Experimental and Theoretical Study of the Interactions between Fe2O3/Al2O3 and CO
Abstract
:1. Introduction
2. Experimental and Theoretical Method
2.1. Preparation of the Oxygen Carrier
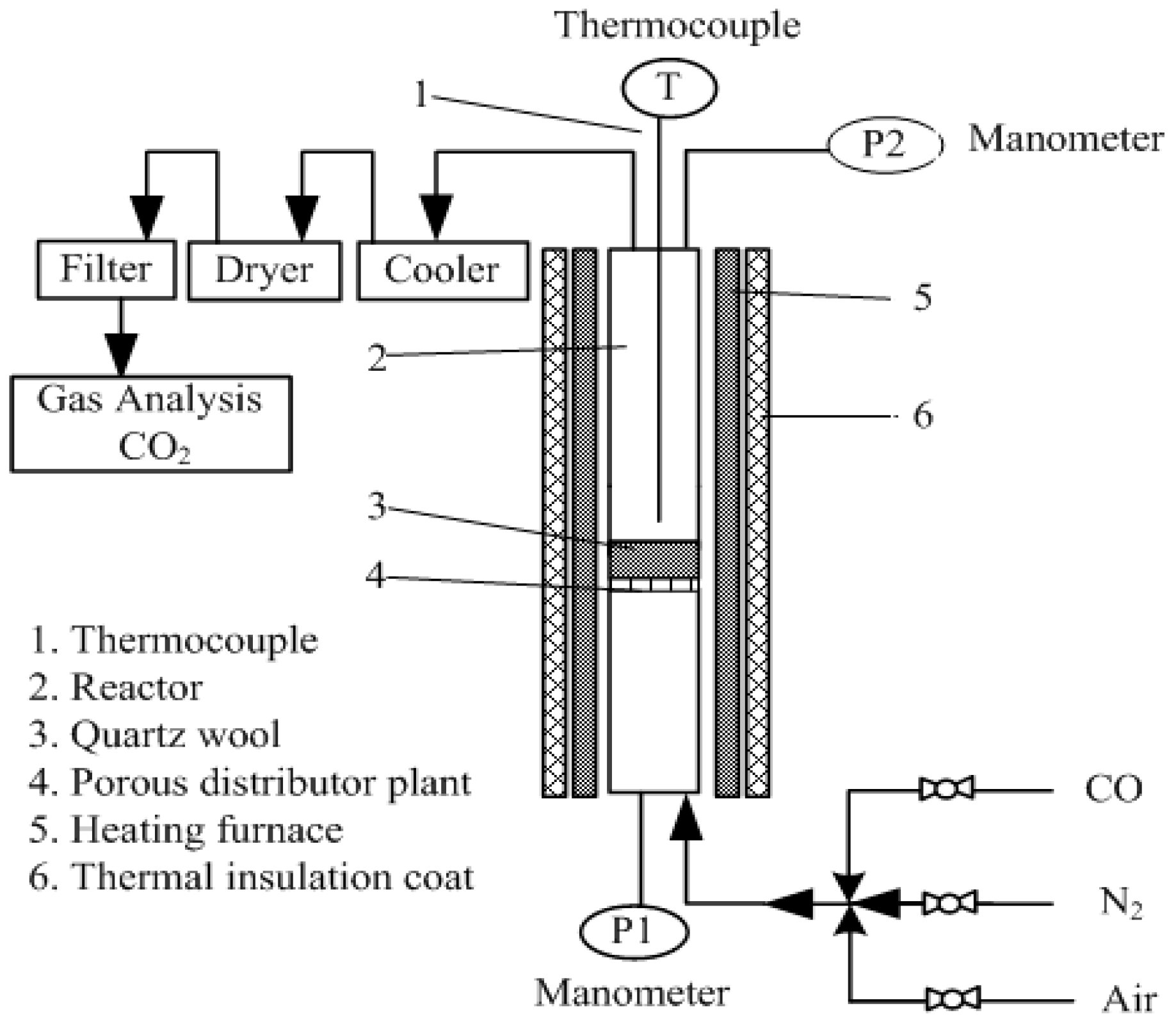
2.2. Combustion Experiment
2.3. Thermal Gravimetric Analysis (TGA)
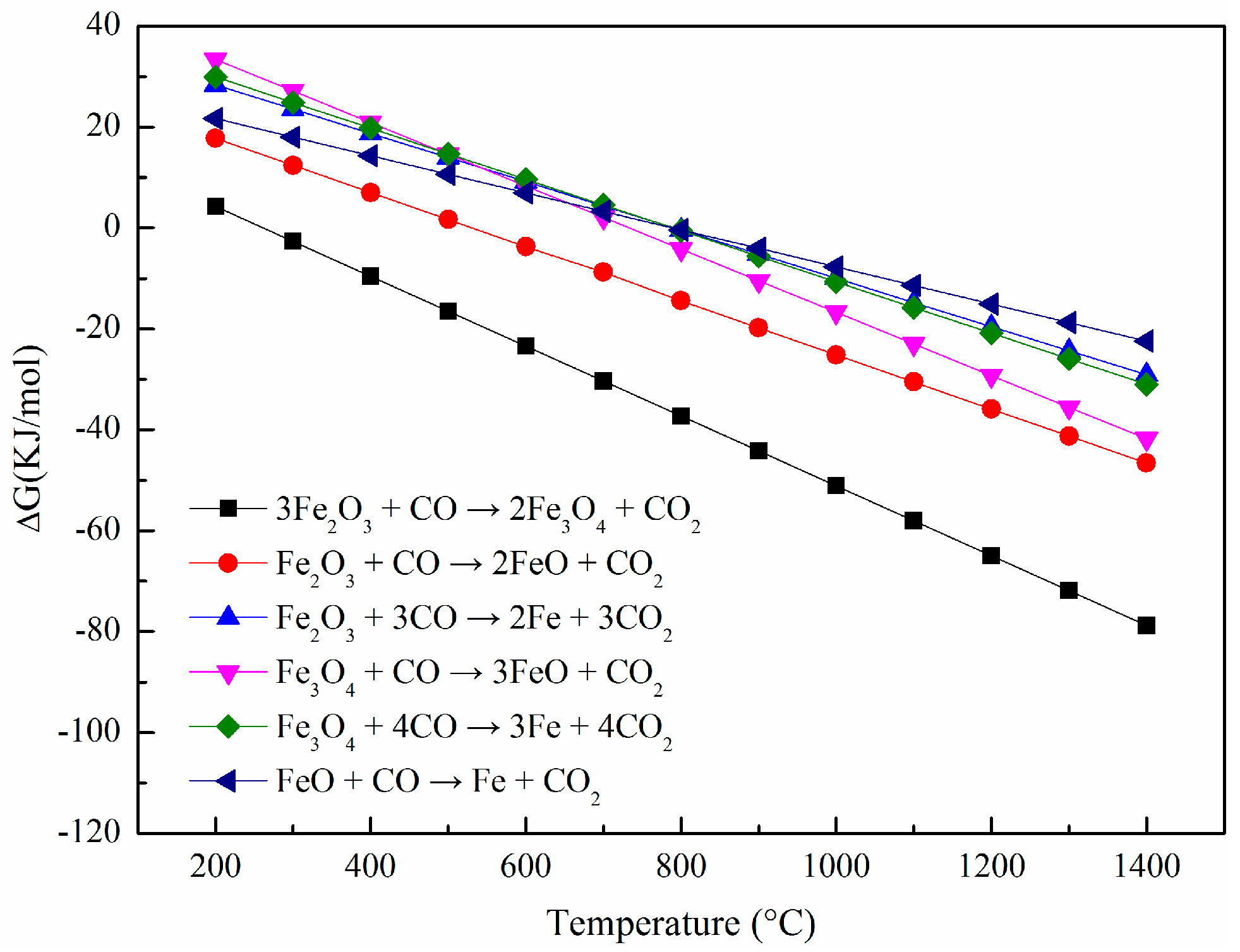
2.4. Thermodynamic Analysis
2.5. Theoretical Method
3. Results and Discussion
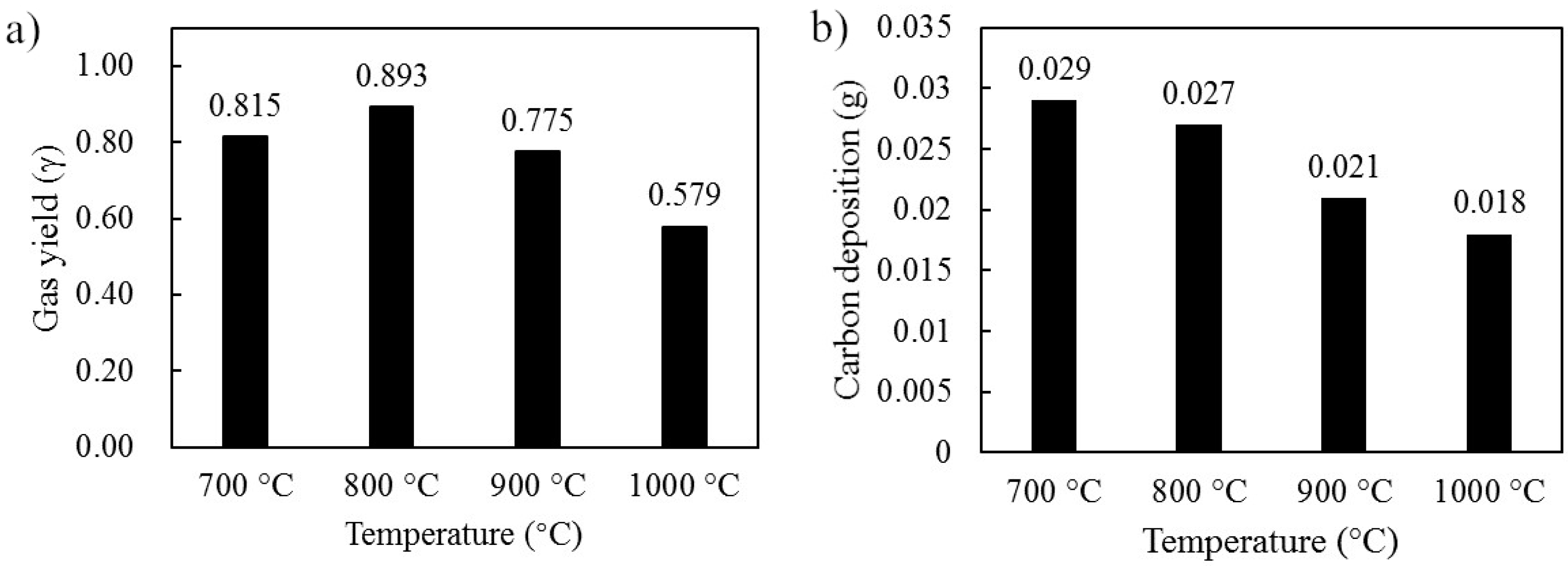
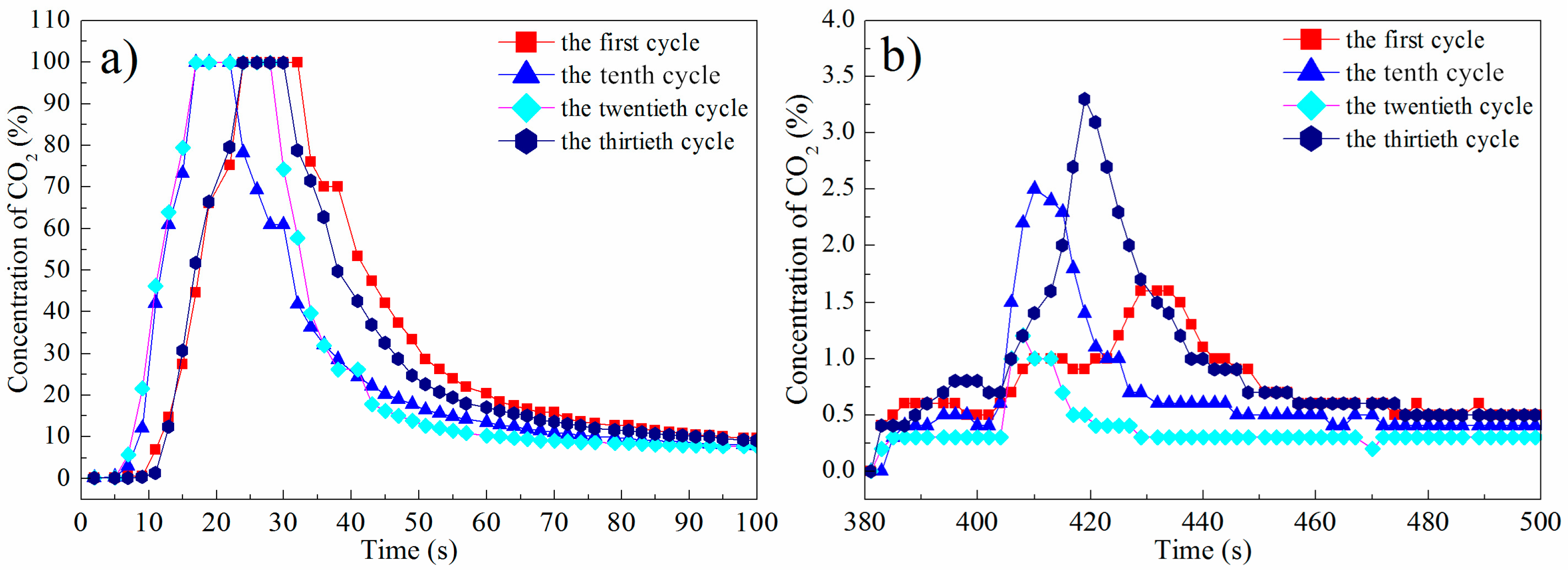
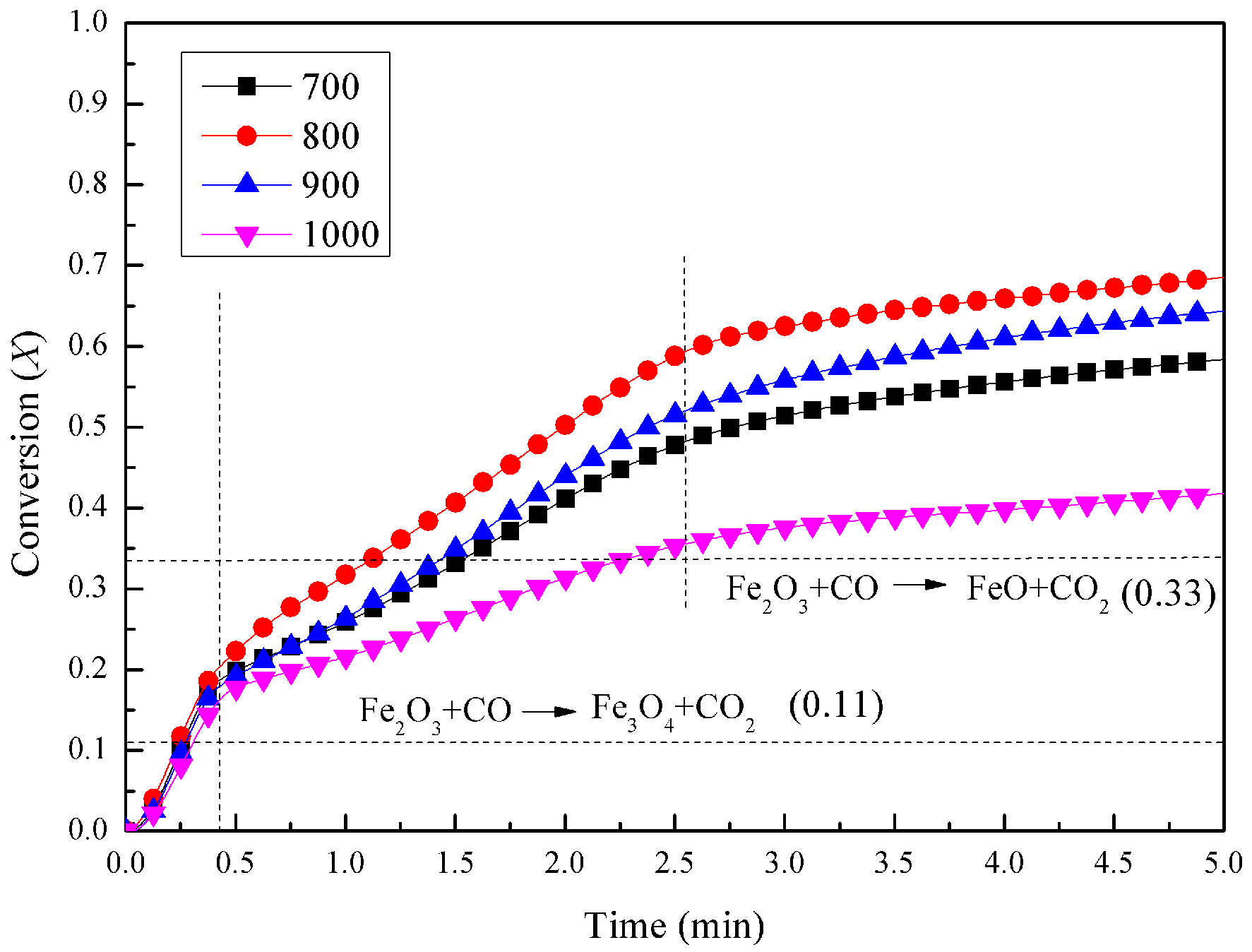
3.1. Experimental Results in the Fluidized-Bed Reactor
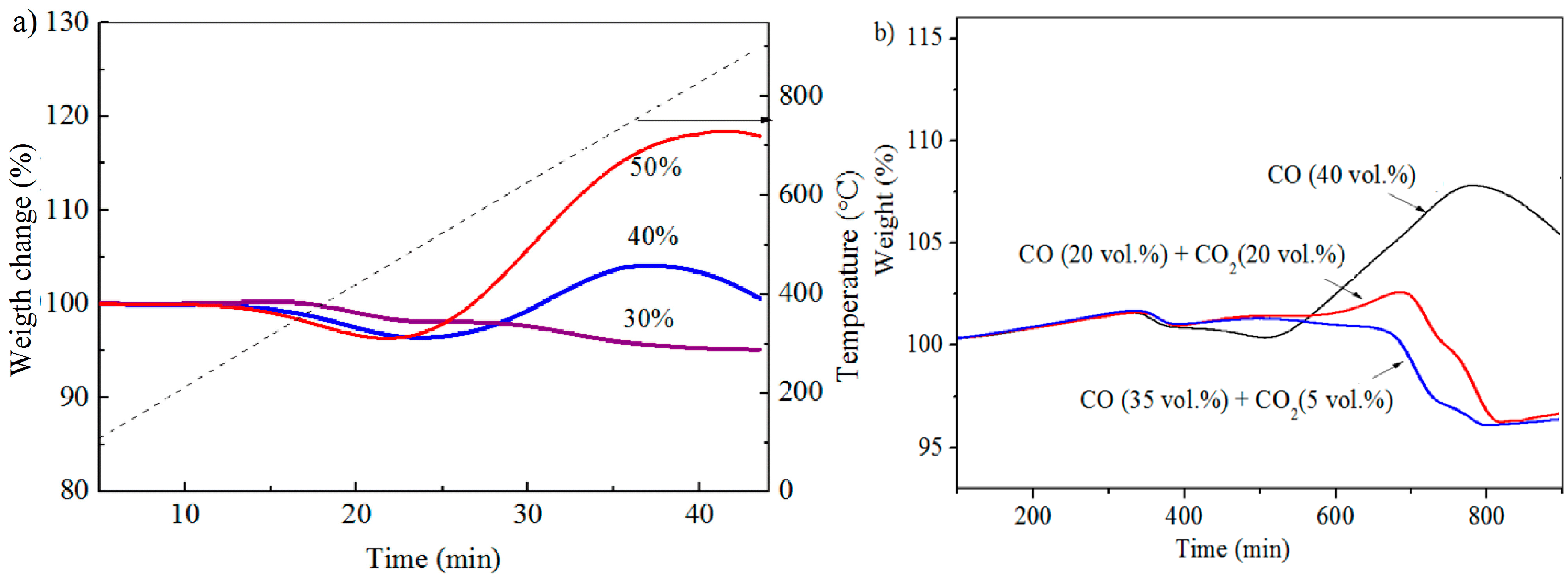
3.2. TGA Experiments
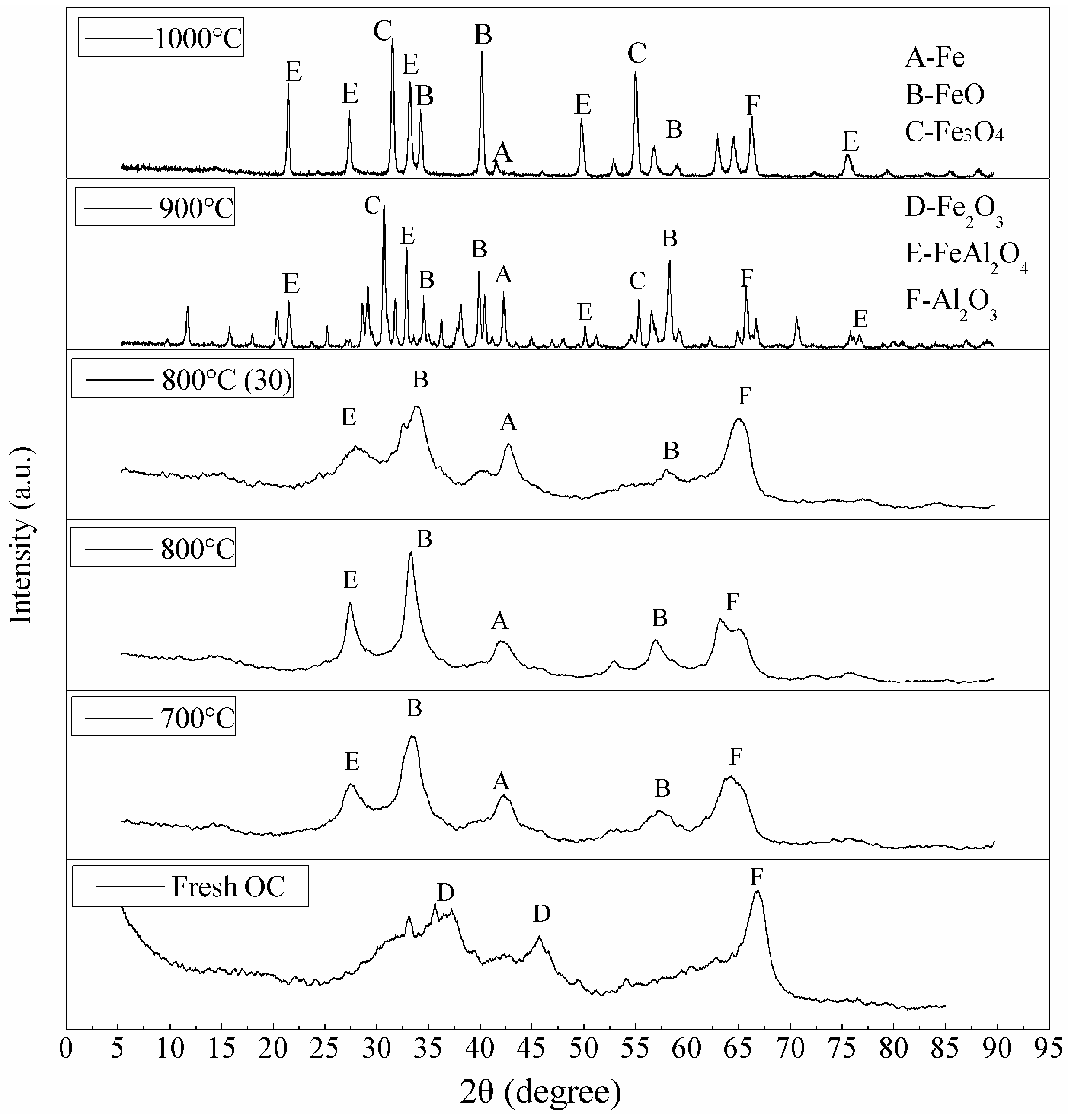
3.3. Characterization of the Oxygen Carrier
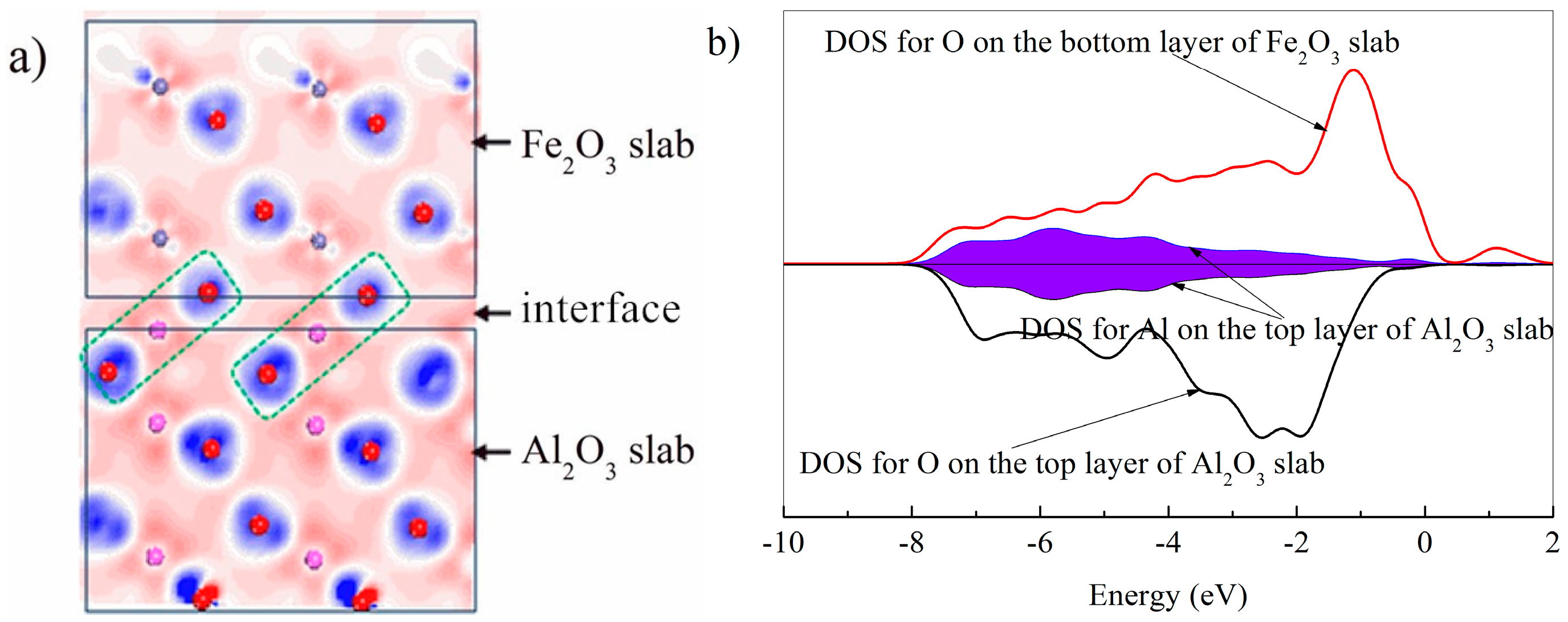
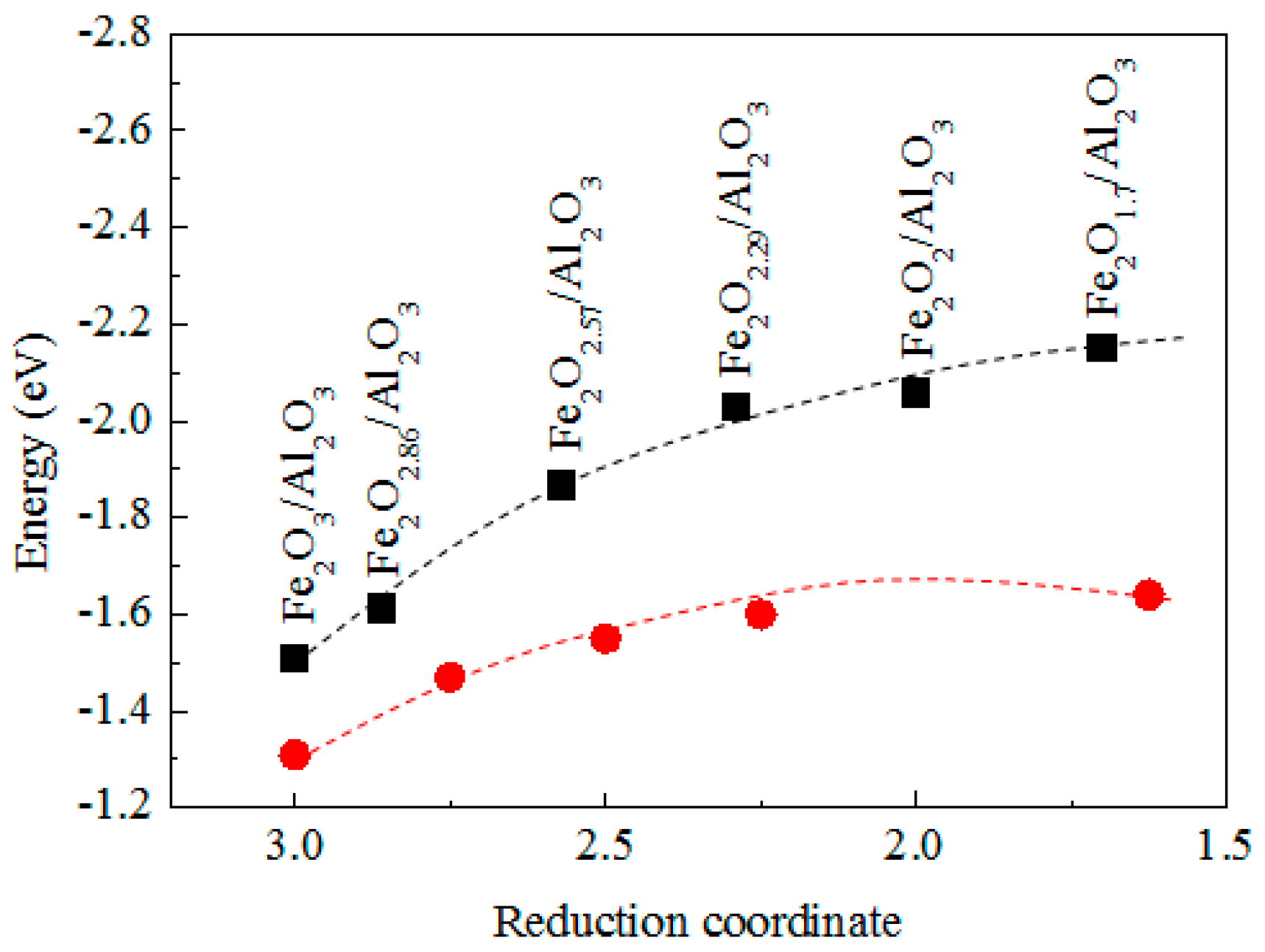
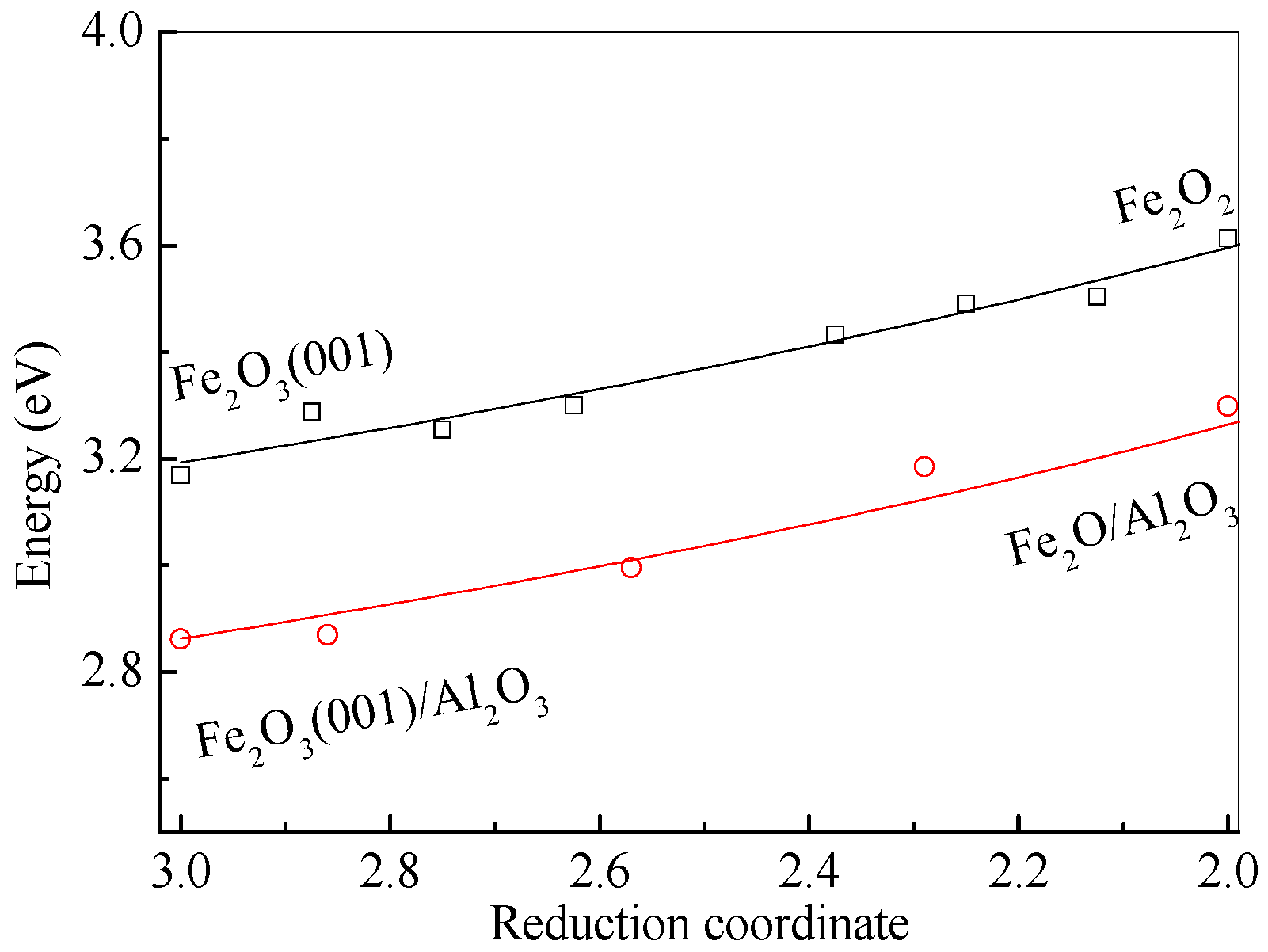
3.4. The Mechanism of CO CLC on Fe2O3/Al2O3
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Diego, L.F.; Garcı́a-Labiano, F.; Adánez, J.; Gayán, P.; Abad, A.; Corbella, B.M.; Marı́a Palacios, J. Development of Cu-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1749–1757. [Google Scholar] [CrossRef]
- Wolf, J.; Anheden, M.; Yan, J.Y. Comparison of nickel- and iron-based oxygen carriers in chemical looping combustion for CO2 capture in power generation. Fuel 2005, 84, 993–1006. [Google Scholar] [CrossRef]
