Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Model and Simulation Details
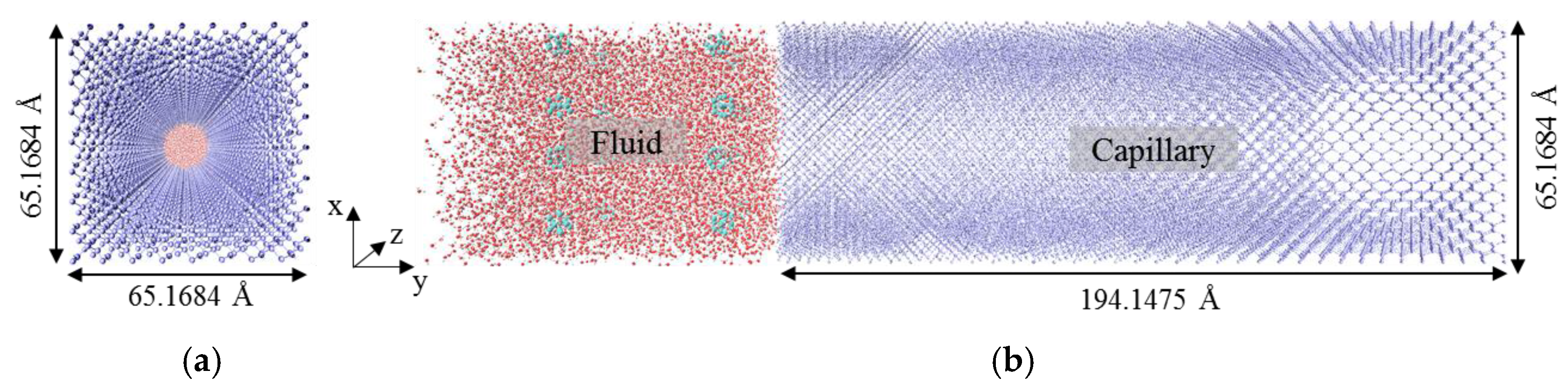
2.1. Modeling of Imbibition System
2.2. Potential Functions and Parameters
3. Results and Discussions
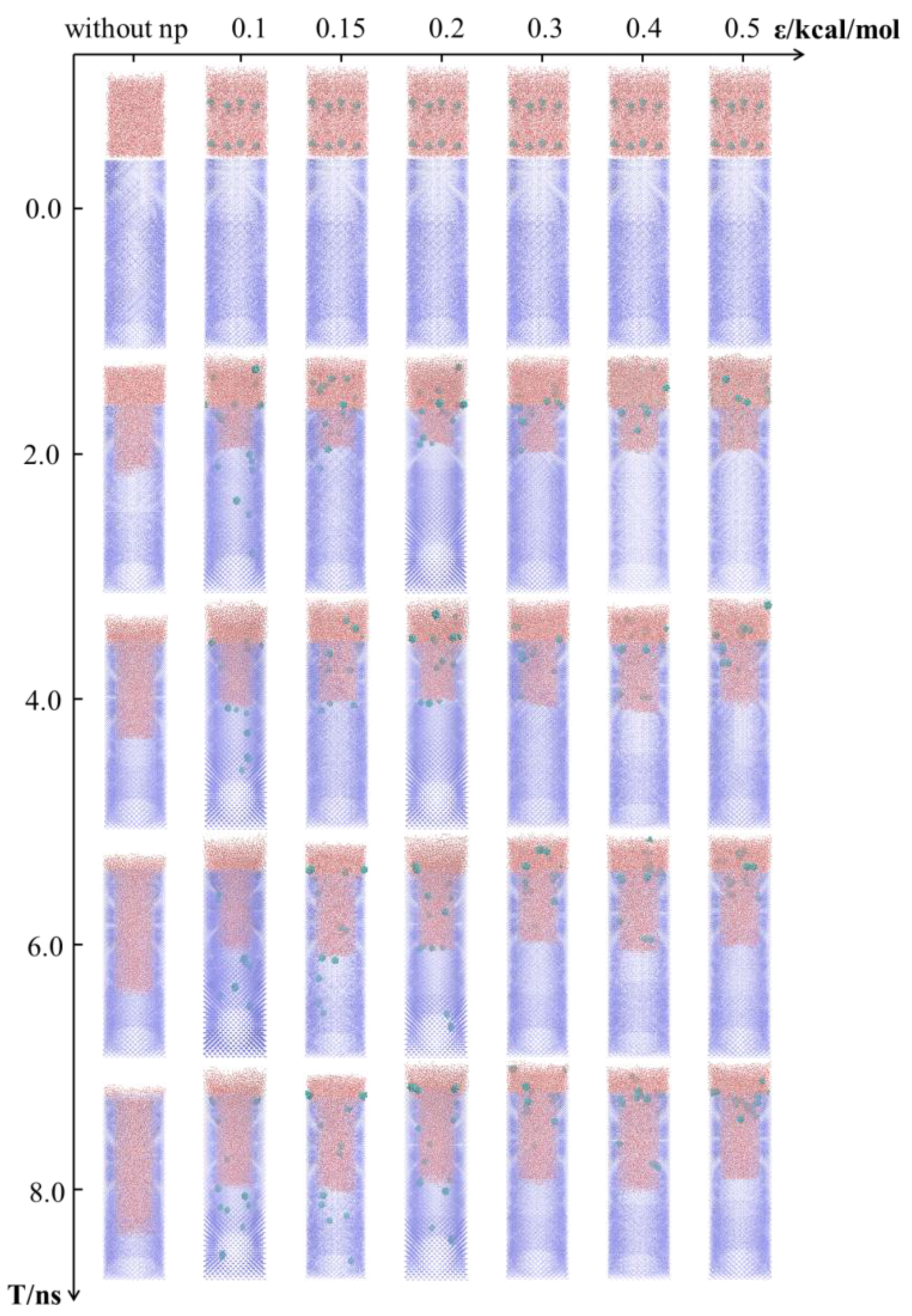
3.1. Imbibition Phenomena of Nanofluids in Reservior
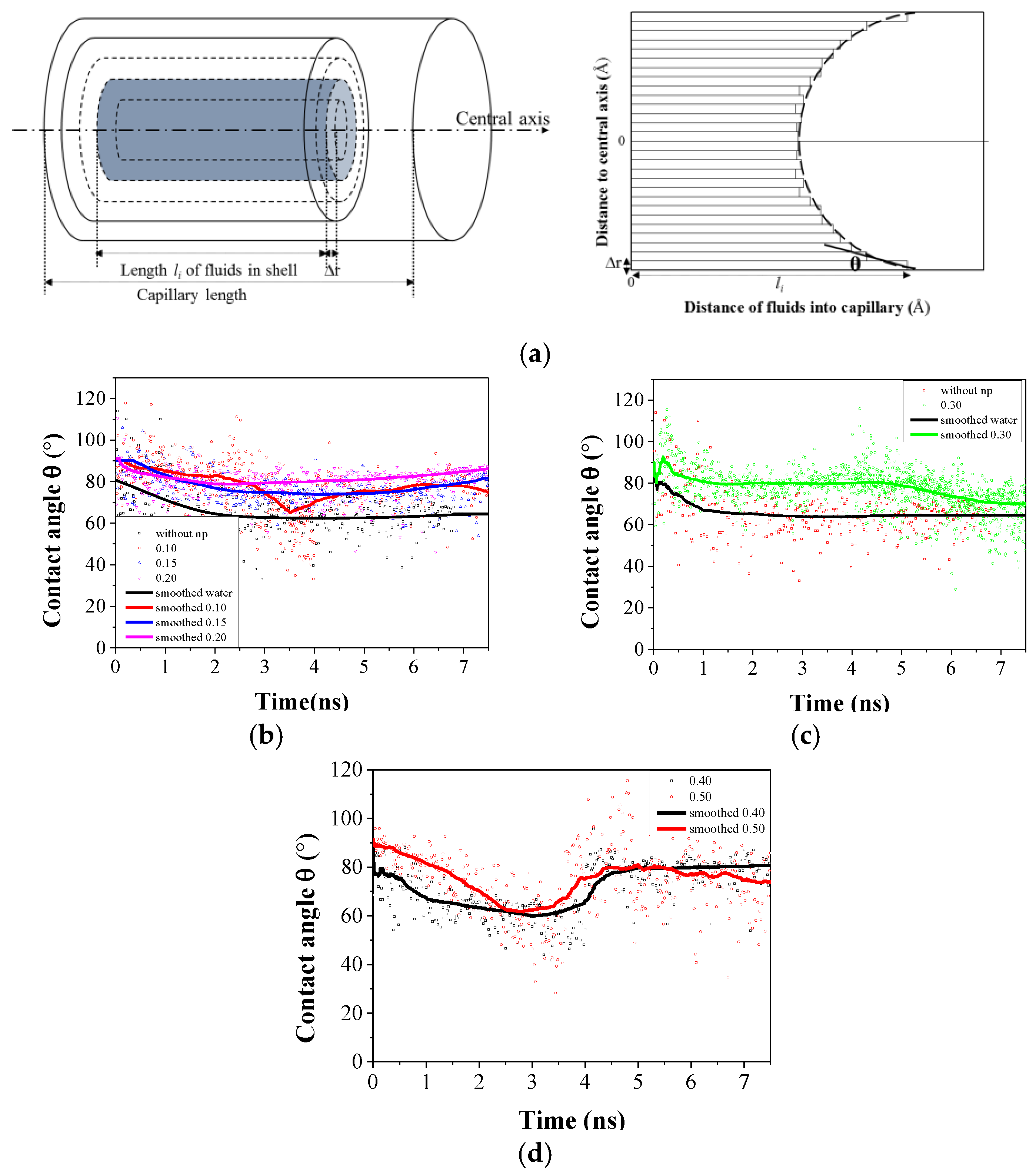
3.2. Wettability of Capillary Alterting by Nanofluids
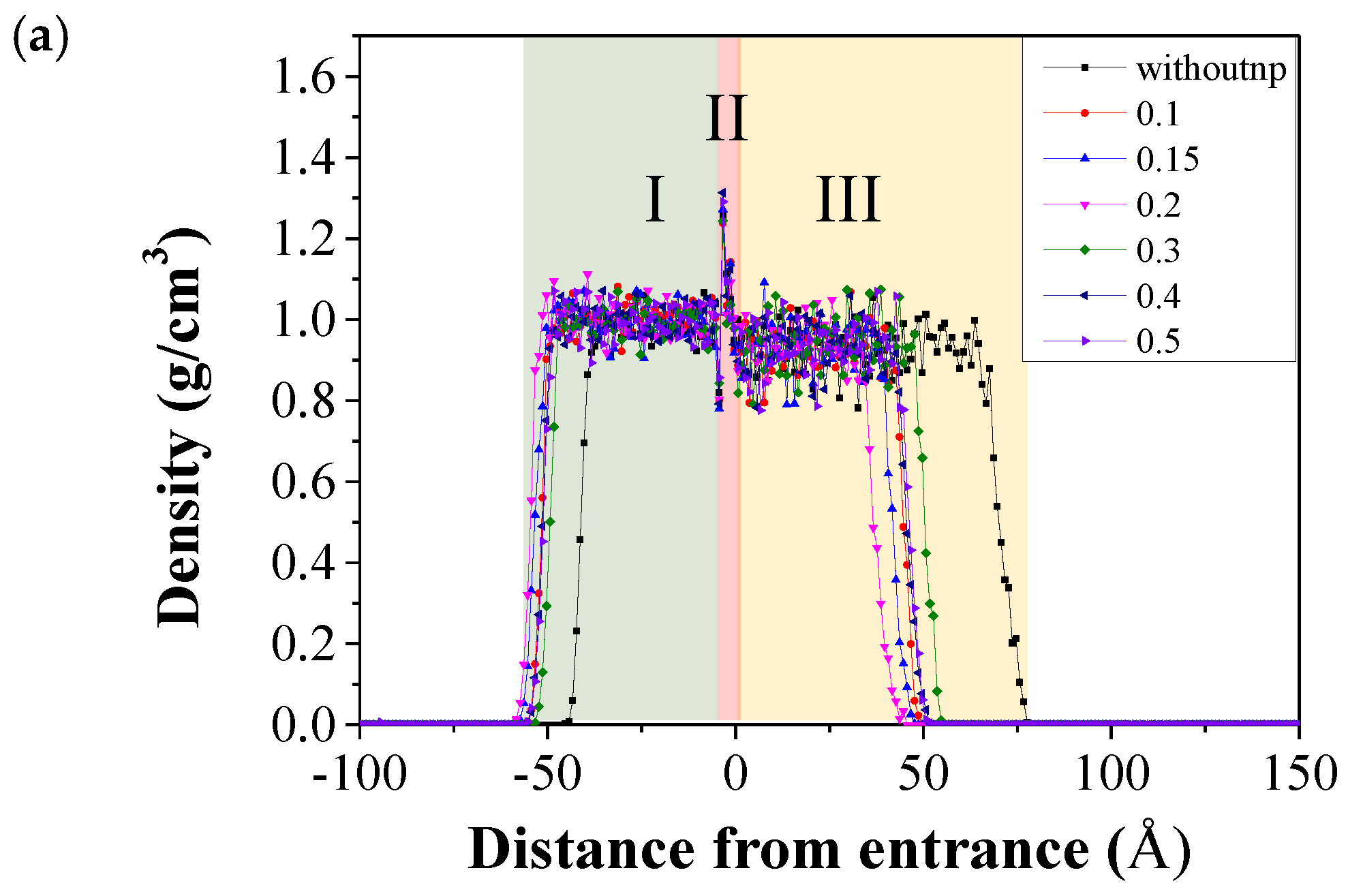
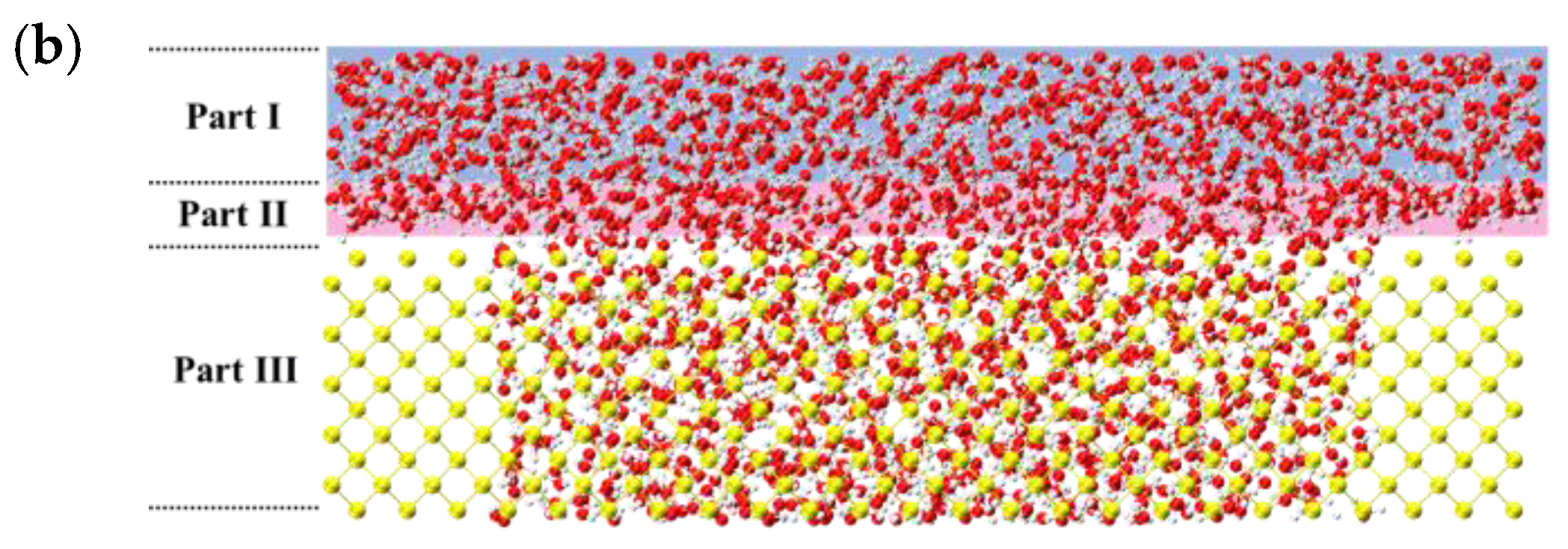
3.3. The Microscale Imbibition Mechanism
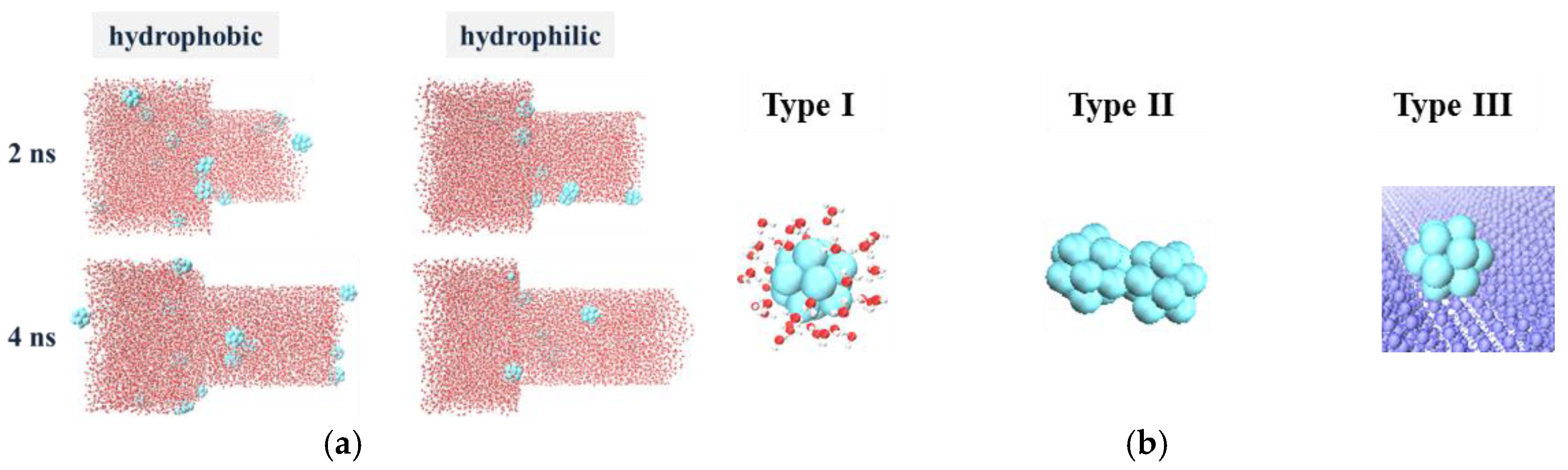
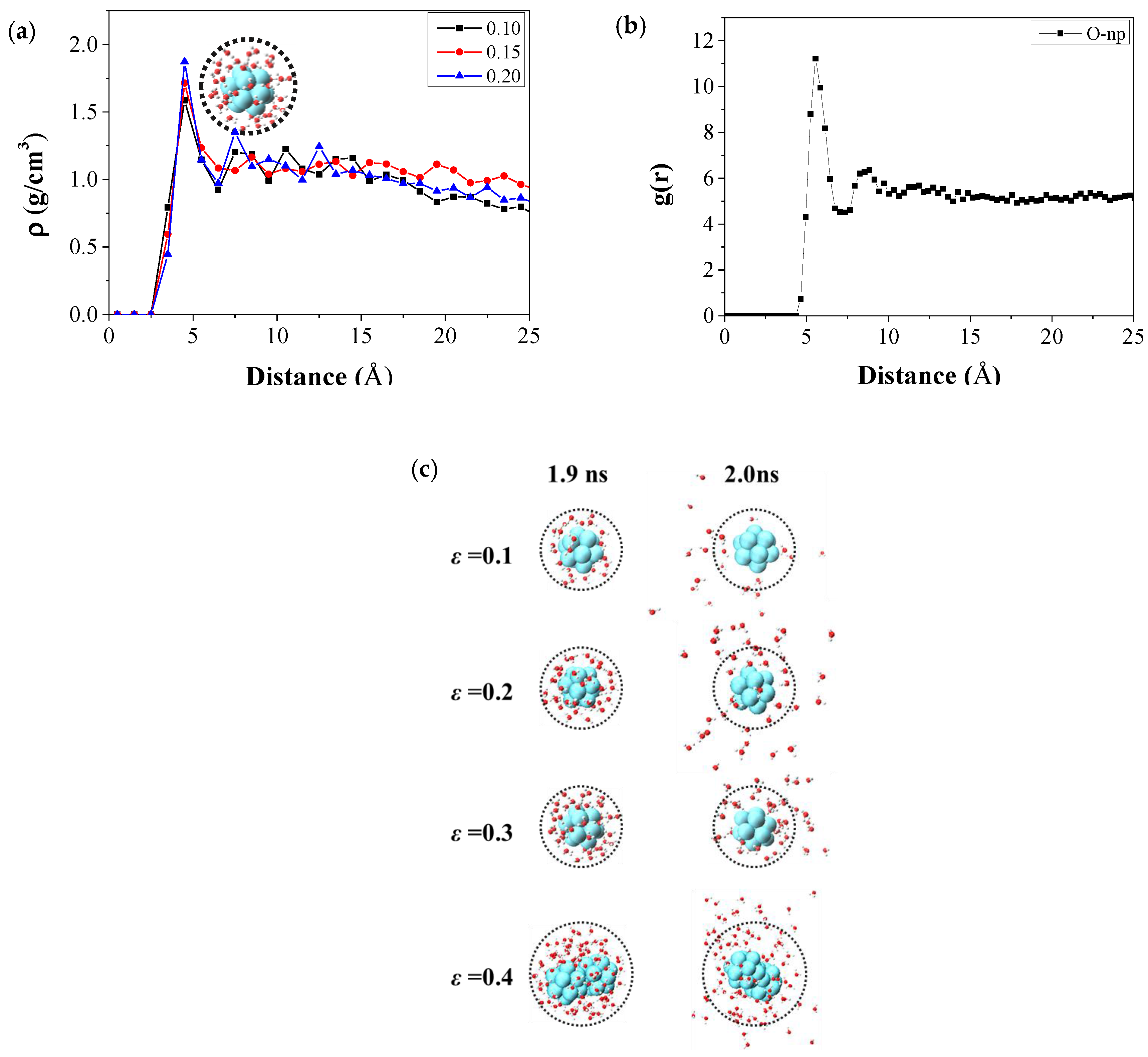
3.3.1. The Micro Behavior of Nanoparticles in Water-Wet Capillary
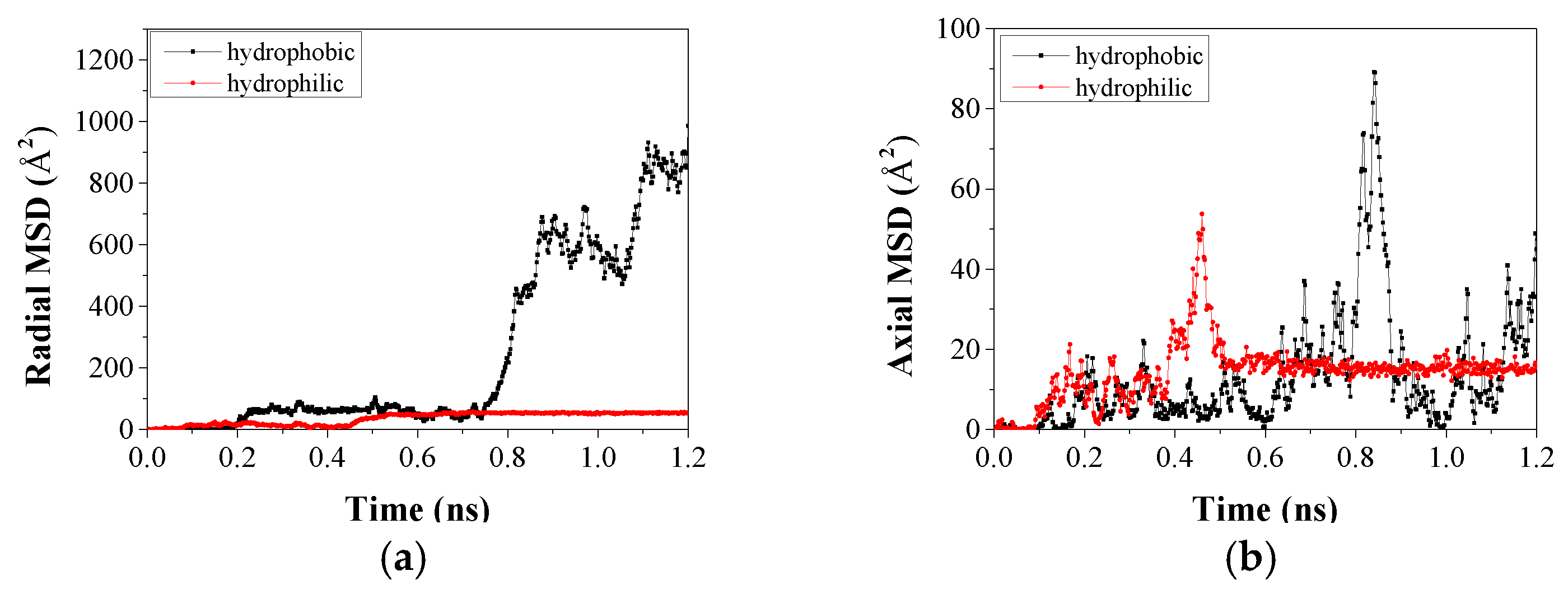
3.3.2. The Micro Imbibition Mechanism of Nanofluids in Water-Wet Capillary
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.; Chu, L. Functional Polymeric Microparticles Engineered from Controllable Microfluidic Emulsions. Acc. Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kondiparty, K.; Nikolov, A.D.; Wasan, D.; Liu, K.L. Dynamic spreading of nanofluids on solids. Part I: Experimental. Langmuir 2012, 28, 14618–14623. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, H.; Yamashita, K.; Endo, A.; Daiguji, H. Adsorption–Desorption and Transport of Water in Two-Dimensional Hexagonal Mesoporous Silica. J. Phys. Chem. C 2013, 117, 21795–21802. [Google Scholar] [CrossRef]
