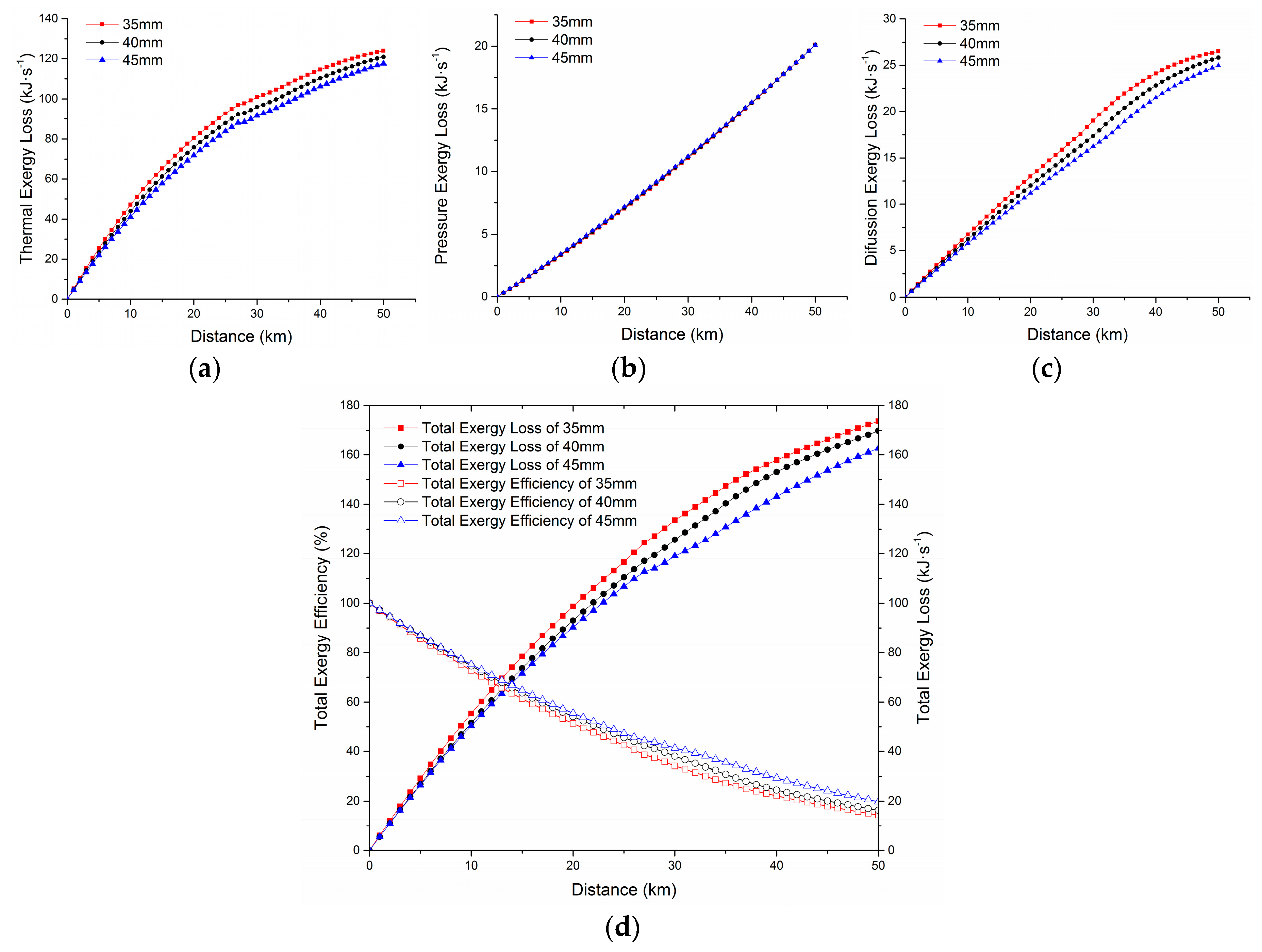
- The orthogonal analysis method was used to analyze the degree of influence of various factors on the total exergy efficiency. The results showed that under different working conditions, the orders of the influence degree on out-station total exergy efficiency were: insulation layer thickness, out-station temperature and out-station pressure. It was confirmed that the highest combination scheme for total exergy efficiency under the 66 × 103 kg·h−1 design flow rate was an out-station temperature of 65 °C, an out-station pressure of 2500 kPa, and an insulation layer thickness of 45 mm.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| E | exergy (kJ) |
| W | work (kJ) |
| R | constant of ideal gas (-) |
| T | temperature (K) |
| V | molar volume (m3/mol) |
| K | constant of the liquid-solid equilibrium (-) |
| H | enthalpy (kJ) |
| C | relative molecular mass (-) |
| M | molar mass (g/mol) |
| G | mass (kg) |
| Q | flow rate (m3/s) |
| S | entropy (kJ/K) |
| e | specific exergy (kJ/kg) |
| c | specific heat capacity (kJ/kg·K) |
| d | relative density (-) |
| p | pressure (kPa) |
| ρ | density (kg/m3) |
| n | mole number (mol) |
| x | molar fraction (-) |
| r | activity coefficient (-) |
| f | fugacity (-) |
| Greek Symbols | |
| ξ | temperature coefficient (-) |
| μ | chemical potential (kJ/mol) |
| δ | solubility parameter (-) |
| η | exergy efficiency (-) |
| ψ | exergy loss coefficient (-) |
| Subscripts | |
| xph | physical |
| xt | thermal |
| xp | pressure |
| 0 | initial state |
| xch | chemical |
| C | chemical reaction |
| D | chemical diffusion |
| i | component |
| x,gain | gained |
| x,pay | paid |
| L | loss |
| Superscripts | |
| 0 | initial state |
| L | liquid |
| S | solid |
| SL | liquid-solid |
| f | fugacity |
References
- Tang, X.; McLellan, B.C.; Zhang, B.S.; Snowden, S.; Hook, M. Trade-off analysis between embodied energy exports and employment creation in China. J. Clean. Prod. 2016, 134, 310–319. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhang, G.Z. Design and Management of Oil Pipeline; China University of Petroleum Press: Dongying, China, 1996; pp. 121–166. ISBN 9787563608669. [Google Scholar]
- Sun, X.L. Analysis on Entropy Production Rate in Pipeline Process of Waxy Crude Oil. Master’s Thesis, North East Petroleum University, Daqing, China, 2013. [Google Scholar]
- Han, G.Z.; Guo, P.S.; Li, S.X.; Hua, B. Thermodynamic exergy and dynamic characteristics of its generalized expressions. J. Eng. Therm. Eng. Power 2007, 22, 409–413. [Google Scholar] [CrossRef]
- Enrico, S.; Göran, W. A brief commented history of exergy from the beginnings to 2004. Int. J. Thermodyn. 2007, 10, 1–26. [Google Scholar] [CrossRef]
- Kenhuanhui of Energy Transformation. Technology for Effective Use of Energy; Chemical Industry Press: Beijing, China, 1984; ISBN 9787109178373. [Google Scholar]
- Zhu, M.S. The Analysis of Energy System by Exergy; Tsinghua University Press: Beijing, China, 1988; ISBN 7-302-00015-8. [Google Scholar]