- Dahl, I.M.; Bakken, E.; Larring, Y.; Spjelkavik, A.I.; Håkonsen, S.F.; Blom, R. On the development of novel reactor concepts for chemical looping combustion. Energy Procedia 2009, 1, 1513–1519. [Google Scholar] [CrossRef]
- De Diego, L.F.; Garcı́a-Labiano, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kwth chemical-looping combustor during 200 h with a CuO-Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar] [CrossRef]
- Dueso, C.; García-Labiano, F.; Adánez, J.; de Diego, L.F.; Gayán, P.; Abad, A. Syngas combustion in a chemical-looping combustion system using an impregnated Ni-based oxygen carrier. Fuel 2009, 88, 2357–2364. [Google Scholar] [CrossRef]
- Shen, L.H.; Zheng, M.; Xiao, J.; Zhang, H.; Xiao, R. Chemical looping combustion of coal in interconnected fluidized beds. Sci. Chin. Ser. E 2007, 50, 230–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Comprehensive study of Fe2O3/Al2O3 reduction with ultralow concentration methane under conditions pertinent to chemical looping combustion. Energy Fuels 2015, 29, 1951–1960. [Google Scholar] [CrossRef]
- Franca, R.V.; Thursfield, A.; Metcalfe, I.S. La0.6Sr0.4CO0.2Fe0.8O3-δ microtubular membranes for hydrogen production from water splitting. J. Membr. Sci. 2012, 389, 173–181. [Google Scholar]
- Echegoyen, Y.; Suelves, I.; Lázaro, M.J.; Sanjuán, M.L.; Moliner, R. Thermo catalytic decomposition of methane over Ni-Mg and Ni-Cu-Mg catalysts. Appl. Catal. A Gen. 2007, 333, 229–237. [Google Scholar] [CrossRef]