- Xu, X.; Vereecke, G.; Chen, C.; Pourtois, G.; Armini, S.; Verellen, N.; Tsai, W.; Kim, D.; Lee, E.; Lin, C.; et al. Capturing Wetting States in Nanopatterned Silicon. ACS Nano 2014, 8, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Fong, N.; Yin, Y.; Svehla, M.; McKenzie, D.R. Science of Water Leaks: Validated Theory for Moisture Flow in Microchannels and Nanochannels. Langmuir 2015, 31, 11740–11747. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Son, H.A.; Choi, S.K.; Kim, J.W.; Sung, W.M.; Kim, H.T. Core Flooding of Complex Nanoscale Colloidal Dispersions for Enhanced Oil Recovery byin SituFormation of Stable Oil-in-Water Pickering Emulsions. Energy Fuels 2016, 30, 2628–2635. [Google Scholar] [CrossRef]
- Bakli, C.; Chakraborty, S. Capillary filling dynamics of water in nanopores. Appl. Phys. Lett. 2012, 101, 153112. [Google Scholar] [CrossRef]
- Cupelli, C.; Henrich, B.; Glatzel, T.; Zengerle, R.; Moseler, M.; Santer, M. Dynamic capillary wetting studied with dissipative particle dynamics. New J. Phys. 2008, 10, 043009. [Google Scholar] [CrossRef]
- Oyarzua, E.; Walther, J.H.; Mejia, A.; Zambrano, H.A. Early regimes of water capillary flow in slit silica nanochannels. Phys. Chem. Chem. Phys. 2015, 17, 14731–14739. [Google Scholar] [CrossRef] [PubMed]
- Berthier, J.; Gosselin, D.; Pham, A.; Delapierre, G.; Belgacem, N.; Chaussy, D. Capillary Flow Resistors: Local and Global Resistors. Langmuir 2016, 32, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Hamraoui, A.; Nylander, T. Analytical approach for the Lucas-Washburn