Investigation of Methane Oxy-Fuel Combustion in a Swirl-Stabilised Gas Turbine Model Combustor
Abstract
:1. Introduction
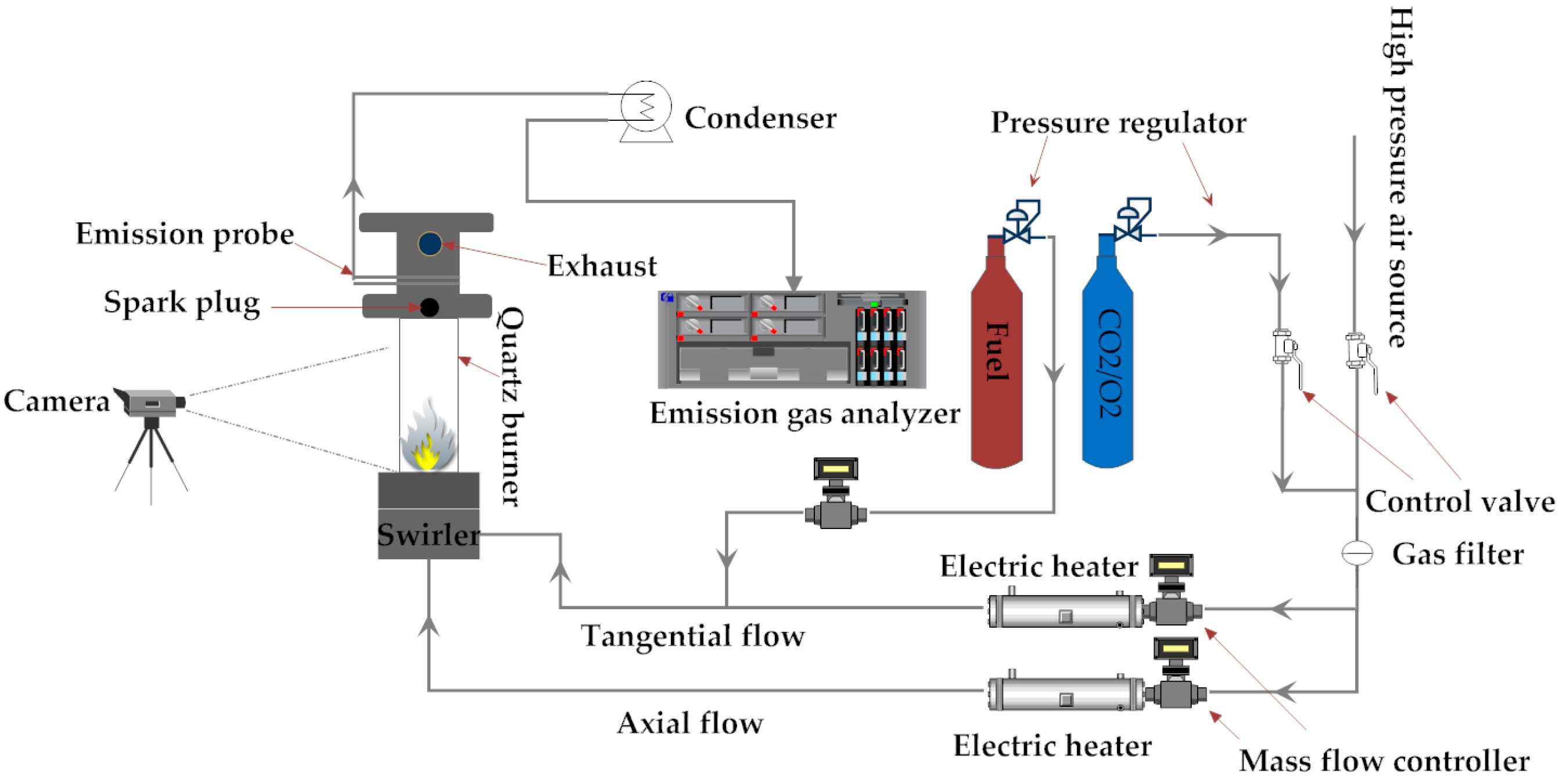
2. Experimental Apparatus and Methods
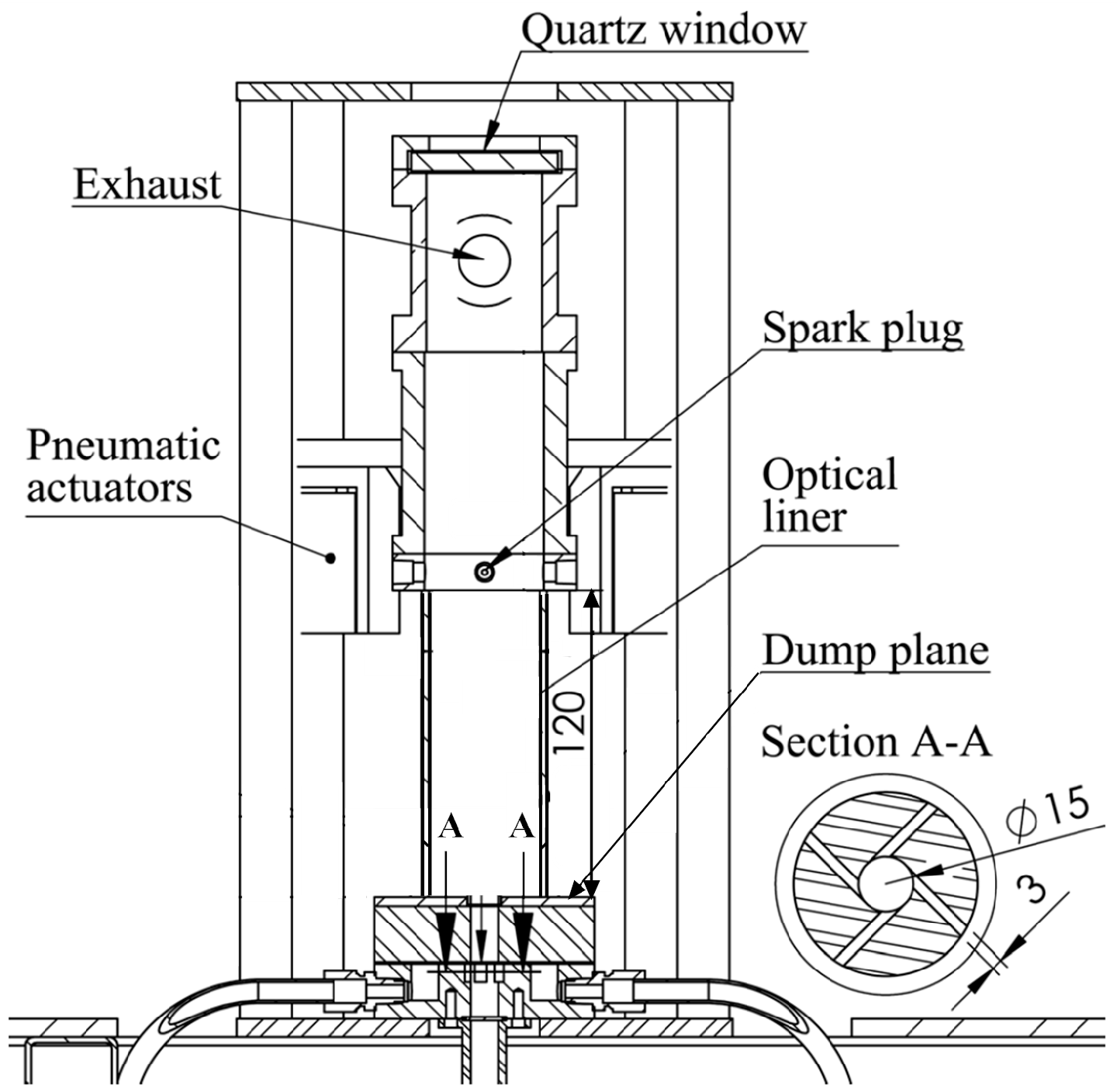
