Ageing and Water-Based Processing of LiFeMnPO4 Secondary Agglomerates and Its Effects on Electrochemical Characteristics
Abstract
:1. Introduction
2. Experimental
2.1. Sample Ageing and Cell Preparation
2.2. Electrochemical Characterization
2.3. Analytical Methods
3. Results
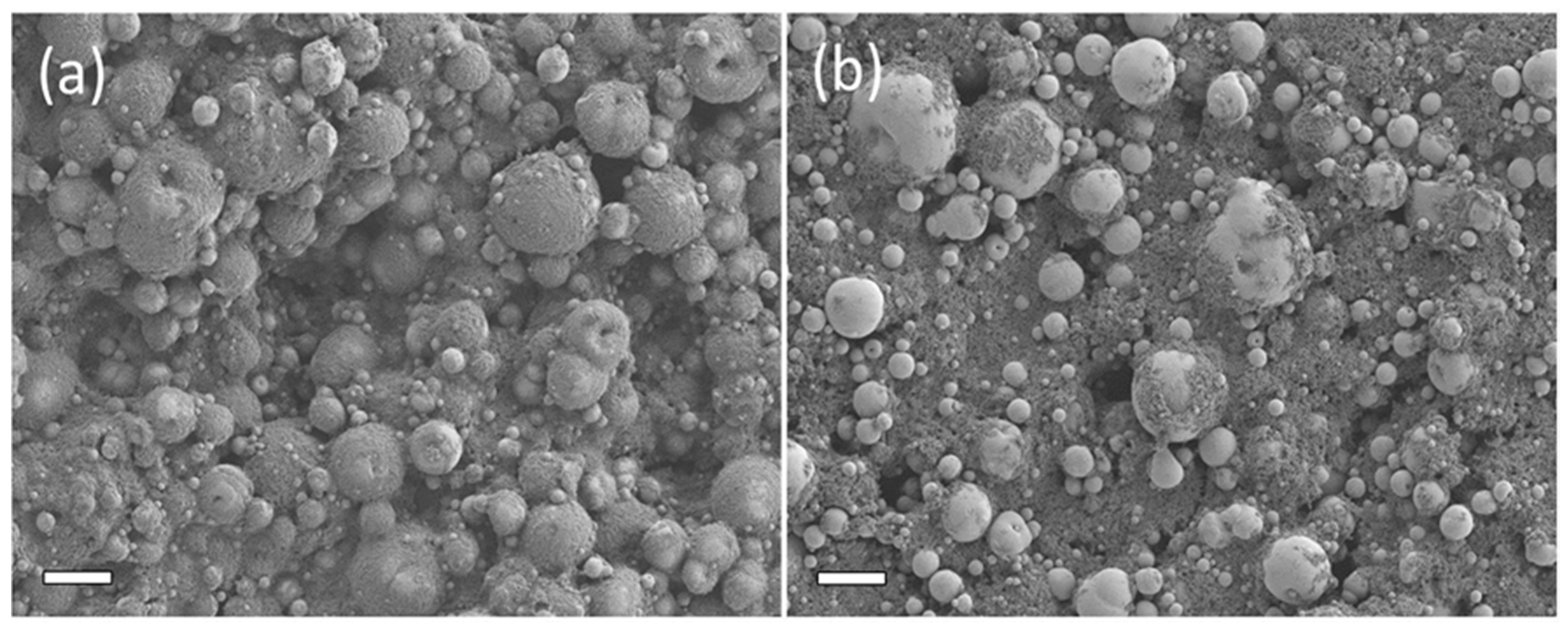
3.1. Powder and Cathode Characterization
3.2. Electrochemical Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Padhi, A.K. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Takahashi, M.; Ohtsuka, H.; Akuto, K.; Sakurai, Y. Confirmation of Long-Term Cyclability and High Thermal Stability of LiFePO4 in Prismatic Lithium-Ion Cells. J. Electrochem. Soc. 2005, 152, A899–A904. [Google Scholar] [CrossRef]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Molenda, J.; Ojczyk, W.; Swierczek, K.; Zajac, W.; Krok, F.; Dygas, J.; Liu, R. Diffusional mechanism of deintercalation in LiFe1−yMnyPO4 cathode material. Solid State Ion. 2006, 177, 2617–2624. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Ancharov, A.I.; Markov, A.V.; Karnaushenko, D.D.; Makukha, V.K. Structural studies of nanosized LiFe0.5Mn0.5PO4 under cycling by in situ synchrotron diffraction. Solid State Ion. 2012, 225, 564–569. [Google Scholar] [CrossRef]
- Li, G.; Kudo, Y.; Liu, K.-Y.; Azuma, H.; Tohda, M. X-ray Absorption Study of LixMnyFe1−yPO4 (0 ≤ x ≤ 1, 0 < y ≤ 1). J. Electrochem. Soc. 2002, 149, A1414–A1418. [Google Scholar] [CrossRef]
- Bramnik, N.N.; Bramnik, K.G.; Nikolowski, K.; Hinterstein, M.; Baehtz, C.; Ehrenberg, H. Synchrotron Diffraction Study of Lithium Extraction from LiMn0.6Fe0.4PO4. Electrochem. Solid State Lett. 2005, 8, A379–A381. [Google Scholar] [CrossRef]
- Nam, K.-W.; Yoon, W.-S.; Zaghib, K.; Yoon Chung, K.; Yang, X.-Q. The phase transition behaviors of Li1−xMn0.5Fe0.5PO4 during lithium extraction studied by in situ X-ray absorption and diffraction techniques. Electrochem. Commun. 2009, 11, 2023–2026. [Google Scholar] [CrossRef]
- Porcher, W.; Moreau, P.; Lestriez, B.; Jouanneau, S.; Le Cras, F.; Guyomard, D. Stability of LiFePO4 in water and consequence on the Li battery behaviour. Ionics 2008, 14, 583–587. [Google Scholar] [CrossRef]
- Cuisinier, M.; Martin, J.-F.; Dupré, N.; Yamada, A.; Kanno, R.; Guyomard, D. Moisture driven aging mechanism of LiFePO4 subjected to air exposure. Electrochem. Commun. 2010, 12, 238–241. [Google Scholar] [CrossRef]
- Martin, J.F.; Yamada, A.; Kobayashi, G.; Nishimura, S.-I.; Kanno, R.; Guyomard, D.; Dupré, N. Air Exposure Effect on LiFePO4. Electrochem. Solid State Lett. 2008, 11, A12–A16. [Google Scholar] [CrossRef]
- Nicholas, P.W.; Liu, Z.; Lu, P.; Olson, K.L.; Moote, J.; Powell, B.R.; Kim, J.-H. Understanding Transition-Metal Dissolution Behavior in LiNi0.5Mn1.5O4 High-Voltage Spinel for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 15947–15957. [Google Scholar] [CrossRef]
- Saulnier, M.; Auclair, A.; Liang, G.; Schougaard, S.B. Manganese dissolution in lithium-ion positive electrode materials. Solid State Ion. 2016, 294, 1–5. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Räsänen, S.; Jokinen, M.; Yliniemi, K.; Worsley, D.A.; Kuusivaara, J.; Juurikivi, J.; Ekqvist, R.; Kallio, T.; Karppinen, M. Water soluble binder for fabrication of Li4Ti5O12 electrodes. J. Power Sources 2013, 226, 134–139. [Google Scholar] [CrossRef]