equation. J. Colloid Interface Sci. 2002, 250, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, K.; Zhuang, L.; Li, X.; Dong, J.; Lu, J. Effective fluid front of the moving meniscus in capillary. Langmuir 2013, 29, 3269–3273. [Google Scholar] [CrossRef] [PubMed]
- Stroberg, W.; Keten, S.; Liu, W.K. Hydrodynamics of capillary imbibition under nanoconfinement. Langmuir 2012, 28, 14488–14495. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.I.; Klushin, L.I.; Milchev, A.; Binder, K. Flow and transport in brush-coated capillaries: A molecular dynamics simulation. Phys. Fluids 2008, 20, 092102. [Google Scholar] [CrossRef]
- Chibbaro, S.; Costa, E.; Dimitrov, D.I.; Diotallevi, F.; Milchev, A.; Palmieri, D.; Pontrelli, G.; Succi, S. Capillary filling in microchannels with wall corrugations: a comparative study of the Concus-Finn criterion by continuum, kinetic, and atomistic approaches. Langmuir 2009, 25, 12653–12660. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.I.; Milchev, A.; Binder, K. Method for wettability characterization based on contact line pinning. Phys. Rev. E 2010, 81, 041603. [Google Scholar] [CrossRef] [PubMed]
- Beu, T.A. Molecular dynamics simulations of ion transport through carbon nanotubes. I. Influence of geometry, ion specificity, and many-body interactions. J. Chem. Phys. 2010, 132, 164513. [Google Scholar] [CrossRef] [PubMed]
- Colosqui, C.E.; Teng, T.; Rahmani, A.M. Wetting Driven by Thermal Fluctuations on Terraced Nanostructures. Phys. Rev. Lett. 2015, 115, 154504. [Google Scholar] [CrossRef] [PubMed]
