Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Leaf Litter Designated for Combustion
Abstract
:1. Introduction
- To investigate the concentrations of PAHs and heavy metals in urban leaf litter from street sweepings;
- To determine the effect of provenience and collection technique on the concentration of PAHs and heavy metals in urban leaf litter from street sweepings;
- To investigate the effect of washing and dehydration through the IFBB system on the heavy metal concentration;
- To develop a linear regression model to predict the heavy metal concentration in urban leaf litter.
2. Materials and Methods
2.1. Collection of Leaf Litter
2.2. Washing Facility and Washing of Leaf Litter
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results and Discussion
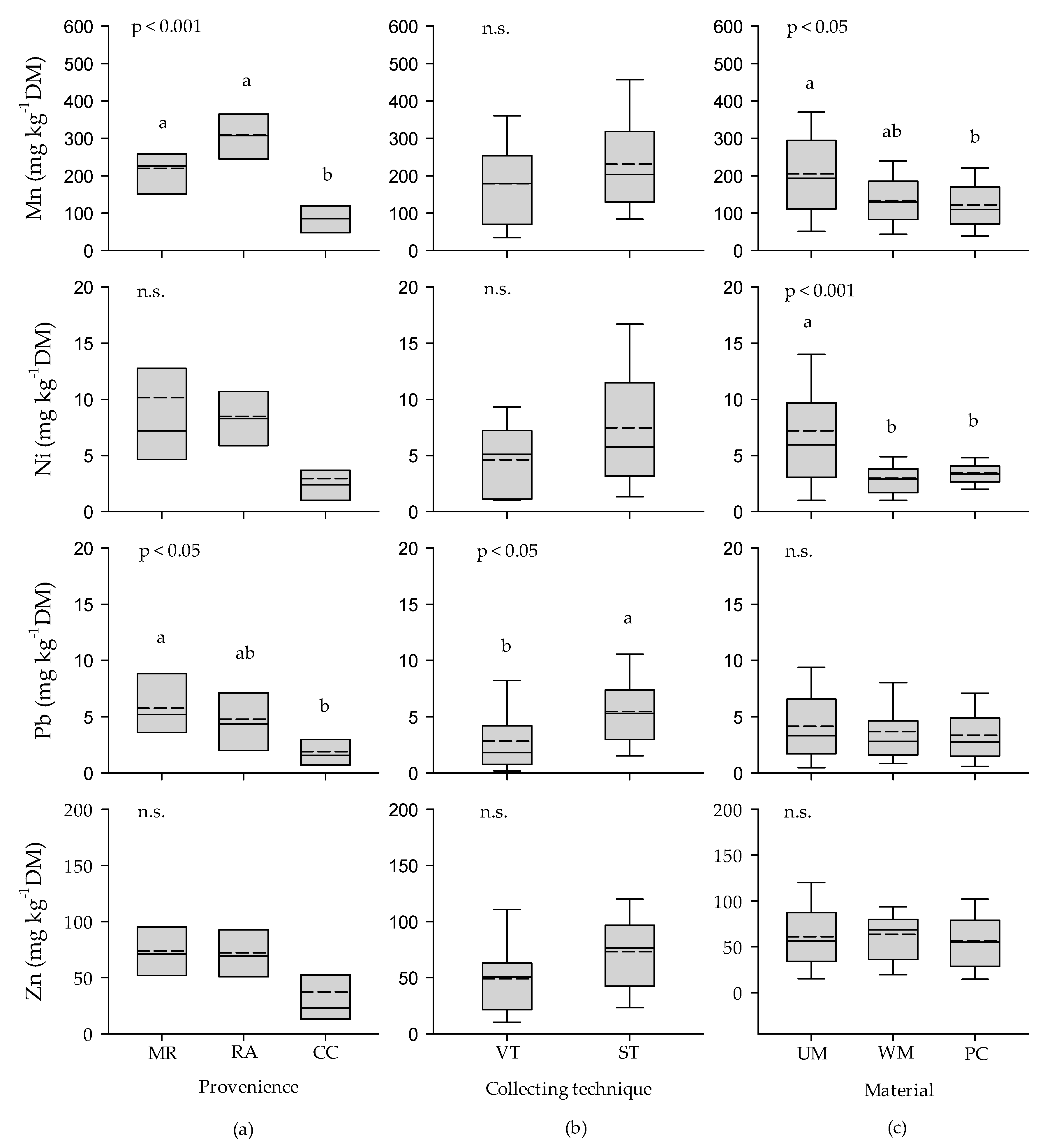
3.1. Heavy Metals
3.1.1. Heavy Metals in Urban Leaf Litter
3.1.2. Ash Content as a Predictor for Heavy Metal Concentration
3.1.3. Relevance of Heavy Metals in Urban Leaf Litter for Combustion
3.2. PAHs
3.2.1. PAHs in Urban Leaf Litter
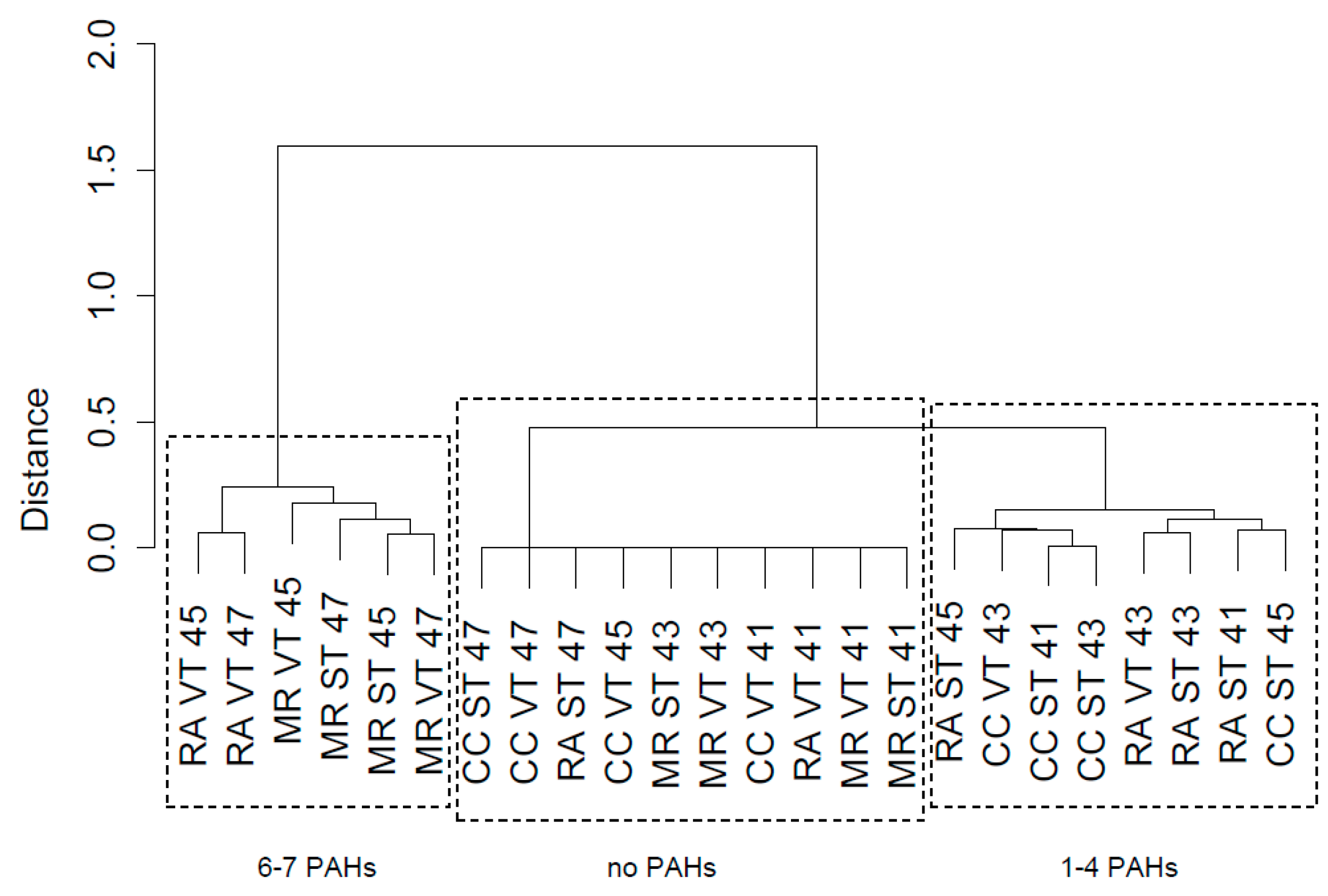
3.2.2. Hierarchical Clustering of PAHs in Urban Leaf Litter
3.2.3. Relevance of PAHs in Urban Leaf Litter for Combustion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Díaz-Porras, D.F.; Gaston, K.J.; Evans, K.L. 110 Years of change in urban tree stocks and associated carbon storage. Ecol. Evol. 2014, 4, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Britt, C.; Johnston, M. Trees in Towns II: A New Survey of Urban Trees in England and Their Conditions and Management; Department of Communities and Local Government: London, UK, 2008.