- Carnerup, M.A.; Saillenfait, A.M.; Jönsson, B.A.G. Concentrations of N-methyl-2-pyrrolidone (NMP) and its metabolites in plasma and urine following oral administration of NMP to rats. Food Chem. Toxicol. 2005, 43, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Engineering. Frontiers of Engineering; National Academy of Engineering: Washington, DC, USA, 2015; Volume 45, pp. 21–24. [Google Scholar]
- Ekstrom, H.; Lindbergh, G. A Model for Predicting Capacity Fade due to SEI Formation in a Commercial Graphite/LiFePO4 Cell. J. Electrochem. Soc. 2015, 162, A1003–A1007. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liu, Z.; Leung, K.; Chen, L.-Q.; Lu, P.; Qi, Y. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Snyder, C.; Apblett, C.; Grillet, A.; Beechem, T.; Duquette, D. Measuring Li+ Inventory Losses in LiCoO2/Graphite Cells Using Raman Microscopy. J. Electrochem. Soc. 2016, 163, A1036–A1041. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Hoelzel, M.; Senyshyn, A.; Juenke, N.; Boysen, H.; Schmahl, W.; Fuess, H. High-resolution neutron powder diffractometer SPODI at research reactor FRM II. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2012, 667, 32–37. [Google Scholar] [CrossRef]
- Hoelzel, M.; Senyshyn, A.; Dolotko, O. SPODI: High resolution powder diffractometer. JLSRF 2015, 1. [Google Scholar] [CrossRef]
- Gilles, R.; Hoelzel, M.; Schlapp, M.; Elf, F.; Krimmer, B.; Boysen, H.; Fuess, H. First test measurements at the new structure powder diffractometer (SPODI) at the FRM-II. Z. Kristallogr. Suppl. 2006, 2006, 183–188. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye–Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Finger, L.W.; Cox, D.E.; Jephcoat, A.P. A correction for powder diffraction peak asymmetry due to axial divergence. J. Appl. Crystallogr. 1994, 27, 892–900. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Kaduk, J.A.; Partenheimer, W. Chemical accuracy and precision in Rietveld analysis: The crystal structure of cobalt(II) acetate tetrahydrate. Powder Diffr. 1997, 12, 27–39. [Google Scholar] [CrossRef]
- Schwarzenbach, D.; Abrahams, S.C.; Flack, H.D.; Gonschorek, W.; Hahn, T.; Huml, K.; Marsh, R.E.; Prince, E.; Robertson, B.E.; Rollett, J.S.; et al. Statistical descriptors in crystallography: Report of the IUCr Subcommittee on Statistical Descriptors. Acta Crystallogr. Sect. A Found. Adv. 1989, 45, 63–75. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Accuracy of crystal structure error estimates. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1986, 42, 112–120. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Dominko, R.; Gaberscek, M.; Drofenik, J.; Bele, M.; Pejovnik, S.; Jamnik, J. The role of carbon black distribution in cathodes for Li ion batteries. J. Power Sources 2003, 119–121, 770–773. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Tang, Y.; Li, R.; Liang, G.; Sham, T.-K.; Sun, X. Surface aging at olivine LiFePO4: A direct visual observation of iron dissolution and the protection role of nano-carbon coating. J. Mater. Chem. A 2013, 1, 1579–1586. [Google Scholar] [CrossRef]