2.1. Flow Paths and Combustor
2.2. Measurement Conditions
2.3. Lean Blowout Measurement
2.4. Emission Measurement
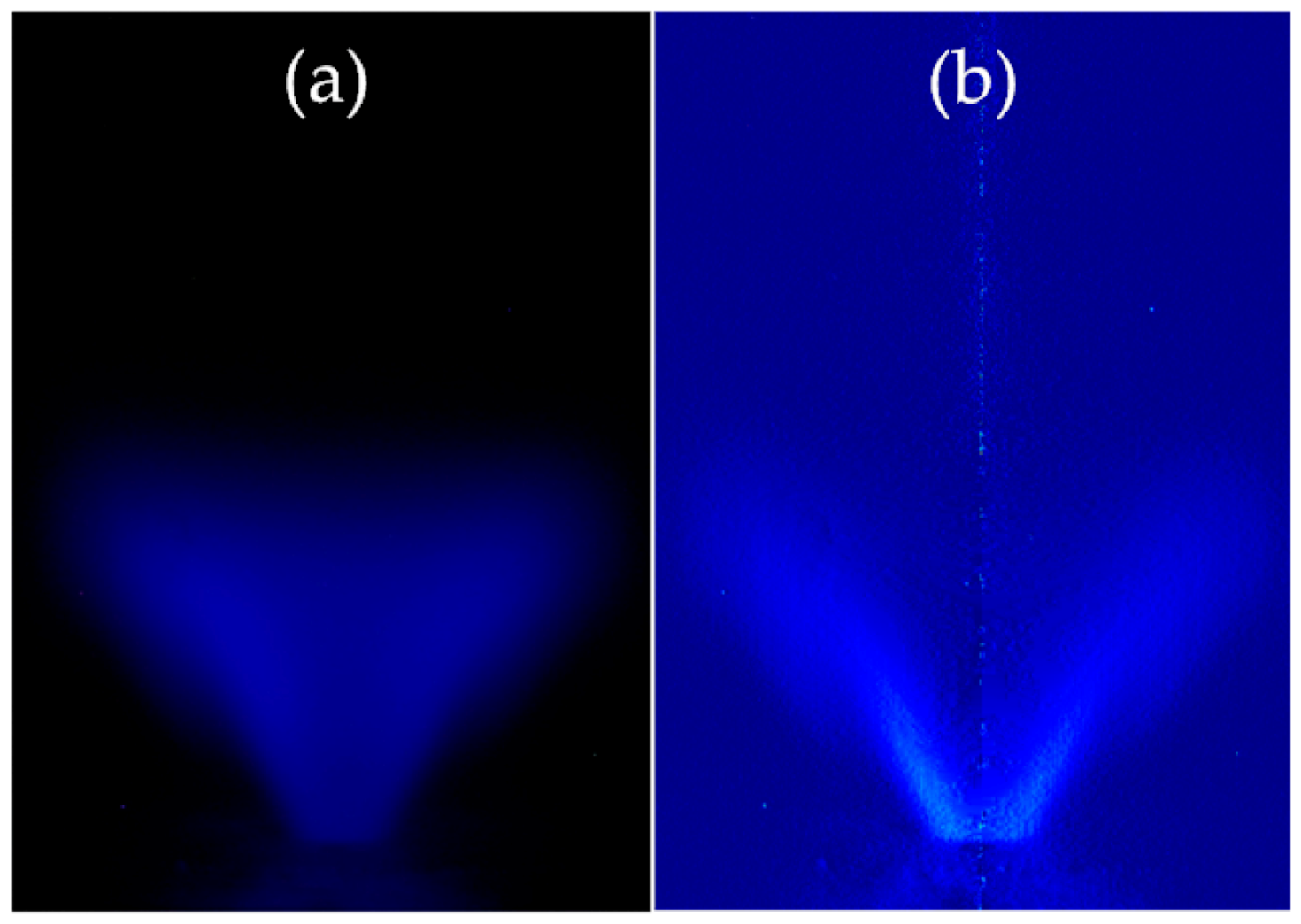
2.5. Flame Visualisation with CH Chemiluminescence Imaging
2.6. Chemical Kinetic Simulation
