Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis
Abstract
:1. Introduction
2. Methods
2.1. Experimental Data and Their Interpolation
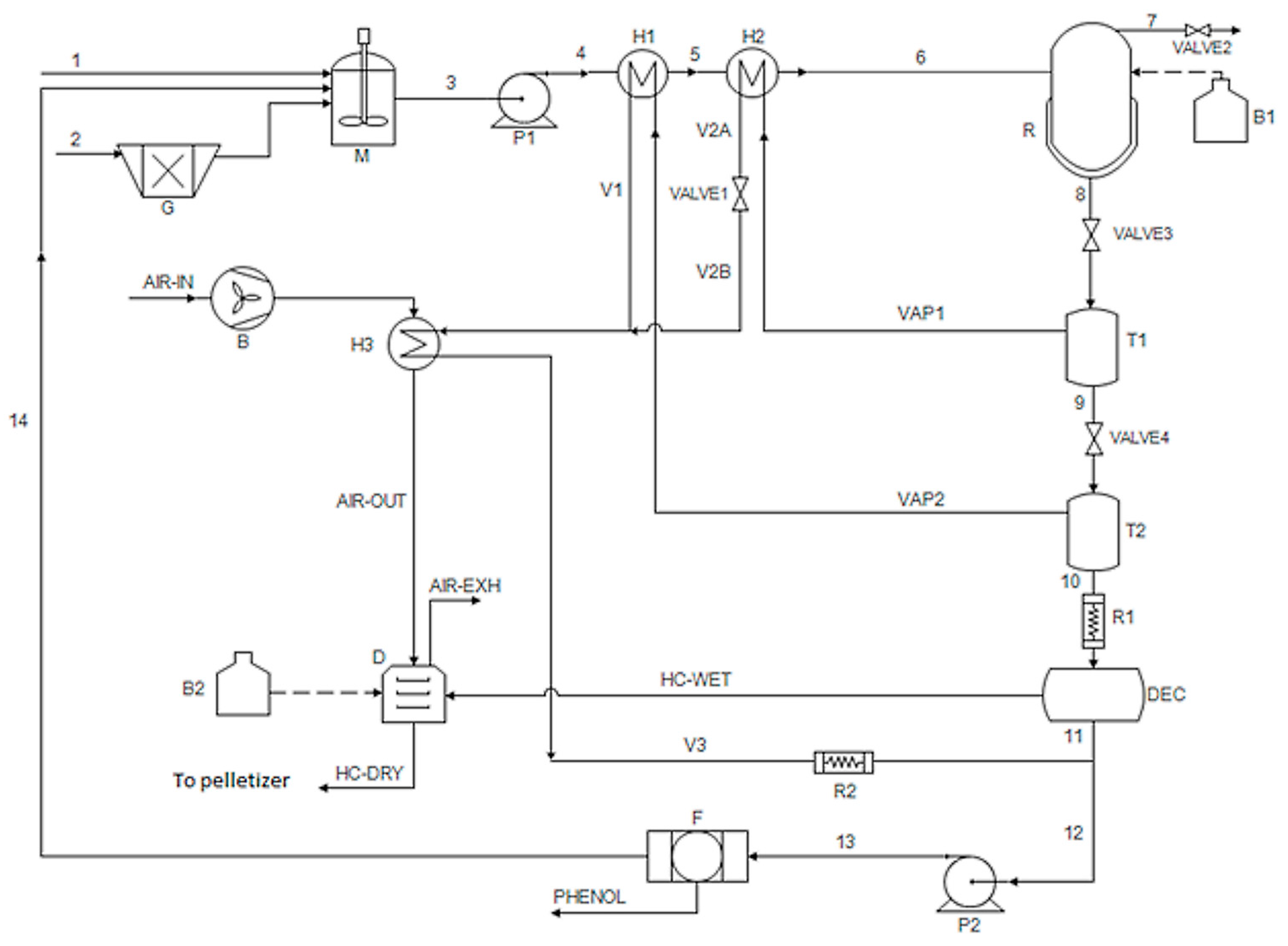
2.2. Conceptual Process Design
2.3. Process Model and Simulations