- Zaghib, K.; Dontigny, M.; Charest, P.; Labrecque, J.F.; Guerfi, A.; Kopec, M.; Mauger, A.; Gendron, F.; Julien, C.M. Aging of LiFePO4 upon exposure to H2O. J. Power Sources 2008, 185, 698–710. [Google Scholar] [CrossRef]
- Starke, B.; Seidlmayer, S.; Jankowsky, S.; Dolotko, O.; Gilles, R.; Pettinger, K.-H. Influence of Particle Morphologies of LiFePO4 on Water- and Solvent-Based Processing and Electrochemical Properties. Sustainability 2017, 9, 888. [Google Scholar] [CrossRef]
- Burdock, G.A. Encyclopedia of Food and Color Additives; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Reardon, S. Food preservatives linked to obesity and gut disease. Nature 2015. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, Y.-K. Microstructural analysis of poly(vinylidene fluoride) using benzene derivative pyrolysis products. J. Anal. Appl. Pyrolysis 2012, 96, 16–23. [Google Scholar] [CrossRef]







| Element Ratio | ||||||
|---|---|---|---|---|---|---|
| Method | P | Li | Fe | Mn | O | C |
| ICP-OES | 1.00 | 2.65 | 0.11 | 0.22 | - | - |
| EDX | 1.00 | - | 0.09 | 0.19 | 3.88 | 0.37 |
| Parameter | Pristine | 23 °C/35% rh | 40 °C/100% rh |
|---|---|---|---|
| a in (Å) | 10.4113(1) | 10.4125(1) | 10.4126(1) |
| b in (Å) | 6.0707(1) | 6.0711(1) | 6.0709(1) |
| c in (Å) | 4.7298(1) | 4.7306(1) | 4.7311(1) |
| V in (Å3) | 298.940(2) | 299.050(2) | 299.072(3) |
| Solvent | ||
|---|---|---|
| Parameter | NMP | H2O |
| a in (Å) | 10.4119(3) | 10.4092(3) |
| b in (Å) | 6.0705(1) | 6.0689(1) |
| c in (Å) | 4.7306(1) | 4.7293(1) |
| V in (Å3) | 299.00(1) | 298.76(1) |
| Relative Capacity and ICL/% | ||||
|---|---|---|---|---|
| Solvent | Storage Condition | Charging | Discharging | ICL |
| NMP | Pristine | 99.3 ± 0.3 | 81.3 ± 1.8 | 18.1 ± 1.4 |
| 23 °C/35% rh | 98.0 ± 0.6 | 81.9 ± 1.1 | 16.4 ± 0.9 | |
| 40 °C/100% rh | 100.0 ± 1.1 | 81.2 ± 2.7 | 18.8 ± 1.6 | |
| Average of all cells | 99.1 ± 1.2 | 81.4 ± 1.5 | 17.7 ± 1.8 | |
| H2O | Pristine | 97.6 ± 1.3 | 80.6 ± 2.8 | 17.4 ± 1.1 |
| 23 °C/35% rh | 98.5 ± 3.1 | 81.3 ± 2.6 | 17.5 ± 3.0 | |
| 40 °C/100% rh | 98.2 ± 1.1 | 82.7 ± 1.5 | 15.7 ± 0.3 | |
| Average of all cells | 98.1 ± 1.9 | 81.5 ± 2.1 | 16.6 ± 1.8 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starke, B.; Seidlmayer, S.; Dolotko, O.; Gilles, R.; Pettinger, K.-H. Ageing and Water-Based Processing of LiFeMnPO4 Secondary Agglomerates and Its Effects on Electrochemical Characteristics. Energies 2017, 10, 2135. https://doi.org/10.3390/en10122135
Starke B, Seidlmayer S, Dolotko O, Gilles R, Pettinger K-H. Ageing and Water-Based Processing of LiFeMnPO4 Secondary Agglomerates and Its Effects on Electrochemical Characteristics. Energies. 2017; 10(12):2135. https://doi.org/10.3390/en10122135
Chicago/Turabian StyleStarke, Benjamin, Stefan Seidlmayer, Oleksandr Dolotko, Ralph Gilles, and Karl-Heinz Pettinger. 2017. "Ageing and Water-Based Processing of LiFeMnPO4 Secondary Agglomerates and Its Effects on Electrochemical Characteristics" Energies 10, no. 12: 2135. https://doi.org/10.3390/en10122135