- Walls, P.L.; Dequidt, G.; Bird, J.C. Capillary Displacement of Viscous Liquids. Langmuir 2016, 32, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.S.; Unsal, E. Spontaneous and forced imbibition of aqueous wettability altering surfactant solution into an initially oil-wet capillary. Langmuir 2009, 25, 12591–12603. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P.S.; Unsal, E. Spontaneous imbibition of surfactant solution into an oil-wet capillary: wettability restoration by surfactant-contaminant complexation. Langmuir 2011, 27, 4412–4429. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.D.; Savory, L.D.; Woods, F.; Clarke, A.; Howe, A.M. Model study of enhanced oil recovery by flooding with aqueous surfactant solution and comparison with theory. Langmuir 2015, 31, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Howe, A.M.; Mitchell, J.; Staniland, J.; Hawkes, L.; Leeper, K. Mechanism of anomalously increased oil displacement with aqueous viscoelastic polymer solutions. Soft Matter 2015, 11, 3536–3541. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Liu, Y.; Zhou, Y.; Yang, L.; Zhang, G.; Liu, Y. Modeling Nanoparticle Targeting to a Vascular Surface in Shear Flow Through Diffusive Particle Dynamics. Nanoscale Res. Lett. 2015, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef] [PubMed]
- El-Amin, M.F.; Salama, A.; Sun, S. Numerical and dimensional analysis of nanoparticles transport with two-phase flow in porous media. J. Pet. Sci. Eng. 2015, 128, 53–64. [Google Scholar] [CrossRef]
- Hu, Z.; Azmi, S.M.; Raza, G.; Glover, P.W.J.; Wen, D. Nanoparticle-Assisted Water-Flooding in Berea Sandstones. Energy Fuels 2016, 30, 2791–2804. [Google Scholar] [CrossRef]
- Lin, Y.; Skaff, H.; Emrick, T.; Dinsmore, A.D.; Russell, T.P. Nanoparticle Assembly and Transport at Liquid-Liquid Interfaces. Science 2003, 299, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Yong, X. Modeling the Assembly of Polymer-Grafted Nanoparticles at Oil-Water Interfaces. Langmuir 2015, 31, 11458–11469. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bhattacharya, A.; Kuksenok, O.; Perchak, D.; Balazs, A.C. Modeling the transport of nanoparticle-filled binary fluids through micropores. Langmuir 2012, 28, 11410–11421. [Google Scholar] [CrossRef] [PubMed]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2014, 5, 181–199. [Google Scholar] [CrossRef]
- Wong, A.S.; Mann, S.K.; Czuba, E.; Sahut, A.; Liu, H.; Suekama, T.C.; Bickerton, T.; Johnston, A.P.; Such, G.K. Self-assembling dual component nanoparticles with endosomal escape capability. Soft Matter 2015, 11, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Supple, S.; Quirke, N. Molecular dynamics of transient oil flows in nanopores I: Imbibition speeds for single wall carbon nanotubes. J. Chem. Phys. 2004, 121, 8571–8579. [Google Scholar] [CrossRef] [PubMed]