2.4. Efficiency Parameters: Thermal Efficiency and Plant Efficiency
3. Results and Discussion
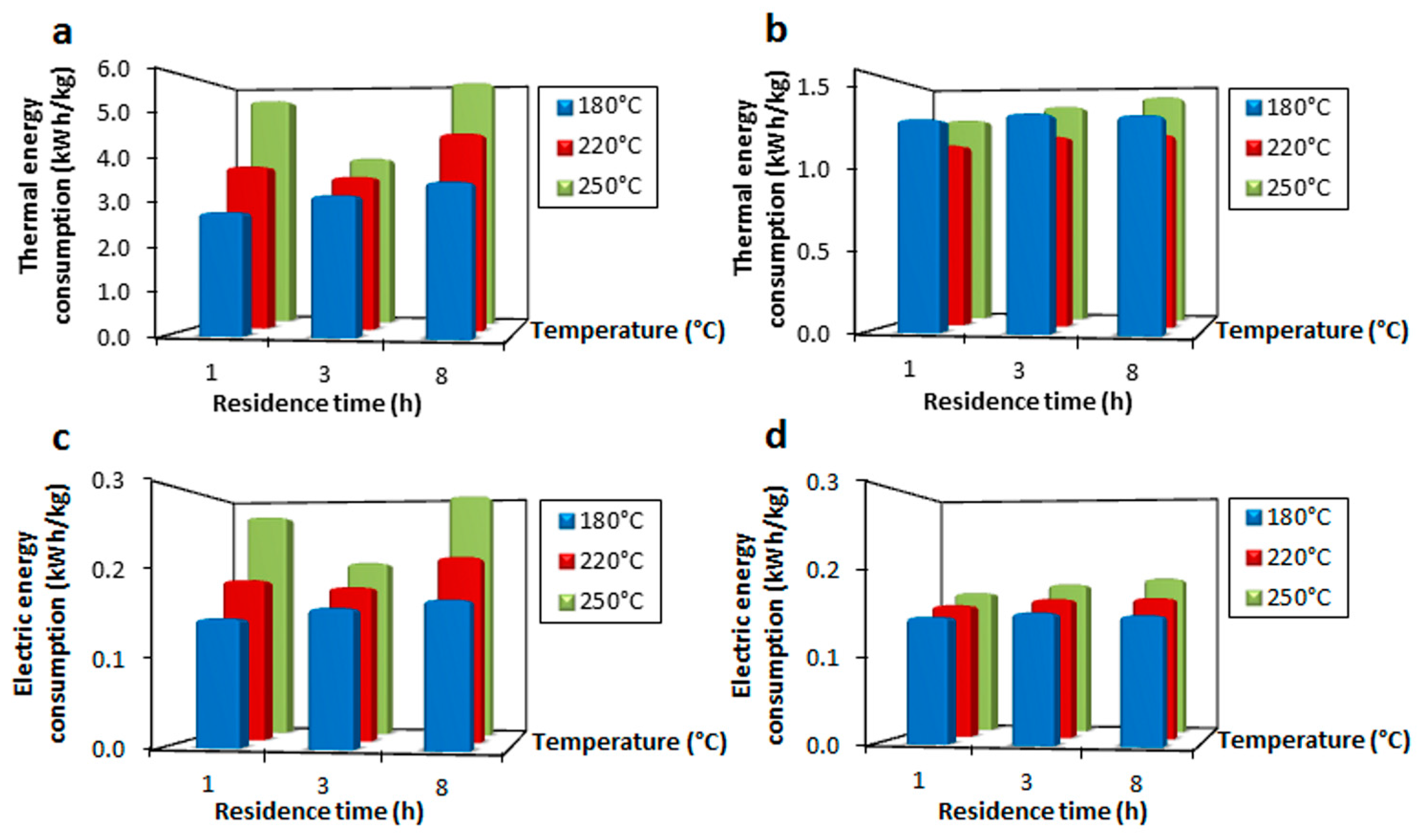
3.1. Electric Energy Consumption
3.2. Thermal Energy Consumption
3.3. Specific Energy Consumption
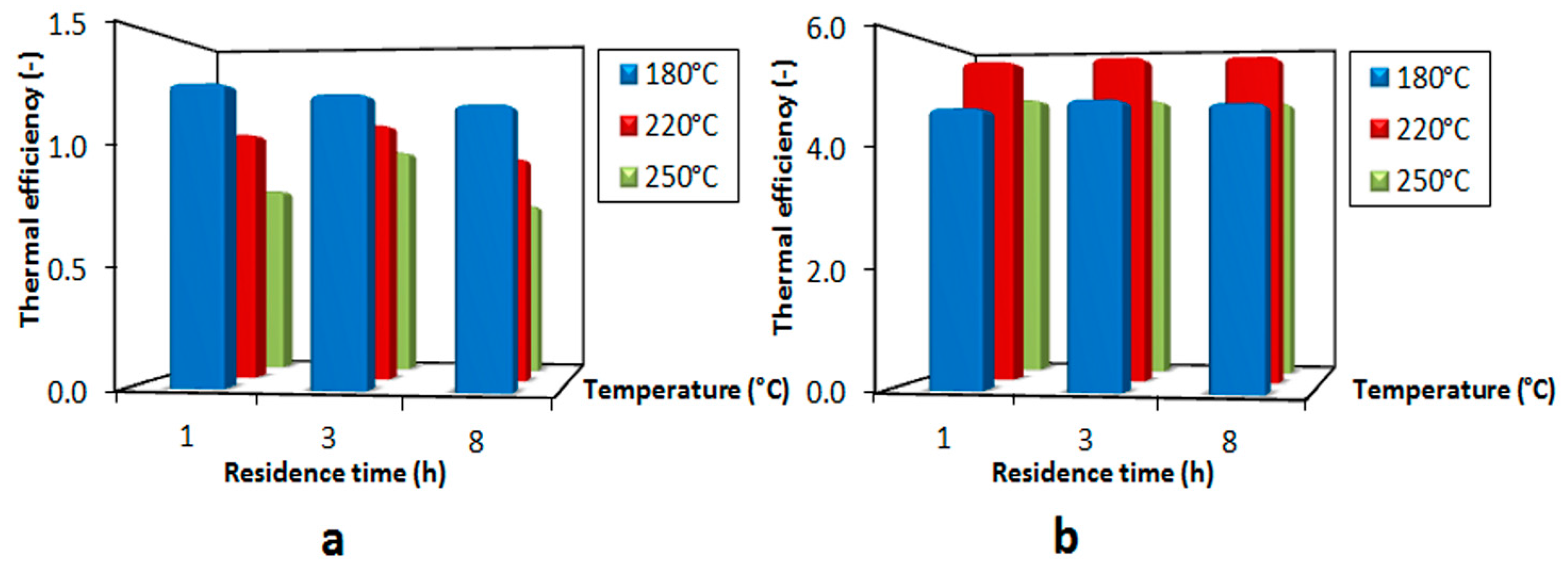