- Mattisson, T.; Leion, H.; Lyngfelt, A. Chemical-looping with oxygen uncoupling using CuO/ZrO2 with petroleum coke. Fuel 2009, 88, 683–690. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Lyngfelt, A. Chemical-looping with oxygen uncoupling using combined Mn-Fe oxides, testing in batch fluidized bed. Energy Procedia 2011, 4, 370–377. [Google Scholar] [CrossRef]
- Bermúdez, J.M.; Fidalgo, B.; Arenillas, A.; Menéndez, J.A. CO2 reforming of coke oven gas over a Ni/γAl2O3 catalyst to produce syngas for methanol synthesis. Fuel 2012, 94, 197–203. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, H.; Zheng, Y.; Liu, Z.; Yan, R.; Zheng, C. Chemical looping combustion of a Chinese anthracite with Fe2O3-based and CuO-based oxygen carriers. Fuel Process. Technol. 2012, 96, 104–115. [Google Scholar] [CrossRef]
- Mei, D.F.; Zhao, H.B.; Ma, Z.J.; Yang, W.J.; Fang, Y.F.; Zheng, C.G. Oxygen release kinetics and mechanism study on Cu-, Co-, Mn-based oxygen carrier. J. Fuel Chem. Technol. 2013, 41, 235–242. [Google Scholar] [CrossRef]
- Chen, D.Q.; Shen, L.H.; Xiao, J.; Song, T.; Gu, H.M.; Zhang, S.W. Experimental investigation of hematite oxygen carrier decorated with NiO for chemical-looping combustion of coal. J. Fuel Chem. Technol. 2012, 40, 267–272. [Google Scholar] [CrossRef]
- Aghabararnejad, M.; Patience, G.S.; Chaouki, J. TGA and kinetic modelling of CO, Mn and Cu oxides for chemical looping gasification (CLG). Can. J. Chem. Eng. 2014, 92, 1903–1910. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Lee, D.H.; Zheng, Y.; Zhao, H.; Zheng, C. Characterization and evaluation of Fe2O3/Al2O3 oxygen carrier prepared by sol–gel combustion synthesis. J. Anal. Appl. Pyrolysis 2011, 91, 105–113. [Google Scholar] [CrossRef]
- Li, K.; Wang, H.; Wei, Y.; Yan, D. Direct conversion of methane to synthesis gas using lattice oxygen of CeO2–Fe2O3 complex oxides. Chem. Eng. J. 2010, 156, 512–518. [Google Scholar] [CrossRef]
- Gayán, P.; Pans, M.A.; Ortiz, M.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Testing of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for a SR–CLC system in a continuous CLC unit. Fuel Process. Technol. 2012, 96, 37–47. [Google Scholar] [CrossRef]
- Gao, Z.P.; Shen, L.H.; Xiao, J.; Zheng, M.; Wu, J.H. Analysis of reactivity of Fe-based oxygen carrier with coal during chemical-looping combustion. J. Fuel Chem. Technol. 2009, 37, 513–520. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zhu, H.; Chen, D.; Wei, G.; Feng, Y.; Zheng, A.; Zhao, Z.; Li, H. Thermodynamic analysis and thermogravimetric investigation on chemical looping gasification of biomass char under different atmospheres with Fe2O3 oxygen carrier. Appl. Energy 2015, 157, 546–553. [Google Scholar] [CrossRef]
- Zhang, Y.; Doroodchi, E.; Moghtaderi, B. Reduction kinetics of Fe2O3/Al2O3 by ultralow concentration methane under conditions pertinent to chemical looping combustion. Energy Fuels 2015, 29, 337–345. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar]