- Chaczykowski, M.; Osiadacz, A.J.; Uilhoorn, F.E. Exergy-based analysis of gas transmission system with application to Yamal-Europe pipeline. Appl. Energy 2011, 88, 2219–2230. [Google Scholar] [CrossRef]
- Ameri, M.; Askari, M. Enhancing the efficiency of crude oil transportation pipeline: A novel approach. Int. J. Exergy 2013, 13, 523–542. [Google Scholar] [CrossRef]
- Cheng, Q.L.; Ding, N.; Yi, X.; Zhao, Y.; Sun, W. Study of exergy and conversion law of irreversible process in crude oil pipeline transportation. J. Therm. Sci. Technol. 2015, 14, 125–129. [Google Scholar] [CrossRef]
- Gooya, R.; Gooya, M.; Dabir, B. Effect of flow and physical parameters on the wax deposition of middle east crude oil under subsea condition: Heat transfer viewpoint. Heat Mass Transf. 2013, 49, 1205–1216. [Google Scholar] [CrossRef]
- Fu, Q.S. Thermodynamic Analysis Method of Energy System; Xi’an Jiaotong University Press: Xi’an, China, 2005; ISBN 9787560519449. [Google Scholar]
- Chen, W.H.; Zhao, Z.C. Thermodynamic modeling of wax precipitation in crude oils. Chin. J. Chem. Eng. 2006, 14, 685–689. [Google Scholar] [CrossRef]
- Xiang, X.Y.; Cheng, Q.L. Entropy, exergy and energy analysis, energy transfer. J. Therm. Sci. Technol. 2004, 3, 275–278. [Google Scholar] [CrossRef]
- Kaushik, S.C.; Singh, O.K. Estimation of chemical exergy of solid, liquid and gaseous fuels used in thermal power plants. J. Therm. Anal. Calorim. 2014, 115, 903–908. [Google Scholar] [CrossRef]
- Gupta, A.; Anand, Y.; Anand, S.; Tyagi, S.K. Thermodynamic optimization and chemical exergy quantification for absorption-based refrigeration system. J. Therm. Anal Calorim. 2015, 122, 893–905. [Google Scholar] [CrossRef]
- Su, W.K. The Comprehensive Composition of Exergy Flow and Exergy Loss Analysis in Waxy Crude Oil Transportation Process. Master’s Thesis, North East Petroleum University, Daqing, China, 2016. [Google Scholar]
- Govin, O.V.; Diky, V.V.; Kabo, G.J.; Blokhin, A.V. Evaluation of the chemical exergy of fuels and petroleum fractions. J. Therm. Anal. Calorim. 2000, 62, 123–133. [Google Scholar] [CrossRef]
- Chen, W.H. The Thermodynamic Research of Wax Precipitation in Crude Oils. Master’s Thesis, Dalian University Technology, Dalian, China, 2006. [Google Scholar]
- Cai, J.M.; Zhang, G.Z.; Xing, X.K.; Luo, J.W. Research developments of wax deposition in waxy crude oil pipelines. Oil Gas Storage Transp. 2002, 21, 12–16. [Google Scholar] [CrossRef]
- Han, G.Z.; Hua, B.; Chen, Q.L.; Yin, Q.H. The general expression of exergy in thermodynamics. Sci. China (Ser. A) 2001, 31, 934–938. [Google Scholar] [CrossRef]
- Weingarten, J.S.; Euchner, J.A. Methods for predicting wax precipitation and deposition. SPE Prod. Eng. 1988, 3, 121–126. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Skovborg, P. Wax precipitation from North-Sea crude oils. 4. Thermodynamic modeling. Energy Fuels 1991, 5, 924–935. [Google Scholar] [CrossRef]