3.4. Thermal Efficiency
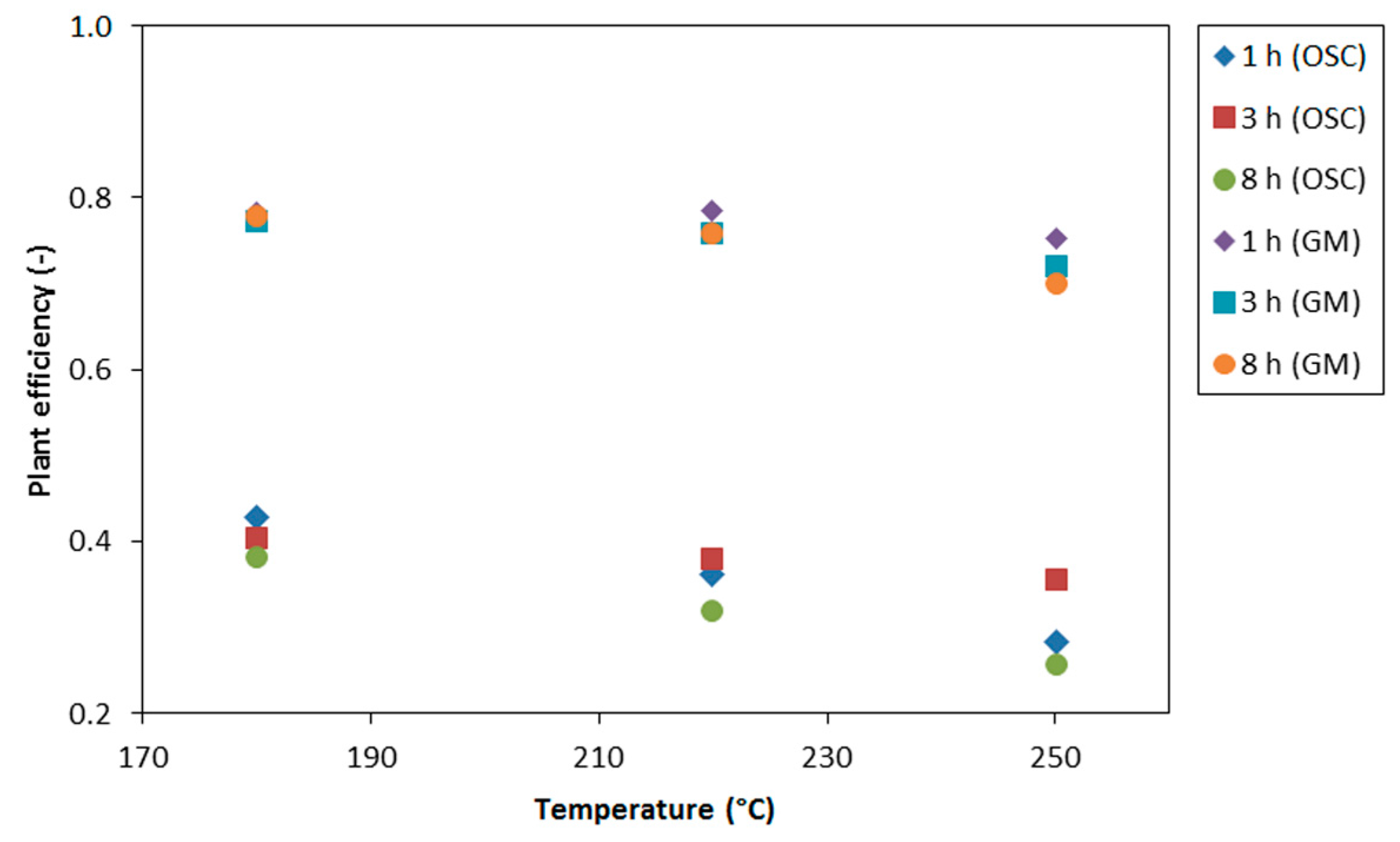
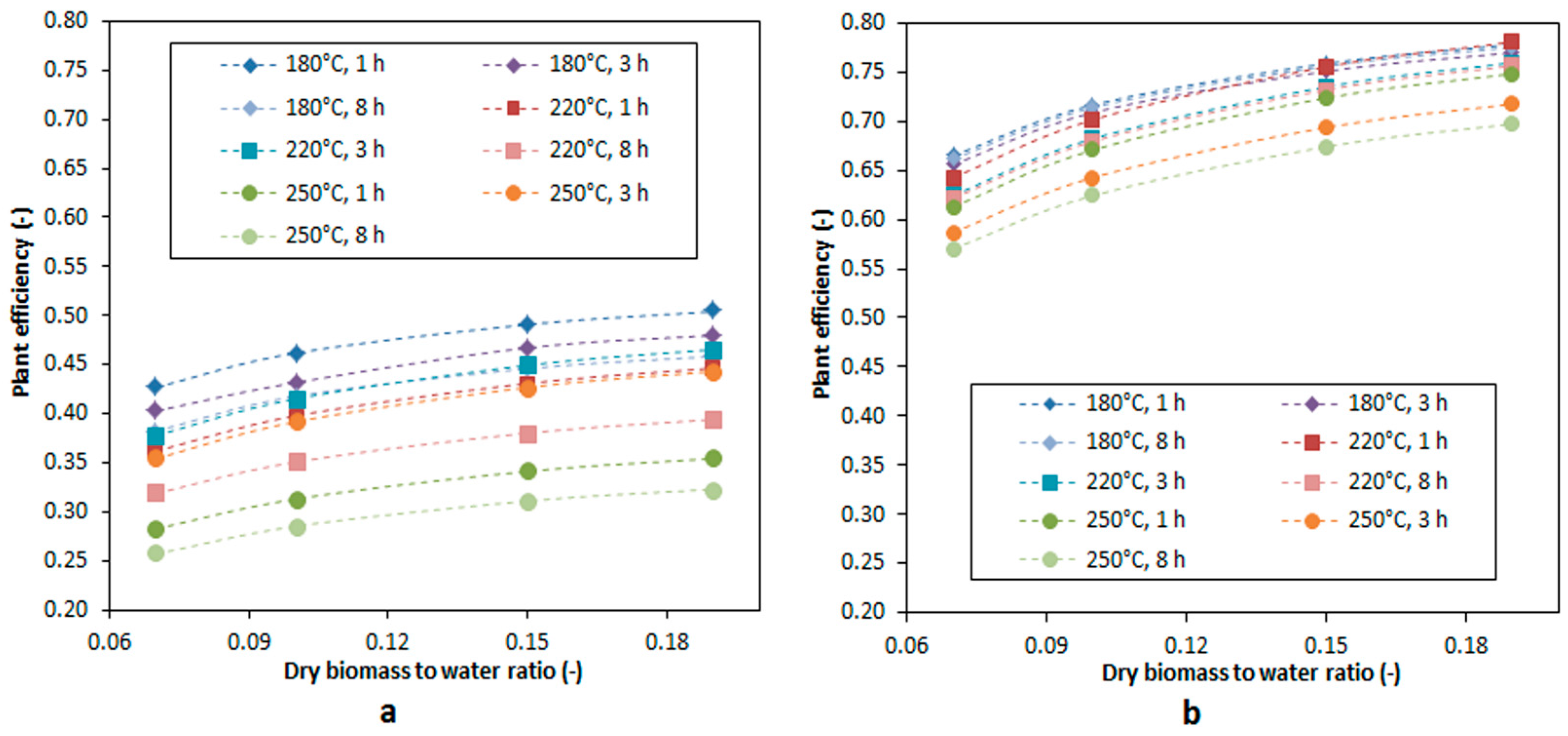
3.5. Plant Efficiency
3.6. Economic Feasibility
3.6.1. Investment Costs
3.6.2. Total Production Costs
3.6.3. Cost Linked to the Capital and Selling Price of HTC Coal
4. Conclusions
- a plant efficiency of 78% was achieved;
- specific thermal energy consumption was equal to 1.17 kWh/kghydrochar (0.31 kWh/kgfeedstock);
- specific electric energy consumption was equal to 0.16 kWh/kghydrochar (0.04 kWh/kgfeedstock);
- the production cost of pelletized hydrochar was equal to 157 €/tonhydrochar;
- the hydrochar break-even value for a plant repayment period of 10 years was equal to 200 €/tonhydrochar, competitive with the price of wood pellets (150–200 €/tonwood).