3. Results and Discussion
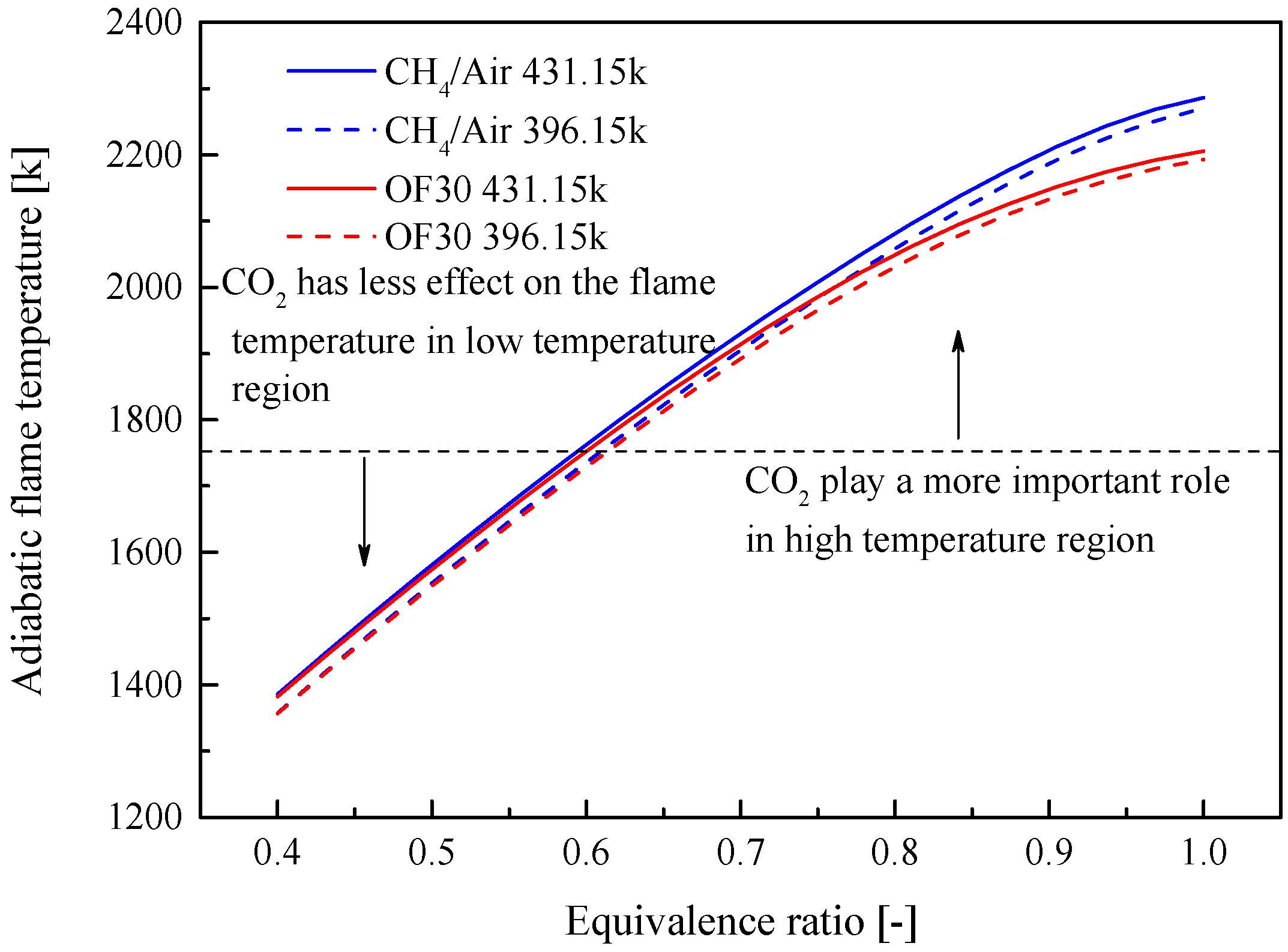
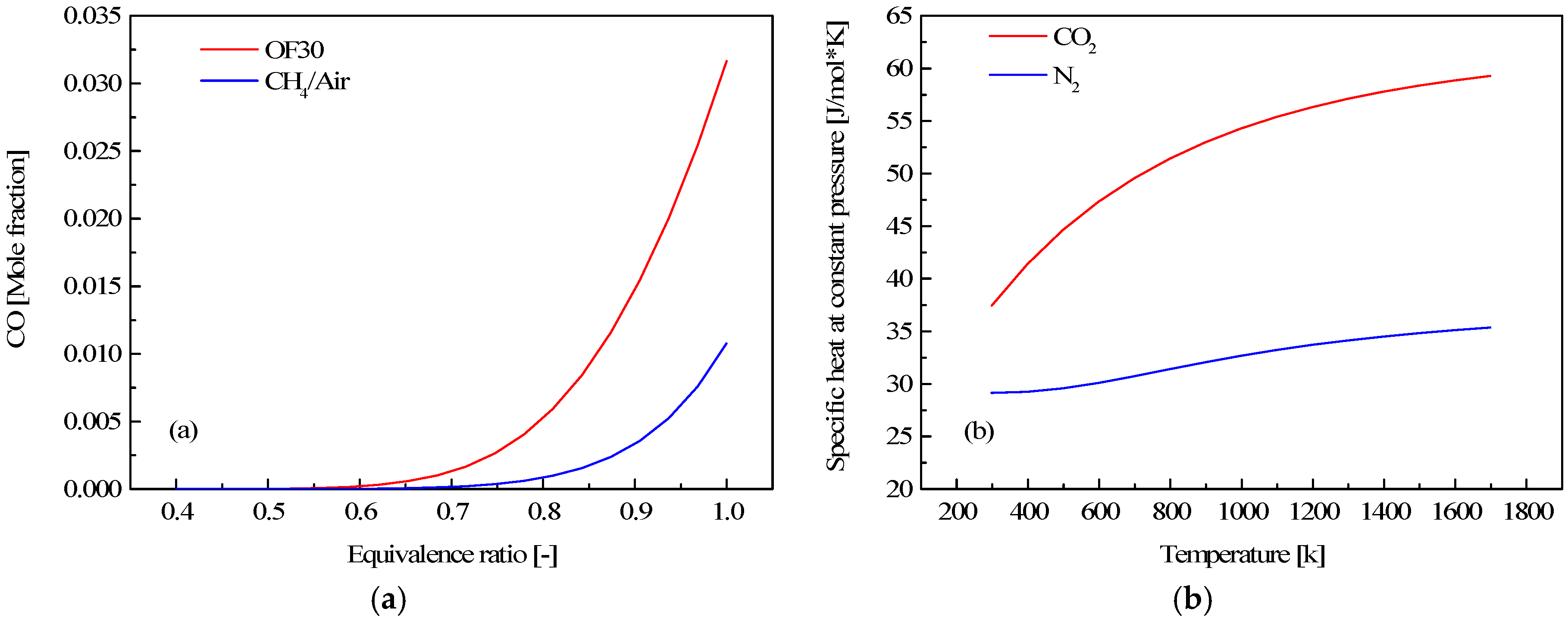
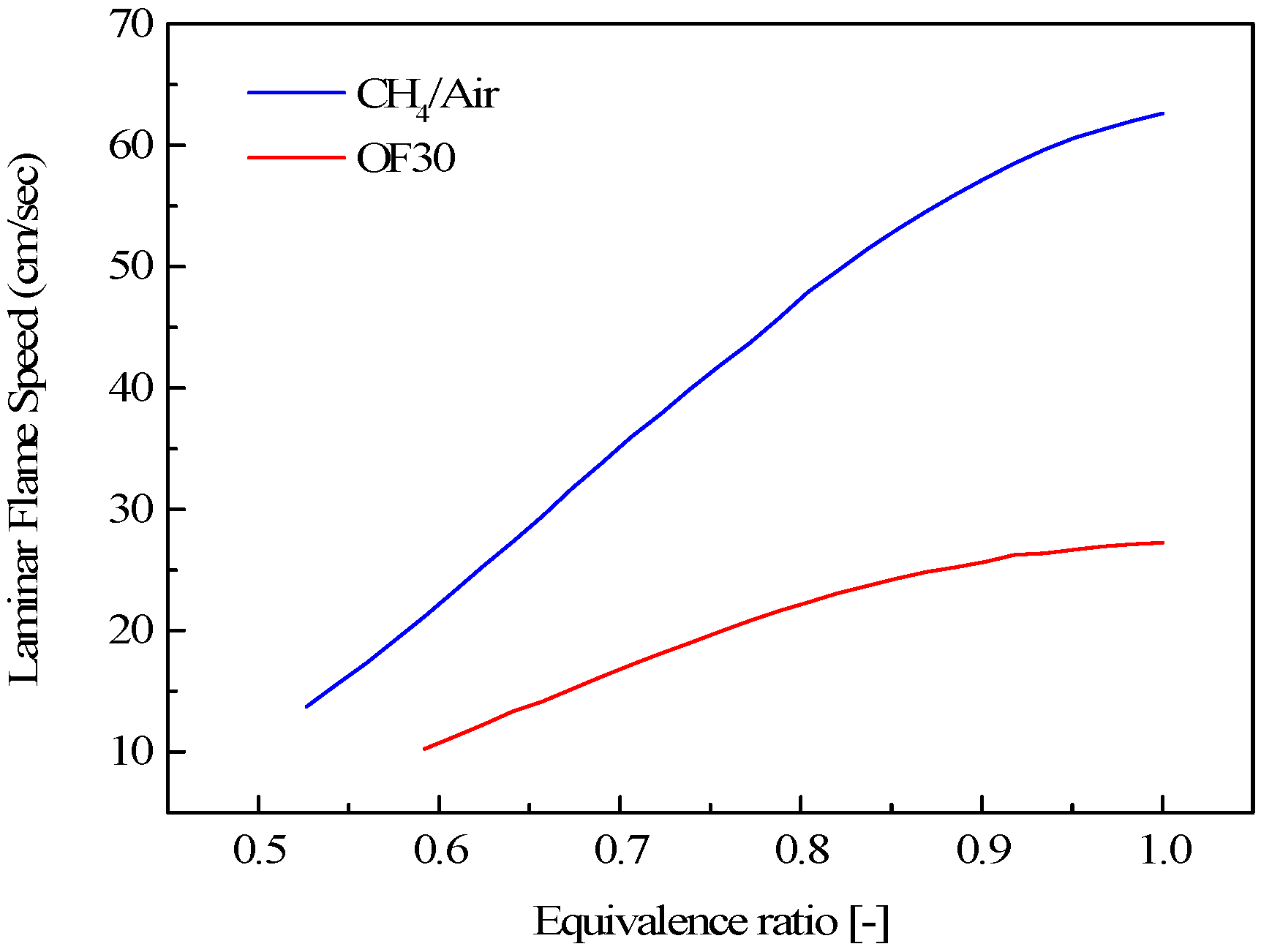
