High-Titer Methane from Organosolv-Pretreated Spruce and Birch
Abstract
:1. Introduction
2. Results and Discussion
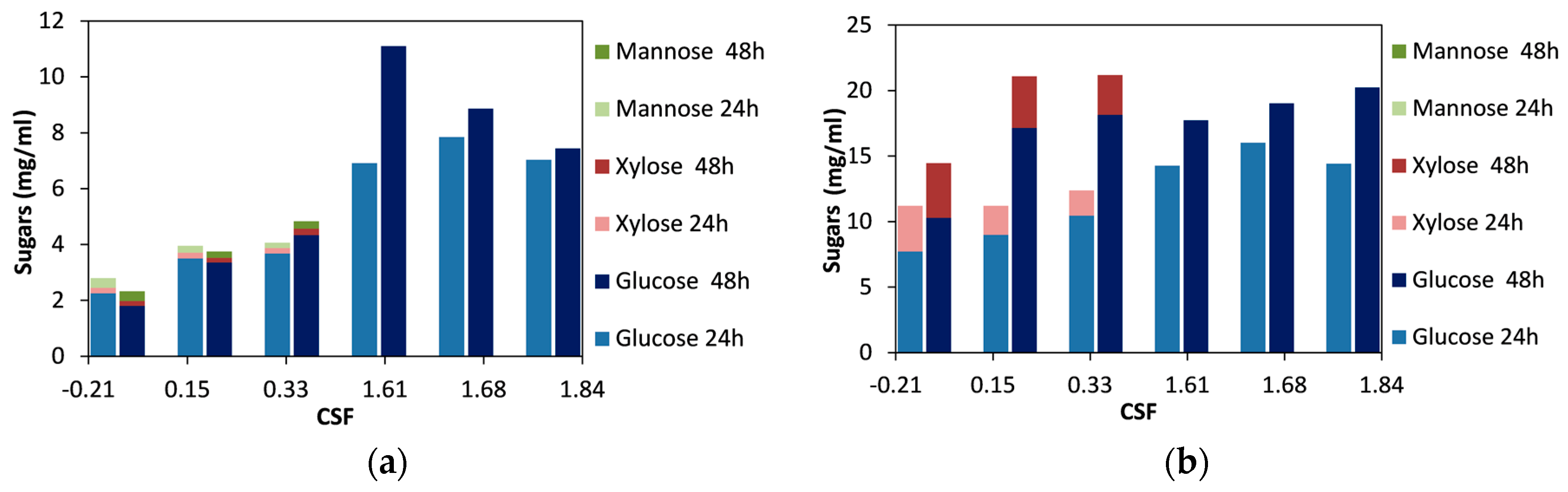
2.1. Effect of Organosolv Pretreatment on Enzymatic Hydrolysis
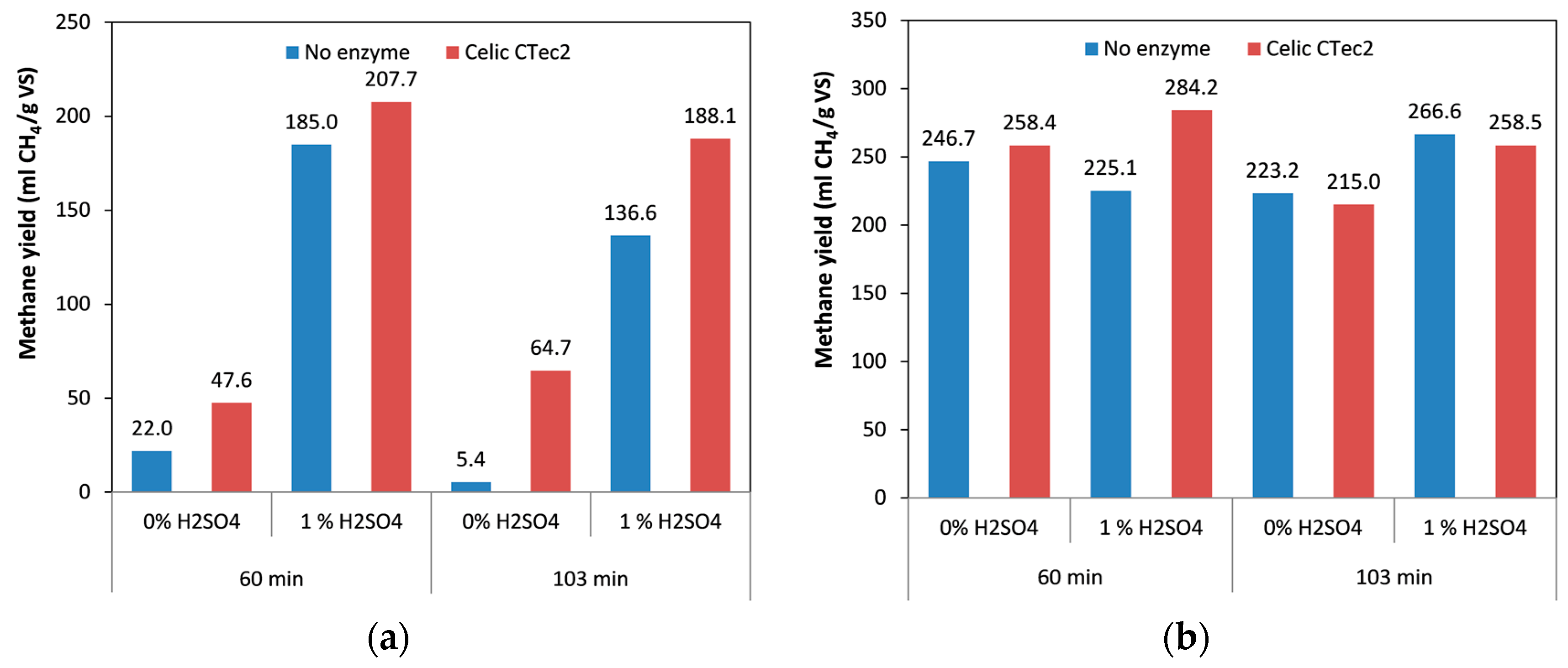
2.2. Effect of Pretreatment Time on Methane Yield
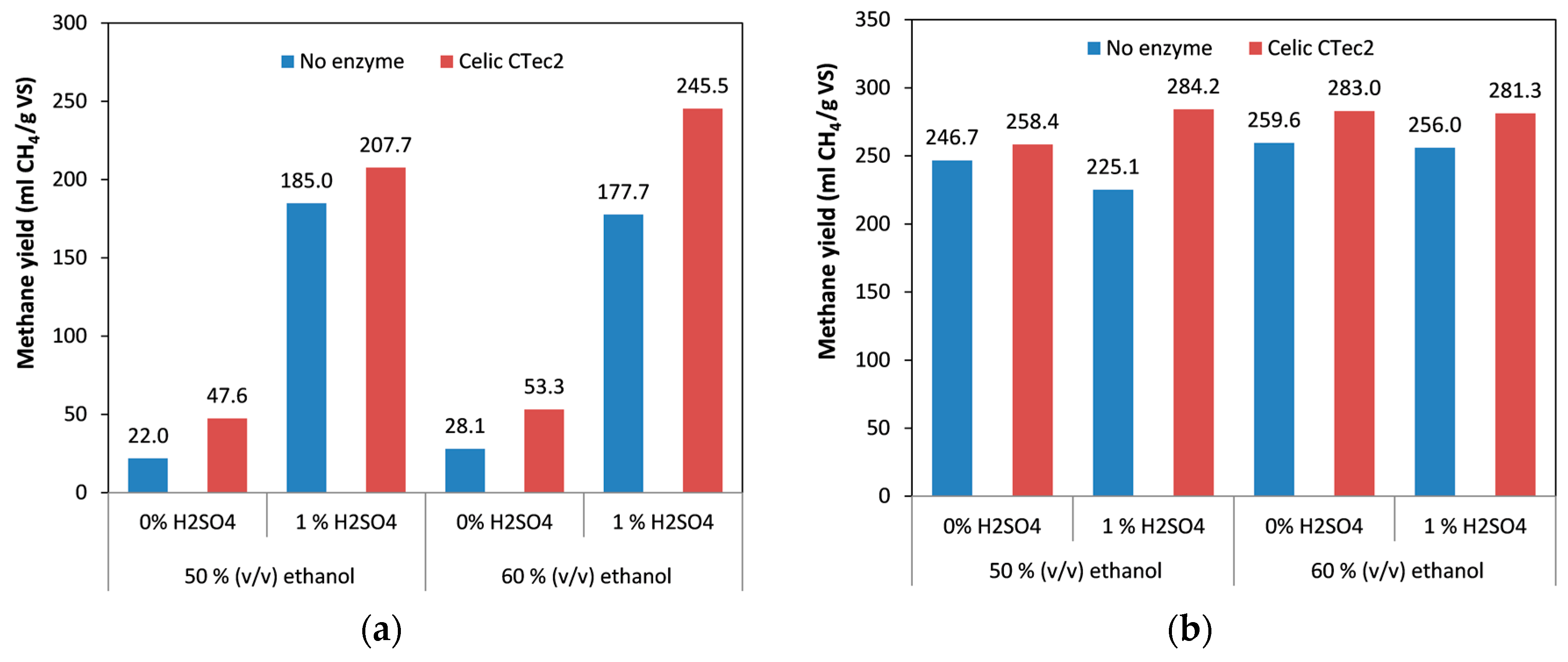
2.3. Effect of Ethanol Content on Methane Yield
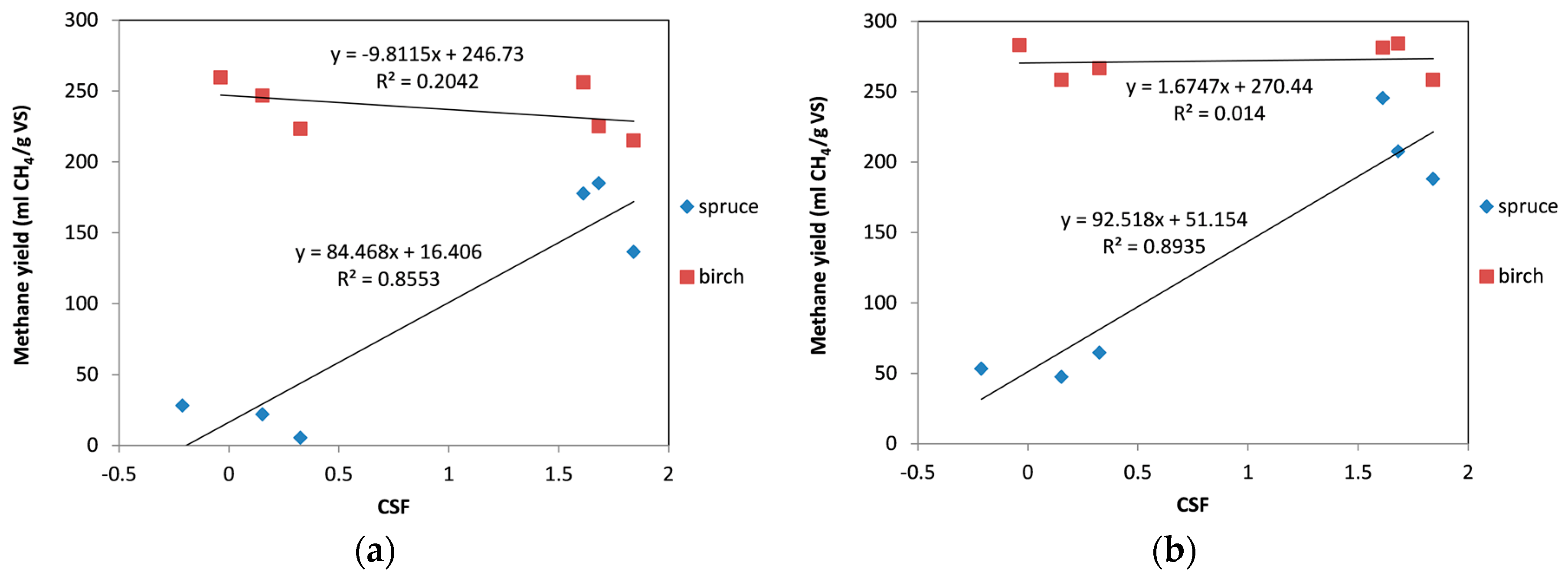
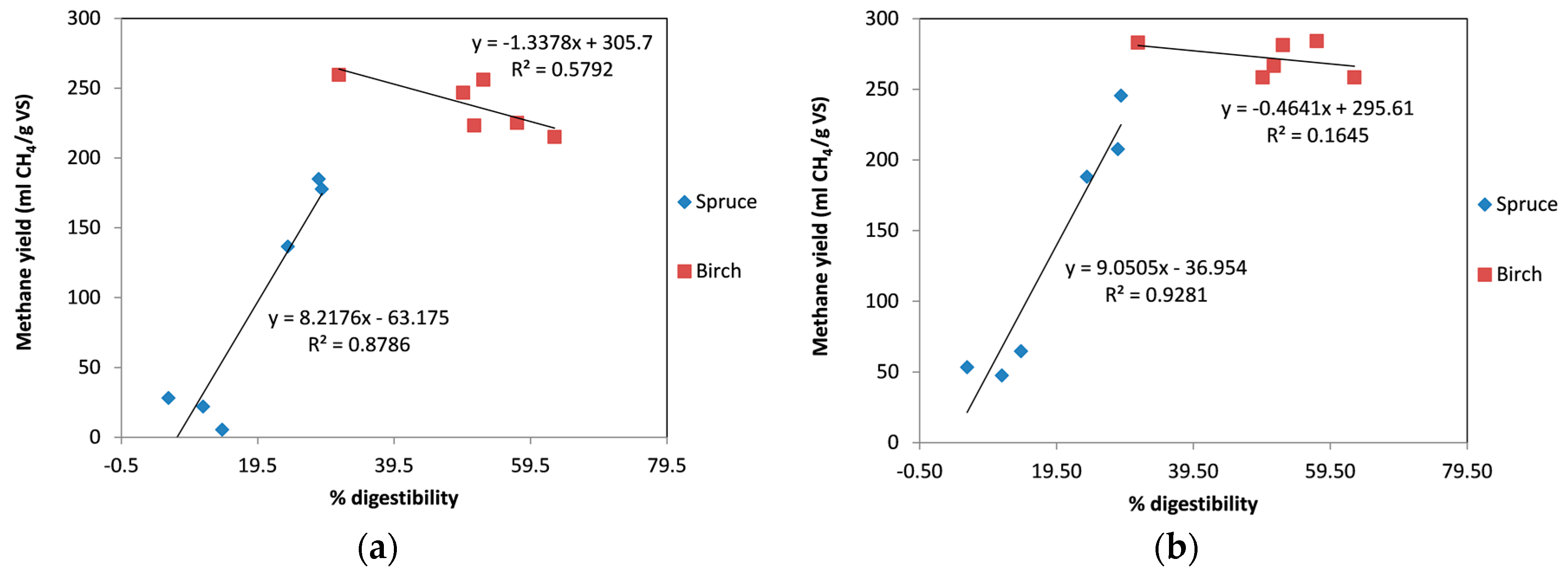
2.4. Correlation between Combined Severity Factor (CSF) and the Methane Yield
3. Materials and Methods
3.1. Feedstock, Inoculum, and Enzymes
3.2. Pretreatment of Forest Material
3.3. Enzymatic Hydrolysis
3.4. Biochemical Methane Potential (BMP) Test
3.5. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Goldemberg, J. The promise of clean energy. Energy Policy 2006, 34, 2185–2190. [Google Scholar] [CrossRef]
- International Energy Agency. Key World Energy Statistics; International Education Association (IEA): Paris, France, 2016. [Google Scholar]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Owen, N.A.; Inderwildi, O.R.; King, D.A. The status of conventional world oil reserves—Hype or cause for concern? Energy Policy 2010, 38, 4743–4749. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Börjesson, P.; Berglund, M. Environmental systems analysis of biogas systems—Part I: Fuel-cycle emissions. Biomass Bioenergy 2006, 30, 469–485. [Google Scholar] [CrossRef]