Supplementary Materials
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Grinder
Appendix A.2. Agitator
Appendix A.3. Pumps
Appendix A.4. Heat Exchangers
Appendix A.5. Reactor
Appendix A.6. Burners
Appendix A.7. Flash Separators
Appendix A.8. Decanter
Appendix A.9. Dryer and Air Blower
Appendix A.10. Pelletizer
References
- Afolabi, O.O.D.; Sohail, M.; Thomas, C.P.L. Microwave Hydrothermal Carbonization of Human Biowastes. Waste Biomass Valoriz. 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Lu, X.; Berge, N.D. Influence of feedstock chemical composition on product formation and characteristics derived from the hydrothermal carbonization of mixed feedstocks. Bioresour. Technol. 2014, 166, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A. Upgrading of Olive Tree Trimmings Residue as Biofuel by Hydrothermal Carbonization and Torrefaction: A Comparative Study. Chem. Eng. Trans. 2016, 50, 13–18. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Volpe, R.; Messineo, A.; Millan, M.; Volpe, M.; Kandiyoti, R. Assessment of olive wastes as energy source: Pyrolysis, torrefaction and the key role of H loss in thermal breakdown. Energy 2015, 82, 119–127. [Google Scholar] [CrossRef]
- Volpe, M.; Panno, D.; Volpe, R.; Messineo, A. Upgrade of citrus waste as a biofuel via slow pyrolysis. J. Anal. Appl. Pyrolysis 2015, 115, 66–76. [Google Scholar] [CrossRef]
- Messineo, A.; Volpe, R.; Asdrubali, F. Evaluation of net energy obtainable from combustion of stabilised olive mill by-products. Energies 2012, 5, 1384–1397. [Google Scholar] [CrossRef]
- Gamgoum, R.; Dutta, A.; Santos, R.M.; Chiang, Y.W. Hydrothermal Conversion of Neutral Sulfite Semi-Chemical Red Liquor into Hydrochar. Energies 2016, 9, 1–18. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Castello, D.; Kruse, A.; Fiori, L. Supercritical water gasification of hydrochar. Chem. Eng. Res. Des. 2014, 92, 1864–1875. [Google Scholar] [CrossRef]
- Titirici, M.-M. Sustainable Carbon Materials from Hydrothermal Processes, 1st ed.; John Wiley & Sons: West Sussex, UK, 2013. [Google Scholar]
- Wang, L.; Zhang, Z.; Qu, Y.; Guo, Y.; Wang, Z.; Wang, X. A novel route for preparation of high-performance porous carbons from hydrochars by KOH activation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 183–187. [Google Scholar] [CrossRef]
- Unur, E.; Brutti, S.; Panero, S.; Scrosati, B. Nanoporous carbons from hydrothermally treated biomass as anode materials for lithium ion batteries. Microporous Mesoporous Mater. 2013, 174, 25–33. [Google Scholar] [CrossRef]
- Hitzl, M.; Corma, A.; Pomares, F.; Renz, M. The hydrothermal carbonization (HTC) plant as a decentral biorefinery for wet biomass. Catal. Today 2015, 257, 154–159. [Google Scholar] [CrossRef]
- Stemann, J.; Erlach, B.; Ziegler, F. Hydrothermal carbonisation of empty palm oil fruit bunches: Laboratory trials, plant simulation, carbon avoidance, and economic feasibility. Waste Biomass Valoriz. 2013, 4, 441–454. [Google Scholar] [CrossRef]
- Stemann, J.; Ziegler, F. Assessment of the Energetic Efficiency of A Continuously Operating Plant for Hydrothermal Carbonisation of Biomass. In World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011; Linköping University Electronic Press: Linköping, Sweden, 2011; pp. 125–132. [Google Scholar]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Purcell, R.; Zielinska, B.; Felix, L. Process Development Unit (PDU) for Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Waste Biomass Valoriz. 2014, 5, 669–678. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of Winemaking By-Products. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Fiori, L.; Florio, L. Gasification and Combustion of Grape Marc: Comparison among Different Scenarios. Waste Biomass Valoriz. 2010, 1, 191–200. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Castello, D.; Kruse, A.; Fiori, L. Biomass gasification in supercritical and subcritical water: The effect of the reactor material. Chem. Eng. J. 2013, 228, 535–544. [Google Scholar] [CrossRef]
- Seider, W.D.; Seader, J.D.; Lewin, D.R. Product and Process Design Principles: Synthesis, Analysis and Design, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2004. [Google Scholar]
- CEPCI, 2015: Economic Indicators. Available online: http://www.chemengonline.com/economic-indicators-cepci/ (accessed on 15 May 2016).
- Autorità per L’energia Elettrica il Gas e il Sistema Idrico: Electrical Energy Prices. Available online: http://www.autorita.energia.it/it/dati/eepcfr2.htm (accessed on 16 May 2016).
- Autorità per L’energia Elettrica il Gas e il Sistema Idrico: Natural Gas Prices. Available online: http://www.autorita.energia.it/it/dati/gpcfr2.htm (accessed on 16 May 2016).