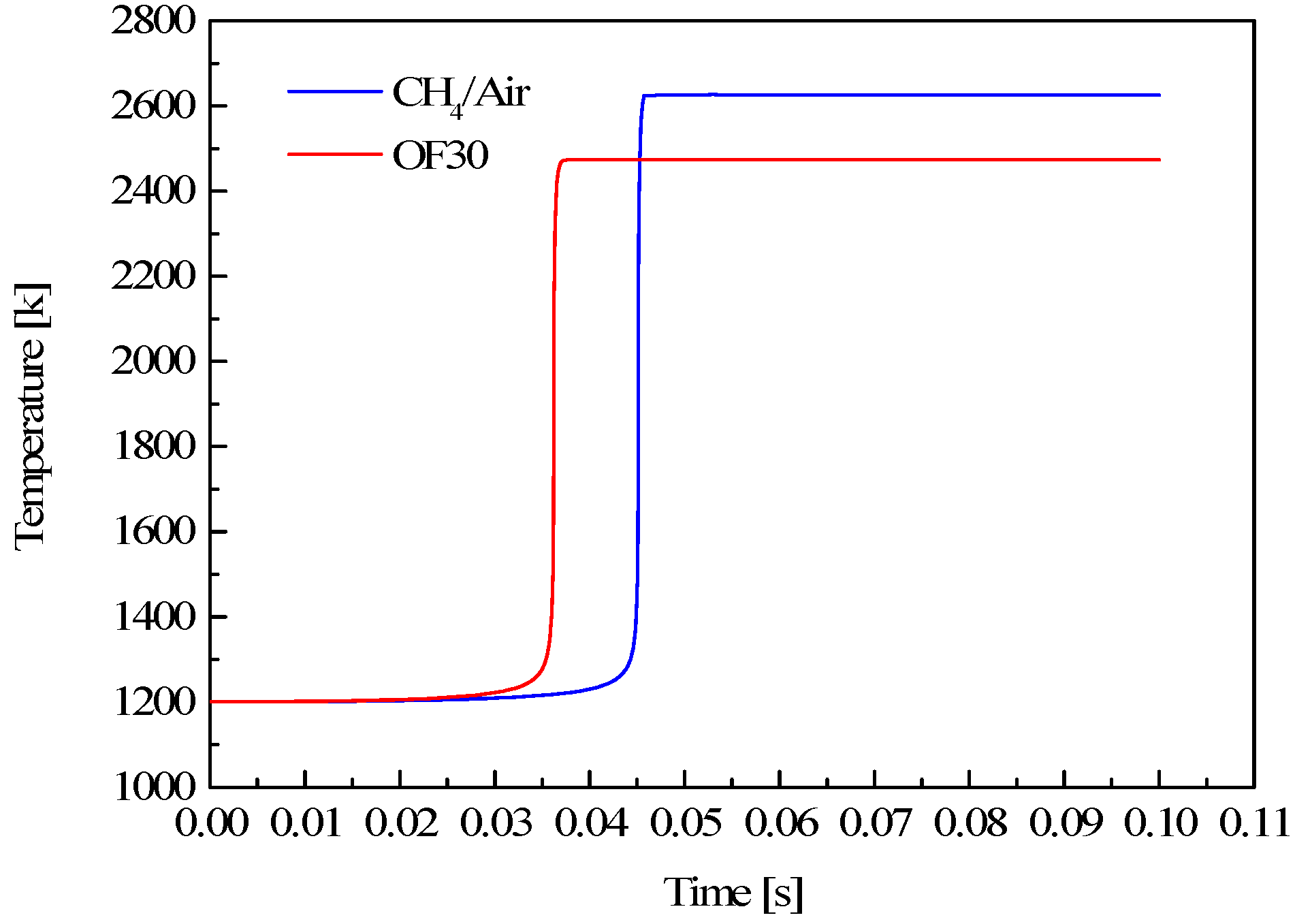
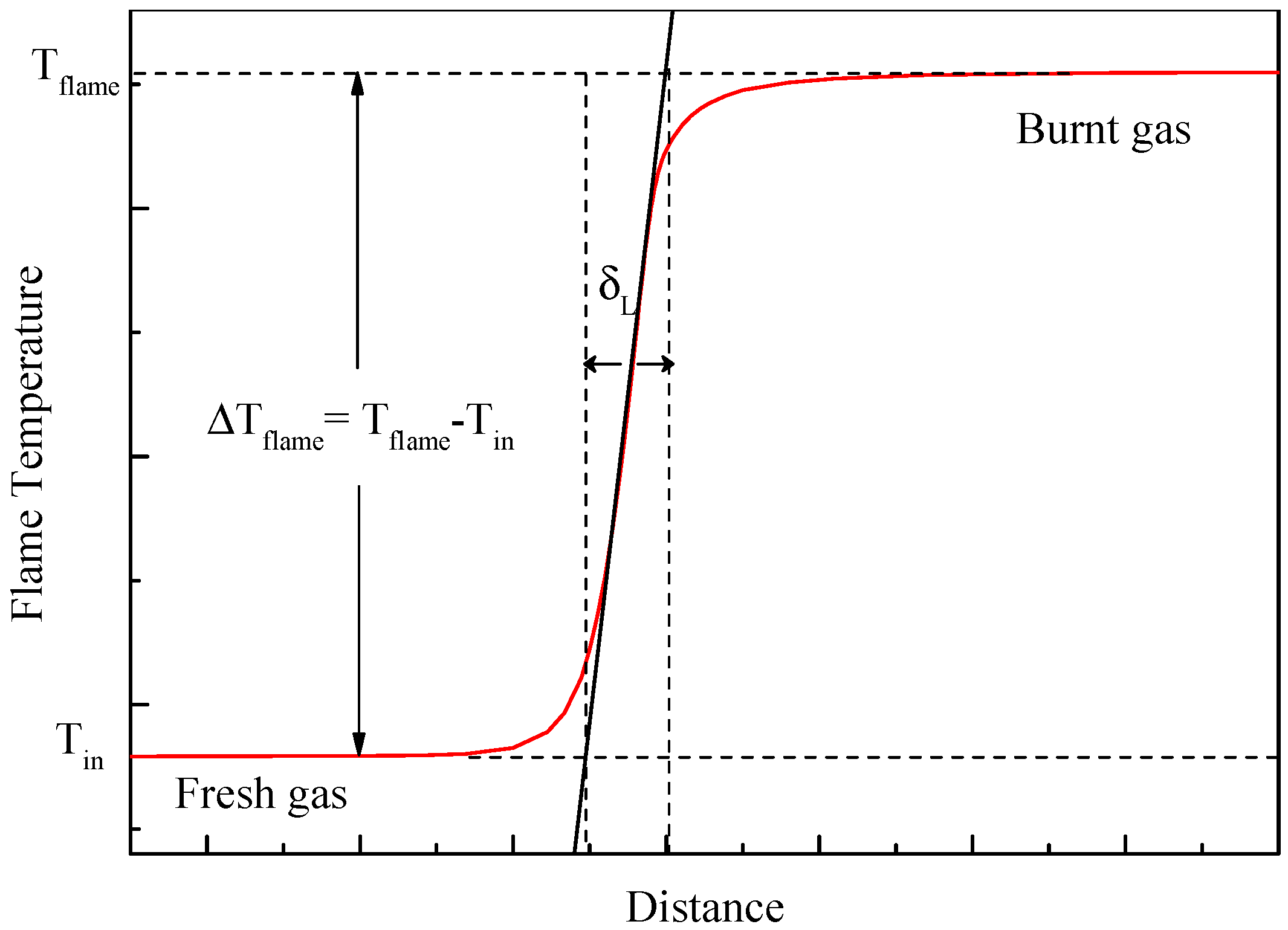
3.1. Effect of CO2 on