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Solvent Pretreatments of Lignocellulosic Materials to Enhance Biogas Production: A Review. Energy Fuels 2016, 30, 1892–1903. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Ethanol Production from Enzymatically Treated Dried Food Waste Using Enzymes Produced On-Site. Sustainability 2015, 7, 1446–1458. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J. Fundamentals of Biomass Pretreatment by Fractionation. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 201–222. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Topakas, E.; Christakopoulos, P. Hydrolysis and fermentation for cellulosic ethanol production. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 633–654. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mtui, G. Anaerobic fermentation of woody biomass treated by various methods. Biotechnol. Bioprocess Eng. 2003, 8, 179–182. [Google Scholar] [CrossRef]
- Take, H.; Andou, Y.; Nakamura, Y.; Kobayashi, F.; Kurimoto, Y.; Kuwahara, M. Production of methane gas from Japanese cedar chips pretreated by various delignification methods. Biochem. Eng. J. 2006, 28, 30–35. [Google Scholar] [CrossRef]
- Horn, S.J.; Estevez, M.M.; Nielsen, H.K.; Linjordet, R.; Eijsink, V.G.H. Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour. Technol. 2011, 102, 7932–7936. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.M.; Linjordet, R.; Morken, J. Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour. Technol. 2012, 104, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, B.; Shafiei, M.; Rajaeifar, M.A.; Tabatabaei, M. Biogas and bioethanol production from pinewood pre-treated with steam explosion and N-methylmorpholine-N-oxide (NMMO): A comparative life cycle assessment approach. Energy 2016, 114, 935–950. [Google Scholar] [CrossRef]