- Luo, M.; Wang, S.; Wang, L.; Lv, M. Reduction kinetics of iron-based oxygen carriers using methane for chemical-looping combustion. J. Power Sources 2014, 270, 434–440. [Google Scholar] [CrossRef]
- Botas, J.A.; Melero, J.A.; Martinez, F.; Pariente, M.I. Assessment of Fe2O3/SiO2 catalysts for the continuous treatment of phenol aqueous solutions in a fixed bed reactor. Catal. Today 2010, 149, 334–340. [Google Scholar] [CrossRef]
- Fang, F.; Li, Z.S.; Cai, N.S.; Tang, X.Y.; Yang, H.T. AFM investigation of solid product layers of MgSO4 generated on MgO surfaces for the reaction of MgO with SO2 and O2. Chem. Eng. Sci. 2011, 66, 1142–1149. [Google Scholar] [CrossRef]
- Cabello, A.; Dueso, C.; Garcia-Labiano, F.; Gayán, P.; Abad, A.; de Diego, L.F.; Adánez, J. Performance of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier with CH4 and H2S in a 500 W-th CLC unit. Fuel 2014, 121, 117–125. [Google Scholar] [CrossRef]
- Hossain, M.M.; Ahmed, S. Cu-based mixed metal oxide catalysts for WGSR: Reduction kinetics and catalytic activity. Can. J. Chem. Eng. 2013, 91, 1450–1458. [Google Scholar] [CrossRef]
- Saha, C.; Zhang, S.; Hein, K.; Xiao, R.; Bhattacharya, S. Chemical looping combustion (CLC) of two Victorian brown coals—Part 1: Assessment of interaction between CuO and minerals inherent in coals during single cycle experiment. Fuel 2013, 104, 262–274. [Google Scholar]