- Provincia Autonoma di Trento. Disciplinare Contenente le Prescrizioni per il Conferimento e Trattamento Presso gli Impianti di Depurazione di Proprietà della Provincia Autonoma di Trento dei Reflui di cui All’art. 95 Comma 5 del t.u.l.p. in Materia di Tutela Dell’ambiente Dagli Inquinamenti. Available online: http://www.appalti.provincia.tn.it/binary.php/pat_pi_bandi/bandi/X02_Allegato_A.1233076172.pdf (accessed on 23 December 2016).
- EU Commission, 2015: Access to Finance: Loans. Available online: http://ec.europa.eu/growth/access-to-finance/data-surveys/index_en.htm (accessed on 14 June 2016).
- European Biomass Industry Association: European Market. Available online: http://www.eubia.org/index.php/about-biomass/biomass-pelleting/economics-applications-and-standards (accessed on 1 May 2016).
- Ingelia Italia Srl. Available online: http://www.ingelia.it/ (accessed on 1 May 2016).
- Global Energy Statistics: Coal Prices. Available online: https://knoema.com/xfakeuc/coal-prices-long-term-forecast-to-2020-data-and-charts (accessed on 1 May 2016).
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 7th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Furukawa, H.; Kato, Y.; Inoue, Y.; Kato, T.; Tada, Y.; Hashimoto, S. Correlation of Power Consumption for Several Kinds of Mixing Impellers. Int. J. Chem. Eng. 2012, 2012, 106496. [Google Scholar] [CrossRef]
- White, F. Fluid Mechanics, 7th ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- NIST 2016. Antoine Equation Parameters. Available online: http://webbook.nist.gov/chemistry (accessed on 15 March 2016).
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Sinnott, R.K. Chemical Engineering Design, 4th ed.; Coulson & Richardson: Oxford, UK, 2005. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Coker, A.K. (Ed.) Chilton: Physical properties of liquids and gases. In Ludwig’s Applied Process Design for Chemical and Petrochemicals Plants, 4th ed.; Elsevier: Burlington, VT, USA, 2007.
- Harada, T.; Hata, T.; Ishihara, S. Thermal constants of wood during the heating process measured with the laser flash method. J. Wood Sci. 1998, 44, 425–431. [Google Scholar] [CrossRef]
- DOW Chemical Company. Heat Transfer Fluids. Available online: http://www.dow.com/heattrans (accessed on 1 March 2016).
- Korus, R. Anaerobic Processes for the Treatment of Food Processing Wastes. In Food Biotechnology, 2nd ed.; Pometto, A., Shetty, K., Paliyath, G., Levin, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 2005; p. 1885. [Google Scholar]
- Rak, A. Energy Efficiency in Mechanical Separation. Available online: http://www.sawea.org/ (accessed on 15 February 2016).
- Obernberger, I.; Thek, G. The Pellet Handbook: The Production and Thermal Utilisation of Pellets, 1st ed.; Earthscan: London, UK, 2010. [Google Scholar]

| Process Parameters | OSC | GM |
|---|---|---|
| Biomass as received (ton/y) | 20,000 | 20,000 |
| DB = Biomass db (ton/y) | 14,000 | 7000 |
| Water added (ton/y) | 200,000 | 23,840 |
| W = Total water (ton/y) | 206,000 | 36,840 |
| Total flow rate (ton/y) | 220,000 | 43,840 |
| DB/W (-) | 0.07 | 0.19 |
| Biomass moisture content (%) | 30 | 65 |