- Supple, S.; Quirke, N. Molecular dynamics of transient oil flows in nanopores. II. Density profiles and molecular structure for decane in carbon nanotubes. J. Chem. Phys. 2005, 122, 104706. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, M.; Piri, M.; Goual, L. Atomistic Molecular Dynamics Simulations of Crude Oil/Brine Displacement in Calcite Mesopores. Langmuir 2016, 32, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Giovambattista, N.; Almeida, A.B.; Alencar, A.M.; Buldyrev, S.V. Validation of Capillarity Theory at the Nanometer Scale by Atomistic Computer Simulations of Water Droplets and Bridges in Contact with Hydrophobic and Hydrophilic Surfaces. J. Phys. Chem. C 2016, 120, 1597–1608. [Google Scholar] [CrossRef]
- Yamashita, K.; Daiguji, H. Molecular Dynamics Simulations of Water Uptake into a Silica Nanopore. J. Phys. Chem. C 2015, 119, 3012–3023. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, X.; Du, J.; Wang, L.; Yan, Y.; Zhang, J. Combined Molecular Dynamics and Quantum Mechanics Study of Oil Droplet Adsorption on Different Self-Assembly Monolayers in Aqueous Solution. J. Phys. Chem. C 2013, 117, 12510–12519. [Google Scholar] [CrossRef]
- Chen, C.; Gao, C.; Zhuang, L.; Li, X.; Wu, P.; Dong, J.; Lu, J. A many-body dissipative particle dynamics study of spontaneous capillary imbibition and drainage. Langmuir 2010, 26, 9533–9538. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, J.; Torsæter, O.; Zhang, Z. Effect of Nanoparticles on Oil-Water Flow in a Confined Nanochannel a Molecular Dynamics Study. In Proceedings of the SPE International Oilfield Nanotechnology Conference, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Blake, T.D.; Coninck, J.D. The influence of pore wettability on the dynamics of imbibition and drainage. Colloids Surf. A 2004, 250, 395–402. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Lim, S.; Horiuchi, H.; Nikolov, A.D.; Wasan, D. Nanofluids alter the surface wettability of solids. Langmuir 2015, 31, 5827–5835. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari Bayat, A.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of Metal Oxide Nanoparticles on Enhanced Oil Recovery from Limestone Media at Several Temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H. Molecular dynamics studies on spreading of nanofluids promoted by nanoparticle adsorption on solid surface. Theor. Appl. Mech. Lett. 2013, 3, 054006. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H. Enhanced oil droplet detachment from solid surfaces in charged nanoparticle suspensions. Soft Matter 2013, 9, 7974–7980. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; Adam Hilger: New York, NY, USA, 1989. [Google Scholar]
- Stukan, M.R.; Ligneul, P.; Crawshaw, J.P.; Boek, E.S. Spontaneous imbibition in nanopores of different roughness and wettability. Langmuir 2010, 26, 13342–13352. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, M.; Piri, M.; Goual, L. Molecular dynamics of wetting layer formation and forced water invasion in angular nanopores with mixed wettability. J. Chem. Phys. 2014, 141, 194703. [Google Scholar] [CrossRef] [PubMed]