- Xin, J.; Wang, F.; He, F.; Zhang, Z. Thermodynamic and equilibrium composition analysis of using iron oxide as an oxygen carrier in nonflame combustion technology. J. Nat. Gas Chem. 2005, 04, 248–253. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in car-parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Yang, T.; Wen, X.D.; Ren, J.; Li, Y.W.; Wang, J.G.; Huo, C.F. Surface structures of Fe3O4(111), (110), and (001): A density functional theory study. J. Fuel Chem. Technol. 2010, 38, 121–128. [Google Scholar] [CrossRef]
- Govind, N.; Petersen, M.; Fitzgerald, G.; King-Smith, D.; Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 2003, 28, 250–258. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Sun, Z.; Li, P.; Yu, J. Density functional theory study on the mechanism of calcium sulfate reductive decomposition by carbon monoxide. Ind. Eng. Chem. Res. 2012, 51, 6563–6570. [Google Scholar] [CrossRef]
- Song, J.; Niu, X.; Ling, L.; Wang, B. A density functional theory study on the interaction mechanism between H2S and the α-Fe2O3(0001) surface. Fuel Process. Technol. 2013, 115, 26–33. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Zhang, S.; Rajendran, S.; Henderson, S.; Zeng, D.; Xiao, R.; Bhattacharya, S. Use of pyrite cinder as an iron-based oxygen carrier in coal-fueled chemical looping combustion. Energy Fuels 2015, 29, 2645–2655. [Google Scholar] [CrossRef]
- Dong, C.; Sheng, S.; Qin, W.; Lu, Q.; Zhao, Y.; Wang, X.; Zhang, J. Density functional theory study on activity of α-Fe2O3 in chemical-looping combustion system. Appl. Surf. Sci. 2011, 257, 8647–8652. [Google Scholar] [CrossRef]