- Yoshida, K.; Miyafuji, H.; Saka, S. Methane production from organic acids obtained by supercritical water treatment of Japanese beech. J. Wood Sci. 2010, 56, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Teghammar, A.; Karimi, K.; Sárvári Horváth, I.; Taherzadeh, M.J. Enhanced biogas production from rice straw, triticale straw and softwood spruce by NMMO pretreatment. Biomass Bioenergy 2012, 36, 116–120. [Google Scholar] [CrossRef]
- Kabir, M.M.; Niklasson, C.; Taherzadeh, M.J.; Horváth, I.S. Biogas production from lignocelluloses by N-methylmorpholine-N-oxide (NMMO) pretreatment: Effects of recovery and reuse of NMMO. Bioresour. Technol. 2014, 161, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.M.; Rajendran, K.; Taherzadeh, M.J.; Sárvári Horváth, I. Experimental and economical evaluation of bioconversion of forest residues to biogas using organosolv pretreatment. Bioresour. Technol. 2015, 178, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced solid-state biogas production from lignocellulosic biomass by organosolv pretreatment. Biomed. Res. Int. 2014, 2014, 350414. [Google Scholar] [CrossRef] [PubMed]
- McDonough, T. The chemistry of organosolv delignification. Tappi J. 1993, 76, 186–193. [Google Scholar]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef]
- Eggeman, T.; Elander, R.T. Process and economic analysis of pretreatment technologies. Bioresour. Technol. 2005, 96, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Saito, T. Lignin-derived advanced carbon materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Mamman, A.S.; Lee, J.M.; Kim, Y.C.; Hwang, I.T.; Park, N.J.; Hwang, Y.K.; Chang, J.S.; Hwang, J.S. Furfural: Hemicellulose/xylose-derived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Strategies for Enhanced