- Martic, G.; Gentner, F.; Seveno, D.; Coulon, D.; Coninck, J.D. A Molecular Dynamics Simulation of Capillary Imbibition. Langmuir 2002, 18, 7971–7976. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Ma, Y.; Sun, X.; Liu, Z.; Zhang, J. The coarse-grained model for a water/oil/solid system: based on the correlation of water/air and water/oil contact angles. RSC Adv. 2015, 5, 51135–51141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liu, H.; Wu, H. Nanoparticle-tuned spreading behavior of nanofluid droplets on the solid substrate. Microfluid. Nanofluid. 2014, 18, 111–120. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xiao, S.; Zhang, Z.; He, J. Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation. Energies 2017, 10, 506. https://doi.org/10.3390/en10040506
Wang X, Xiao S, Zhang Z, He J. Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation. Energies. 2017; 10(4):506. https://doi.org/10.3390/en10040506
Chicago/Turabian StyleWang, Xiao, Senbo Xiao, Zhiliang Zhang, and Jianying He. 2017. "Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation" Energies 10, no. 4: 506. https://doi.org/10.3390/en10040506
APA StyleWang, X., Xiao, S., Zhang, Z., & He, J. (2017). Effect of Nanoparticles on Spontaneous Imbibition of Water into Ultraconfined Reservoir Capillary by Molecular Dynamics Simulation. Energies, 10(4), 506. https://doi.org/10.3390/en10040506