- Piepenschneider, M.; Nurmatov, N.; Bühle, L.; Hensgen, F.; Wachendorf, M. Chemical Properties and Ash Slagging Characteristics of Solid Fuels from Urban Leaf Litter. Waste Biomass Valoriz. 2016, 7, 625–633. [Google Scholar] [CrossRef]
- McLaughlin, M.; Parker, D.; Clarke, J. Metals and micronutrients—Food safety issues. Field Crops Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Imperato, M.; Adamo, P.; Naimo, D.; Arienzo, M.; Stanzione, D.; Violante, P. Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ. Pollut. 2003, 124, 247–256. [Google Scholar] [CrossRef]
- Xia, X.; Chen, X.; Liu, R.; Liu, H. Heavy metals in urban soils with various types of land use in Beijing, China. J. Hazard. Mater. 2011, 186, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Solgi, E.; Roohi, N.; Kouroshi-Gholampour, M. A comparative study of metals in roadside soils and urban parks from Hamedan metropolis, Iran. Environ. Nanotechnol. Monit. Manag. 2016, 6, 169–175. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, M. Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ. Monit. Assess. 2006, 120, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kachenko, A.G.; Singh, B. Heavy Metals Contamination in Vegetables Grown in Urban and Metal Smelter Contaminated Sites in Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass-a brief review. J. Hazard. Mater. 2013, 256, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Narodoslawsky, M.; Obernberger, I. From waste to raw material—the route from biomass to wood ash for cadmium and other heavy metals. J. Hazard. Mater. 1996, 50, 157–168. [Google Scholar] [CrossRef]