| Biomass | Temperature (°C) | Time (h) | Electric Power (kW) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grinder | Mixer | Pump P1 | Decanter | Pump P2 | Blower | Pelletizer | TOT 1 | |||
| OSC | 1 | 25.60 | 9.48 | 40.99 | 19.16 | 3.62 | 30.68 26.23 23.54 | 78.82 67.4 60.47 | 229.19 211.73 201.15 | |
| 180 | 3 | |||||||||
| 8 | ||||||||||
| 1 | 25.60 | 9.48 | 61.94 | 17.81 | 3.67 | 21.99 23.87 17.88 | 56.70 61.60 46.10 | 216.91 224.37 200.73 | ||
| 220 | 3 | |||||||||
| 8 | ||||||||||
| 1 | 25.60 | 9.48 | 88.50 | 16.84 | 3.71 | 15.51 22.11 13.66 | 40.10 57.20 35.20 | 219.71 245.78 212.29 | ||
| 250 | 3 | |||||||||
| 8 | ||||||||||
| GM | 1 | 23.91 | 9.29 | 8.63 | 3.18 | 0.66 | 14.43 13.52 14.12 | 37.42 34.94 36.47 | 107.27 103.54 105.89 | |
| 180 | 3 | |||||||||
| 8 | ||||||||||
| 1 | 23.91 | 9.29 | 12.98 | 2.95 | 0.67 | 13.08 12.8 11.97 | 33.52 31.12 30.87 | 106.04 103.09 101.90 | ||
| 220 | 3 | |||||||||
| 8 | ||||||||||
| 1 | 23.91 | 9.29 | 18.68 | 2.79 | 0.67 | 12.12 10.93 10.22 | 31.34 28.24 26.62 | 108.68 103.96 101.40 | ||
| 250 | 3 | |||||||||
| 8 | ||||||||||
| Temperature (°C) | Time (h) | Thermal Power (kW) | |||||
|---|---|---|---|---|---|---|---|
| OSC | GM | ||||||
| Burner B1 | Burner B2 | TOT | Burner B1 | Burner B2 | TOT | ||
| 180 | 1 | 3502.0 | 816.9 | 4318.9 | 445.9 | 513.7 | 959.6 |
| 220 | 3724.2 | 585.4 | 4309.6 | 429.8 | 348.2 | 778.0 | |
| 250 | 3992.3 | 412.9 | 4405.2 | 516.1 | 321.0 | 837.1 | |
| 180 | 3 | 3495.2 | 698.4 | 4193.6 | 444.4 | 473.3 | 917.7 |
| 220 | 3737.8 | 635.7 | 4373.5 | 426.8 | 319.5 | 746.3 | |
| 250 | 4032.4 | 588.8 | 4621.2 | 514.1 | 289.3 | 803.4 | |
| 180 | 8 | 3489.2 | 626.7 | 4115.9 | 446.3 | 497.3 | 943.6 |
| 220 | 3715.4 | 476.1 | 4191.5 | 428.3 | 316.1 | 744.4 | |
| 250 | 3993.4 | 363.8 | 4357.2 | 514.2 | 272.5 | 786.7 | |
| Type of Unit | Cost (€) |
|---|---|
| Heat exchangers (H1, H2, H3) | 32,983 |
| Agitator | 5110 |
| Direct fired heaters | 354,063 |
| Reactor | 436,051 |
| Flash tanks | 145,276 |
| Pumps | 57,237 |
| Centrifuge | 55,028 |
| Crusher | 10,739 |
| Dryer | 131,535 |
| Filter | 1359 |
| Pelletizer | 30,907 |
| Total cost for on-site equipment | 1,260,288 |
| Type of Unit | Cost (€) |
|---|---|
| Total depreciable capital (TDC) | 1,260,288 |
| On-site equipment | 21,526 |
| Utility plants | 230,727 |
| Contractor’s fee and contingencies | 30,251 |
| Land | 30,251 |
| Plant start up | 151,254 |
| Working capital | 79,765 |
| Total capital investment (TCI) | 1,773,811 |
| Operating Costs | Annual Cost (€) |
|---|---|
| Electricity | 90,431 |
| Methane | 243,346 |
| Labor related operations | 160,000 |
| Maintenance | 121,003 |
| Property taxes and insurance | 15,125 |
| General expenses | 76,176 |
| Waste water treatment | 126,903 |
| Total production costs | 832,984 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. https://doi.org/10.3390/en10020211
Lucian M, Fiori L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies. 2017; 10(2):211. https://doi.org/10.3390/en10020211
Chicago/Turabian StyleLucian, Michela, and Luca Fiori. 2017. "Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis" Energies 10, no. 2: 211. https://doi.org/10.3390/en10020211