- Li, Y.P.; Jia, M.; Chang, Y.C.; Kokjohn, S.L.; Reitz, R.D. Thermodynamic energy and exergy analysis of three different engine combustion regimes. Appl. Energy 2016, 180, 849–858. [Google Scholar] [CrossRef]
- Su, L.S.; Zhang, J.B.; Wang, C.J.; Zhang, Y.; Li, Z.; Song, Y.; Jin, T.; Ma, Z. Identifying main factors of capacity fading in lithium ion cells using orthogonal design of experiments. Appl. Energy 2015, 163, 201–210. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, C.J.; Yang, X.F.; Gao, D.K.; Xu, L.Z.; Zhang, Y.Y. Temperature field analysis of liquid stratification for LNG tank based on orthogonal ridgelet finite element method. J. Therm. Anal. Calorim. 2016, 125, 557–562. [Google Scholar] [CrossRef]

| Items | Numerical Value |
|---|---|
| Operating time, day | 350 |
| Thickness of insulating layer, mm | 40 |
| Thermal conductivity of insulating layer, W·m−1·°C−1 | 0.025 |
| Thermal conductivity of soil, W·m−1·°C−1 | 1.4 |
| Pipeline thermal conductivity, W·m−1·°C−1 | 45.24 |
| Pipe length, km | 50 |
| Pipeline diameter, mm | 207.9 |
| Top of the pipe buried depth, mm | 1600 |
| Environment temperature, °C | −4 |
| Absolute roughness, mm | 0.15 |
| Thickness of pipeline, mm | 5.6 |
| Out-station pressure, kPa | 2300 |
| Out-station temperature, °C | 70 |
| Flow rate, kg/h | 63,000 |
| Density of waxy crude oil at 20 °C, kg·m−3 | 844.1 |
| Wax content of waxy crude oil, % | 20.2 |
| Wax precipitation point, °C | 48 |
| Abnormal point, °C | 36.2 |
| Conditions | Out-Station Temperature °C | Out-Station Pressure kPa | Flow Rate 103 kg·h−1 | Thickness of Insulating Layer mm |
|---|---|---|---|---|
| Condition1 | 70 | 2500 | 66 | 45 |
| Condition 2 | 65 | 2300 | 63 | 40 |
| Condition 3 | 60 | 2100 | 60 | 35 |
| Working Condition | Out-Station Temperature (°C) | Out-Station Pressure (kPa) | Thickness of Insulation Layer (mm) | Total Energy Efficiency (%) | ||
|---|---|---|---|---|---|---|
| Level | ||||||
| Test Number | ||||||
| 1 | 70 | 2500 | 45 | 18.94 | ||
| 2 | 70 | 2300 | 40 | 17.17 | ||
| 3 | 65 | 2500 | 40 | 18.15 | ||
| 4 | 65 | 2300 | 45 | 18.73 | ||
| Condition Category | Out-Station Temperature | Out-Station Pressure | Thickness of Insulation Layer |
|---|---|---|---|
| T1j | 36.11 | 37.09 | 37.67 |
| T2j | 36.88 | 35.90 | 35.32 |
| Rj | 0.77 | 1.19 | 2.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Q.; Gan, Y.; Su, W.; Liu, Y.; Sun, W.; Xu, Y. Research on Exergy Flow Composition and Exergy Loss Mechanisms for Waxy Crude Oil Pipeline Transport Processes. Energies 2017, 10, 1956. https://doi.org/10.3390/en10121956
Cheng Q, Gan Y, Su W, Liu Y, Sun W, Xu Y. Research on Exergy Flow Composition and Exergy Loss Mechanisms for Waxy Crude Oil Pipeline Transport Processes. Energies. 2017; 10(12):1956. https://doi.org/10.3390/en10121956
Chicago/Turabian StyleCheng, Qinglin, Yifan Gan, Wenkun Su, Yang Liu, Wei Sun, and Ying Xu. 2017. "Research on Exergy Flow Composition and Exergy Loss Mechanisms for Waxy Crude Oil Pipeline Transport Processes" Energies 10, no. 12: 1956. https://doi.org/10.3390/en10121956