Biogas Generation through Anaerobic Digestion of Forest Material—An Overview. BioResources 2016, 11, 1–18. [Google Scholar]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Bansal, P.; Realff, M.J.; Bommarius, A.S. SO2-catalyzed steam explosion: The effects of different severity on digestibility, accessibility, and crystallinity of lignocellulosic biomass. Biotechnol. Prog. 2013, 29, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.; Crestini, C.; Rova, U.; Christakopoulos, P. Isolation and Characterization of Organosolv and Alkaline Lignins from Hardwood and Softwood Biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Yu, R.W.; Lam, D.; Saddler, J.N. Pretreatment of lodgepole pine killed by mountain pine beetle using the ethanol organosolv process: Fractionation and process optimization. Ind. Eng. Chem. Res. 2007, 46, 2609–2617. [Google Scholar] [CrossRef]
- Araque, E.; Parra, C.; Freer, J.; Contreras, D.; Rodríguez, J.; Mendonça, R.; Baeza, J. Evaluation of organosolv pretreatment for the conversion of Pinus radiata D. Don to ethanol. Enzym. Microb. Technol. 2008, 43, 214–219. [Google Scholar] [CrossRef]
- Park, N.; Kim, H.-Y.; Koo, B.-W.; Yeo, H.; Choi, I.-G. Organosolv pretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2010, 101, 7046–7053. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Patel, A.K.; Adsul, M. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Ge, X.; Zhang, J. Effects of lignin and surfactant on adsorption and hydrolysis of cellulases on cellulose. Biotechnol. Biofuels 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Rova, U.; Christakopoulos, P. Sequential parametric optimization of methane production from different sources of forest raw material. Front. Microbiol. 2015, 6, 1163. [Google Scholar] [CrossRef] [PubMed]