- Brown, J.N.; Peake, B.M. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Sci. Total Environ. 2006, 359, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, B.; Aboal, J.R.; Fernández, J.A.; Real, C.; Carballeira, A. Influence of roads and inhabited areas on metal concentrations in terrestrial mosses. Atmos. Environ. 2010, 44, 3432–3441. [Google Scholar] [CrossRef]
- Cicek, A.; Malkoc, S.; Koparal, A.S. An Investigation on the Usability of Grass in Short Term Detection of Traffic-Related Pollution. Arab. J. Sci. Eng. 2012, 37, 1239–1245. [Google Scholar] [CrossRef]
- De Nicola, F.; Maisto, G.; Prati, M.; Alfani, A. Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environ. Pollut. 2008, 153, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Dećkowska, A.; Pierścieniak, M. Traffic Pollutant Indicators: Common Dandelion (Teraxacum officinale), Scots Pine (Pinus silvestris), Small-Leaved Lime (Tilia cordata). Pol. J. Environ. Stud. 2011, 1, 87–92. [Google Scholar]
- Nabulo, G.; Black, C.R.; Craigon, J.; Young, S.D. Does consumption of leafy vegetables grown in peri-urban agriculture pose a risk to human health? Environ. Pollut. 2012, 162, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Martin, I.; Roberts, J.; Griffiths, E. Leaf Litter in Street Sweepings: Investigation into Collection and Treatment; Environment Agency: Bristol, UK, 2013.