- Claridge, J.B.; Green, M.L.H.; Tsang, S.C.; York, A.P.E.; Ashcroft, A.T.; Battle, P.D. A study of carbon deposition on catalysts during the partial oxidation of methane to synthesis gas. Catal. Lett. 1993, 22, 299–305. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Cleeton, J.P.E.; Scott, S.A.; Dennis, J.S.; Li, Z.; Cai, N. Interaction between Fe-based oxygen carriers and N-heptane during chemical looping combustion. Proc. Combust. Inst. 2013, 34, 2839–2846. [Google Scholar] [CrossRef]
- Dong, C.Q.; Liu, X.L.; Qin, W.; Lu, Q.; Wang, X.Q.; Shi, S.M.; Yang, Y.P. Deep reduction behavior of iron oxide and its effect on direct CO oxidation. Appl. Surf. Sci. 2012, 258, 2562–2569. [Google Scholar] [CrossRef]
- Kierzkowska, A.M.; Bohn, C.D.; Scott, S.A.; Cleeton, J.P.; Dennis, J.S.; Muller, C.R. Development of iron oxide carriers for chemical looping combustion using sol-gel. Ind. Eng. Chem. Res. 2010, 49, 5383–5391. [Google Scholar] [CrossRef]
- Hafizi, A.; Rahimpour, M.R.; Hassanajili, S. Calcium promoted Fe/Al2O3 oxygen carrier for hydrogen production via cyclic chemical looping steam methane reforming process. Int. J. Hydrogen Energy 2015, 40, 16159–16168. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Liu, Y.; Cheng, Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Ind. Eng. Chem. Res. 2012, 51, 12773–12781. [Google Scholar] [CrossRef]