- Sonakya, V.; Raizada, N.; Kalia, V.C. Microbial and enzymatic improvement of anaerobic digestion of waste biomass. Biotechnol. Lett. 2001, 23, 1463–1466. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Lyberatos, G. Effect of Pretreatment of Sweet Sorghum Biomass on Methane Generation. Waste Biomass Valoriz. 2013, 4, 583–591. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Yu, R.W.; Saddler, J.N. The bioconversion of mountain pine beetle-killed lodgepole pine to fuel ethanol using the organosolv process. Biotechnol. Bioeng. 2008, 101, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Van Heiningen, A.R.P. The swelling of pulp fibers derived from the ethanol-based organosolv process. Tappi J. 1997, 80, 211–213. [Google Scholar]
- Pan, X.; Xie, D.; Kang, K.-Y.; Yoon, S.-L.; Saddler, J.N. Effect of organosolv ethanol pretreatment variables on physical characteristics of hybrid poplar substrates. Appl. Biochem. Biotechnol. 2007, 137–140, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Janzon, R.; Schütt, F.; Oldenburg, S.; Fischer, E.; Körner, I.; Saake, B. Steam pretreatment of spruce forest residues: Optimal conditions for biogas production and enzymatic hydrolysis. Carbohydr. Polym. 2014, 100, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, L.; Yuan, K.; Huang, H.; Yan, Z. Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour. Technol. 2013, 150, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, K.; Kabir, M.M.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M. Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. BioResources 2010, 5, 928–938. [Google Scholar]