- Ellis, D.; Keyse, C. Conversion of Street Leaf Sweepings: Final Report; Waste and Resources Action Programme: Cardiff, UK, 2016. [Google Scholar]
- Kosse, P.; Lübken, M.; Wichern, M. Urban lignocellulosic biomass can significantly contribute to energy production in municipal wastewater treatment plants—A GIS-based approach for a metropolitan area. Biomass Bioenergy 2015, 81, 568–573. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Amon, T.; Hobbs, P. Bioenergy from permanent grassland—A review: 2. Combustion. Bioresour. Technol. 2009, 100, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, J.; Döhling, F.; Berger, F. Foliage and Grass as Fuel Pellets–Small Scale Combustion of Washed and Mechanically Leached Biomass. Energies 2016, 9, 361. [Google Scholar] [CrossRef]
- Piepenschneider, M.; Bühle, L.; Wachendorf, M. Solid Fuel Generation from Urban Leaf Litter in Mixture with Grass Cuttings: Chemical Composition, Energetic Characteristics, and Impact of Preprocessing. Bioenergy Res. 2016, 9, 57–66. [Google Scholar] [CrossRef]
- Wachendorf, M.; Richter, F.; Fricke, T.; Graß, R.; Neff, R. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. I. Effects of hydrothermal conditioning and mechanical dehydration on mass flows of organic and mineral plant compounds, and nutrient balances. Grass Forage Sci. 2009, 64, 132–143. [Google Scholar] [CrossRef]
- Hensgen, F.; Buhle, L.; Donnison, I.; Frasier, M.; Vale, J.; Corton, J.; Heinsoo, K.; Melts, I.; Wachendorf, M. Mineral concentrations in solid fuels from European semi-natural grasslands after hydrothermal conditioning and subsequent mechanical dehydration. Bioresour. Technol. 2012, 118, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Hensgen, F.; Richter, F.; Wachendorf, M. Integrated generation of solid fuel and biogas from green cut material from landscape conservation and private households. Bioresour. Technol. 2011, 102, 10441–10450. [Google Scholar] [CrossRef] [PubMed]
- Piepenschneider, M.; de Moor, S.; Hensgen, F.; Meers, E.; Wachendorf, M. Element concentrations in urban grass cuttings from roadside verges in the face of energy recovery. Environ. Sci. Pollut. Res. Int. 2015, 22, 7808–7820. [Google Scholar] [CrossRef] [PubMed]
- Apeagyei, E.; Bank, M.S.; Spengler, J.D. Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos. Environ. 2011, 45, 2310–2323. [Google Scholar] [CrossRef]
- Tyler, G. Changes in the concentrations of major, minor and rare-earth elements during leaf senescence and decomposition in a Fagus sylvatica forest. For. Ecol. Manag. 2005, 206, 167–177. [Google Scholar] [CrossRef]
- Heckman, J.; Kluchinski, D. Chemical Composition of Municipal Leaf Waste and Hand-Collected Urban Leaf Litter. J. Environ. Q. 1996, 25, 355–362. [Google Scholar] [CrossRef]