- Li, J.; Lin, C.; Qin, W.; Xiao, X.; Wei, L. Synergetic effect of mercury adsorption on the catalytic decomposition of CO over perfect and reduced Fe2O3 [001] surface. Acta Phys. Chim. Sin. 2016, 32, 2717–2723. [Google Scholar]
- Qin, W.; Lin, C.F.; Long, D.T.; Wang, J.Y.; Dong, C.Q. Activity of Fe2O3 with a high index facet for bituminous coal chemical looping combustion: A theoretical and experimental study. RSC Adv. 2016, 6, 85551–85558. [Google Scholar] [CrossRef]
- Qin, W.; Wang, Y.; Lin, C.; Hu, X.; Dong, C. Possibility of morphological control to improve the activity of oxygen carriers for chemical looping combustion. Energy Fuels 2015, 29, 1210–1218. [Google Scholar] [CrossRef]
- Lin, C.F.; Qin, W.; Dong, C.Q. Reduction effect of α-Fe2O3 on carbon deposition and CO oxidation during chemical-looping combustion. Chem. Eng. J. 2016, 301, 257–265. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Qin, W.; Dong, C. Experimental and Theoretical Study of the Interactions between Fe2O3/Al2O3 and CO. Energies 2017, 10, 598. https://doi.org/10.3390/en10050598
Liang Z, Qin W, Dong C. Experimental and Theoretical Study of the Interactions between Fe2O3/Al2O3 and CO. Energies. 2017; 10(5):598. https://doi.org/10.3390/en10050598
Chicago/Turabian StyleLiang, Zhiyong, Wu Qin, and Changqing Dong. 2017. "Experimental and Theoretical Study of the Interactions between Fe2O3/Al2O3 and CO" Energies 10, no. 5: 598. https://doi.org/10.3390/en10050598