- Mohsenzadeh, A.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J. Chem. Technol. Biotechnol. 2012, 87, 1209–1214. [Google Scholar] [CrossRef]
- Goshadrou, A.; Karimi, K.; Taherzadeh, M.J. Ethanol and biogas production from birch by NMMO pretreatment. Biomass Bioenergy 2013, 49, 95–101. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]

| Feedstock | Pretreatment | VS (% w/w) | TS (% w/w) |
|---|---|---|---|
| Spruce | 60 min, 50% EtOH | 93.15 | 93.25 |
| 60 min, 50% EtOH, 1% H2SO4 | 97.92 | 98.02 | |
| 60 min, 60% EtOH, | 97.09 | 97.23 | |
| 60 min, 60% EtOH, 1% H2SO4 | 97.68 | 97.70 | |
| 103 min, 50% EtOH | 97.25 | 97.42 | |
| 103 min 50% EtOH, 1% H2SO4 | 97.75 | 97.83 | |
| Birch | 60 min, 50% EtOH | 93.52 | 93.71 |
| 60 min, 50% EtOH, 1% H2SO4 | 98.26 | 98.30 | |
| 60 min, 60% EtOH, | 98.29 | 98.52 | |
| 60 min, 60% EtOH, 1% H2SO4 | 98.57 | 98.63 | |
| 103 min, 50% EtOH | 98.64 | 99.05 | |
| 103 min 50% EtOH, 1% H2SO4 | 99.13 | 99.32 | |
| EtOH; ethanol |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsakas, L.; Nitsos, C.; Vörös, D.; Rova, U.; Christakopoulos, P. High-Titer Methane from Organosolv-Pretreated Spruce and Birch. Energies 2017, 10, 263. https://doi.org/10.3390/en10030263
Matsakas L, Nitsos C, Vörös D, Rova U, Christakopoulos P. High-Titer Methane from Organosolv-Pretreated Spruce and Birch. Energies. 2017; 10(3):263. https://doi.org/10.3390/en10030263
Chicago/Turabian StyleMatsakas, Leonidas, Christos Nitsos, Dimitrij Vörös, Ulrika Rova, and Paul Christakopoulos. 2017. "High-Titer Methane from Organosolv-Pretreated Spruce and Birch" Energies 10, no. 3: 263. https://doi.org/10.3390/en10030263