- Seling, S.; Fischer, P. Schadstoffbelastung von Strassenbegleitgrün: II. Gehalte des Falllaubs an Schwermetallen (Cd, Cr, Cu, Hg, Ni, Pb, Pt, Zn). Müll und Abfall 2003, 35, 410–413. [Google Scholar]
- DIN Deutsches Institut für Normung e. V. Feste Biobrennstoffe—Brennstoffspezifikationen und -klassen—Teil 6: Nicht-holzartige Pellets für nichtindustrielle Verwendung; Deutsche Fassung; Beuth: Berlin, Germany, 2012. [Google Scholar]
- Charlesworth, S.; Everett, M.; McCarthy, R.; Ordóñez, A.; de Miguel, E. A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry, West Midlands, UK. Environ. Int. 2003, 29, 563–573. [Google Scholar] [CrossRef]
- Harrison, R.M.; Smith, D.; Luhana, L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Fuel Energy Abstr. 1996, 37, 388. [Google Scholar]
- Howsam, M.; Jones, K.; Ineson, P. PAHs associated with the leaves of three deciduous tree species. I—Concentrations and profiles. Environ. Pollut. 2000, 108, 413–424. [Google Scholar] [CrossRef]
- Kosnar, Z.; Mercl, F.; Perna, I.; Tlustos, P. Investigation of polycyclic aromatic hydrocarbon content in fly ash and bottom ash of biomass incineration plants in relation to the operating temperature and unburned carbon content. Sci. Total Environ. 2016, 563, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Martin-Gullon, I.; Conesa, J.A.; Font, R. Emissions from pyrolysis and combustion of olive oil solid waste. J. Anal. Appl. Pyrolysis 2005, 74, 512–517. [Google Scholar] [CrossRef]
- Lee, R.G.M.; Coleman, P.; Jones, J.L.; Jones, K.C.; Lohmann, R. Emission Factors and Importance of PCDD/Fs, PCBs, PCNs, PAHs and PM 10 from the Domestic Burning of Coal and Wood in the U.K. Environ. Sci. Technol. 2005, 39, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Krugly, E.; Martuzevicius, D.; Puida, E.; Buinevicius, K.; Stasiulaitiene, I.; Radziuniene, I.; Minikauskas, A.; Kliucininkas, L. Characterization of Gaseous- and Particle-Phase Emissions from the Combustion of Biomass-Residue-Derived Fuels in a Small Residential Boiler. Energy Fuels 2014, 28, 5057–5066. [Google Scholar] [CrossRef]

| Element | Urban Leaf Litter from Roads | Forest Leaf Litter 1 | Urban Leaf Litter 2 | Urban Leaf Litter from Roadside Verges 3 | Deciduous Wood 4 | Standard for Pellets from Non-Woody Materials 5 |
|---|---|---|---|---|---|---|
| As | 0.56 ± 0.08 | 0.07 | - | - | <0.1 | ≤1.0 |
| Cd | 0.13 ± 0.02 | 0.14 | 1.7 | 0.33 | 0.1 | ≤0.5 |
| Cr 6 | 6.04 ± 1.02 | 0.68 | 7.6 | 4.9 | 1.0 | ≤50.0 |
| Cu | 11.84 ± 1.04 | 6.3 | 9.2 | 29 | 2.0 | ≤20.0 |
| Fe | 7 154.46 ± 1271.02 | 110.0 | 1 461.0 | - | 25.0 | - |
| Mn | 204.99 ± 24.47 | 1 850.0 | 550.0 | - | 83.0 | - |
| Ni 6 | 7.20 ± 1.33 | 1.12 | 7.2 | 1.8 | 0.5 | ≤10.0 |
| Pb | 4.14 ± 0.63 | 1.3 | 28.4 | 20 | 2.0 | ≤10.0 |
| Zn | 61.04 ± 6.95 | 36.0 | 81.0 | 89 | 10.0 | ≤100.0 |
| Element | Material | Number of Samples with Detected Concentrations | Minimum Value | Maximum Value | Median |