Some Fundamental Combustion Characteristics
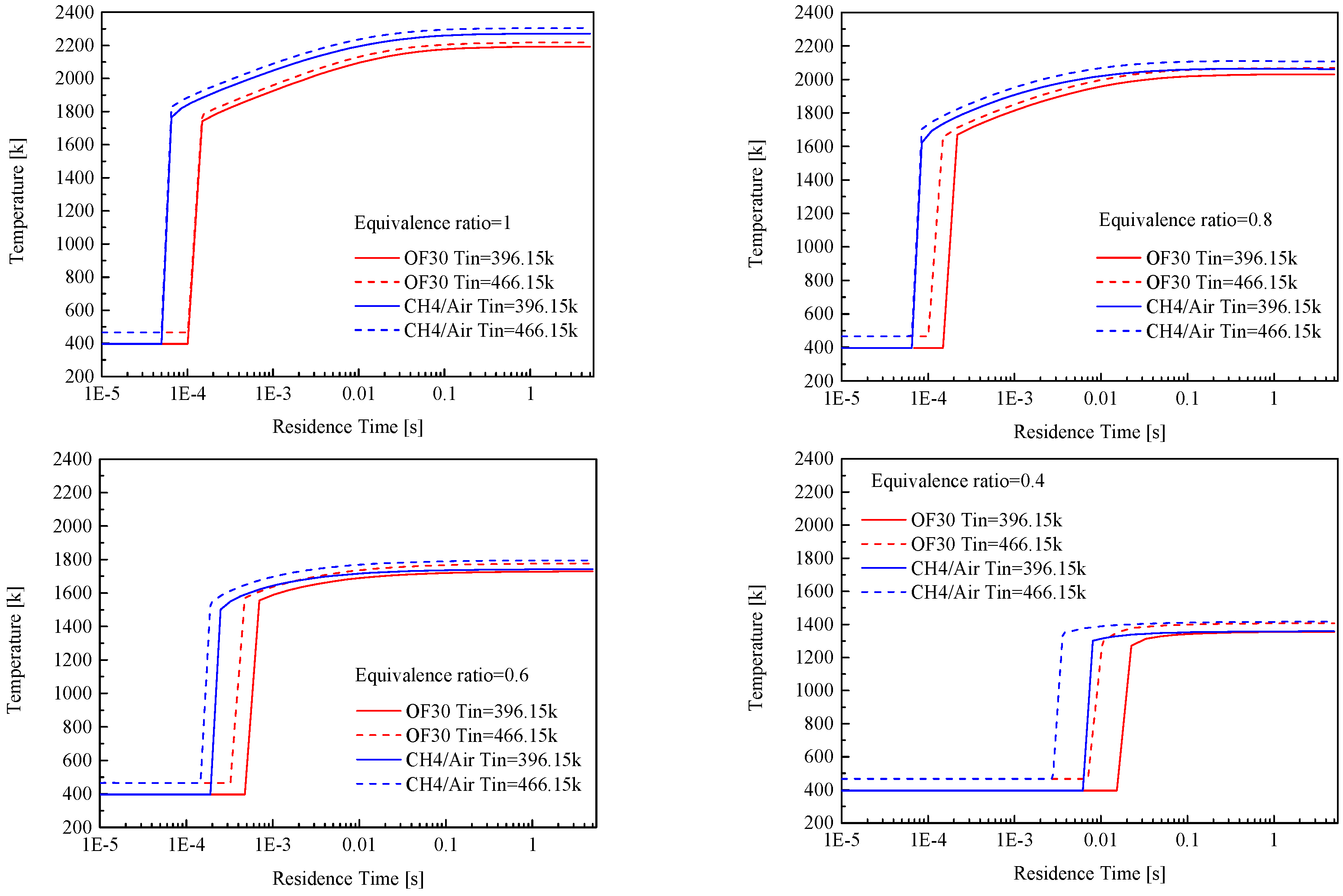
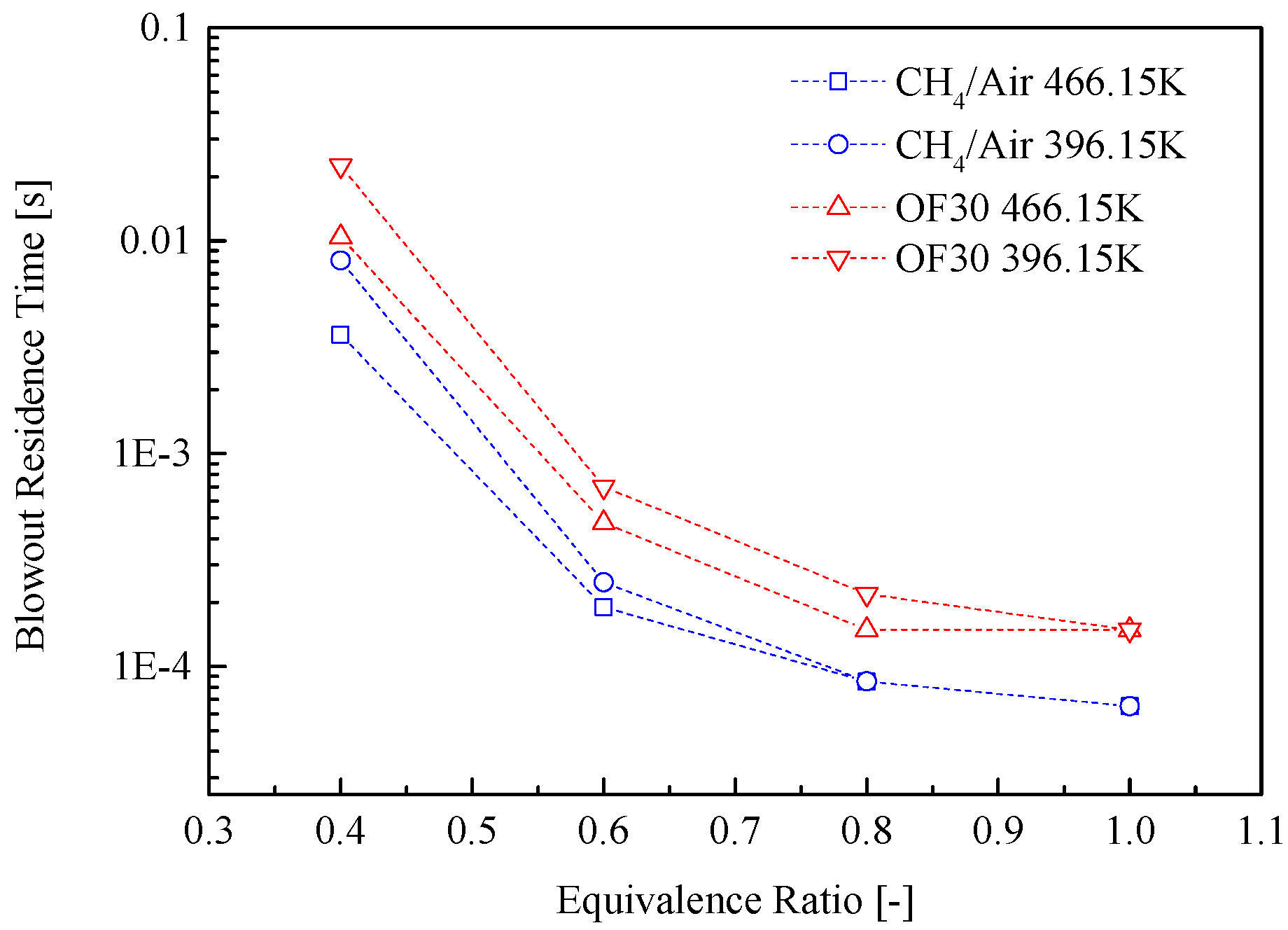