|---|---|---|---|---|---|
| Tl | UM | 15 | <0.01 | 0.05 | 0.02 |
| WM | 13 | <0.01 | 0.04 | 0.02 | |
| PC | 6 | <0.01 | 0.04 | <0.01 | |
| Hg | UM | 10 | <0.01 | 0.06 | 0.02 |
| WM | 7 | <0.01 | 0.06 | <0.01 | |
| PC | 10 | <0.01 | 0.06 | <0.01 |
| Element | Intercept | Slope | R2 | Significance |
|---|---|---|---|---|
| As | −0.173 | 0.031 | 0.77 | *** |
| Cd | 0.010 | 0.005 | 0.44 | *** |
| Cr | −0.237 | 0.278 | 0.39 | ** |
| Cu | 6.629 | 0.219 | 0.21 | * |
| Fe | −4606.400 | 495.620 | 0.71 | *** |
| Mn | 8.919 | 8.263 | 0.53 | *** |
| Ni | 0.061 | 0.317 | 0.29 | * |
| Pb | −0.947 | 0.215 | 0.53 | *** |
| Zn | 0.785 | 2.539 | 0.62 | *** |
| PAH | Chemical Formula | Number of Samples with Detected Concentration | Minimum | Maximum | Median | Literature Value 1,2 |
|---|---|---|---|---|---|---|
| Naphthalene | C10H8 | 0 | <0.05 | <0.05 | <0.05 | 0.102 |
| Acenaphthylene | C12H8 | 0 | <0.05 | <0.05 | <0.05 | 0.052 |
| Acenaphthene | C12H10 | 0 | <0.05 | <0.05 | <0.05 | 0.007 |
| Fluorene | C13H10 | 0 | <0.05 | <0.05 | <0.05 | 0.038 |
| Phenanthrene | C14H10 | 12 | <0.05 | 0.360 | <0.05 | 0.262 |
| Anthracene | C14H10 | 0 | <0.05 | <0.05 | <0.05 | 0.025 |
| Fluoranthene | C16H10 | 13 | <0.05 | 0.668 | 0.174 | 0.328 |
| Pyrene | C16H10 | 8 | <0.05 | 0.520 | <0.05 | 0.419 |
| Benz(a)anthracene | C18H12 | 6 | <0.05 | 0.349 | <0.05 | 0.054 |
| Chrysene | C18H12 | 6 | <0.05 | 0.282 | <0.05 | 0.205 |
| Benzo(b)fluoranthene | C20H12 | 9 | <0.05 | 0.520 | <0.05 | 0.127 a |
| Benzo(k)fluoranthene | C20H12 | 1 | <0.05 | 0.150 | <0.05 | 0.127 a |
| Benzo(a)pyrene | C20H12 | 5 | <0.05 | 0.282 | <0.05 | 0.047 |
| Indeno(123-cd)pyrene | C22H12 | 2 | <0.05 | 0.179 | <0.05 | 0.003 |
| Dibenz(ah)anthracene | C22H14 | 0 | <0.05 | <0.05 | <0.05 | 0.045 |
| Benzo(ghi)perylene | C22H12 | 4 | <0.05 | 0.249 | <0.05 | 0.002 |
| Sum | - | - | <0.05 | 3.053 | 0.267 | 2.10 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsche, M.; Nurmatov, N.; Hensgen, F.; Wachendorf, M. Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Leaf Litter Designated for Combustion. Energies 2017, 10, 298. https://doi.org/10.3390/en10030298
Nitsche M, Nurmatov N, Hensgen F, Wachendorf M. Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Leaf Litter Designated for Combustion. Energies. 2017; 10(3):298. https://doi.org/10.3390/en10030298
Chicago/Turabian StyleNitsche, Meike, Nodirjon Nurmatov, Frank Hensgen, and Michael Wachendorf. 2017. "Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Leaf Litter Designated for Combustion" Energies 10, no. 3: 298. https://doi.org/10.3390/en10030298
APA StyleNitsche, M., Nurmatov, N., Hensgen, F., & Wachendorf, M. (2017). Heavy Metals and Polycyclic Aromatic Hydrocarbons in Urban Leaf Litter Designated for Combustion. Energies, 10(3), 298. https://doi.org/10.3390/en10030298