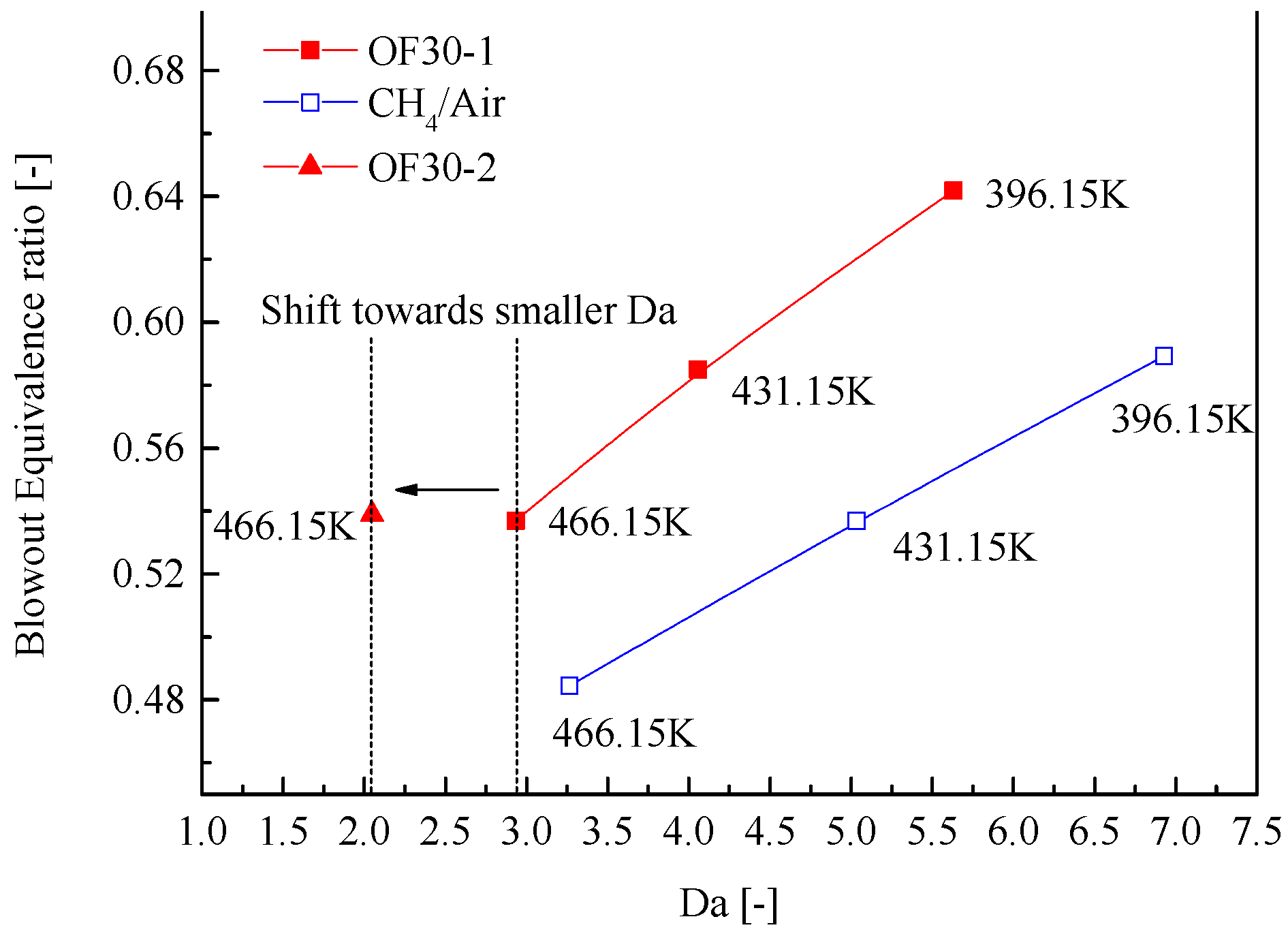
3.2. Lean Blowout Limits
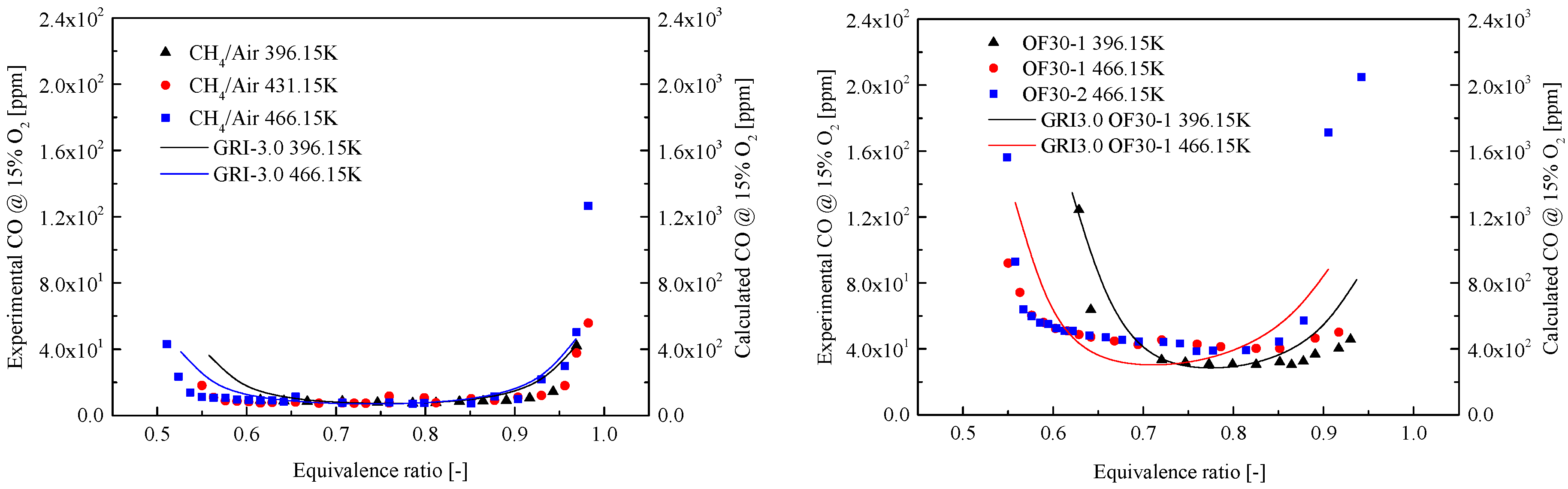
3.3. CO Emission
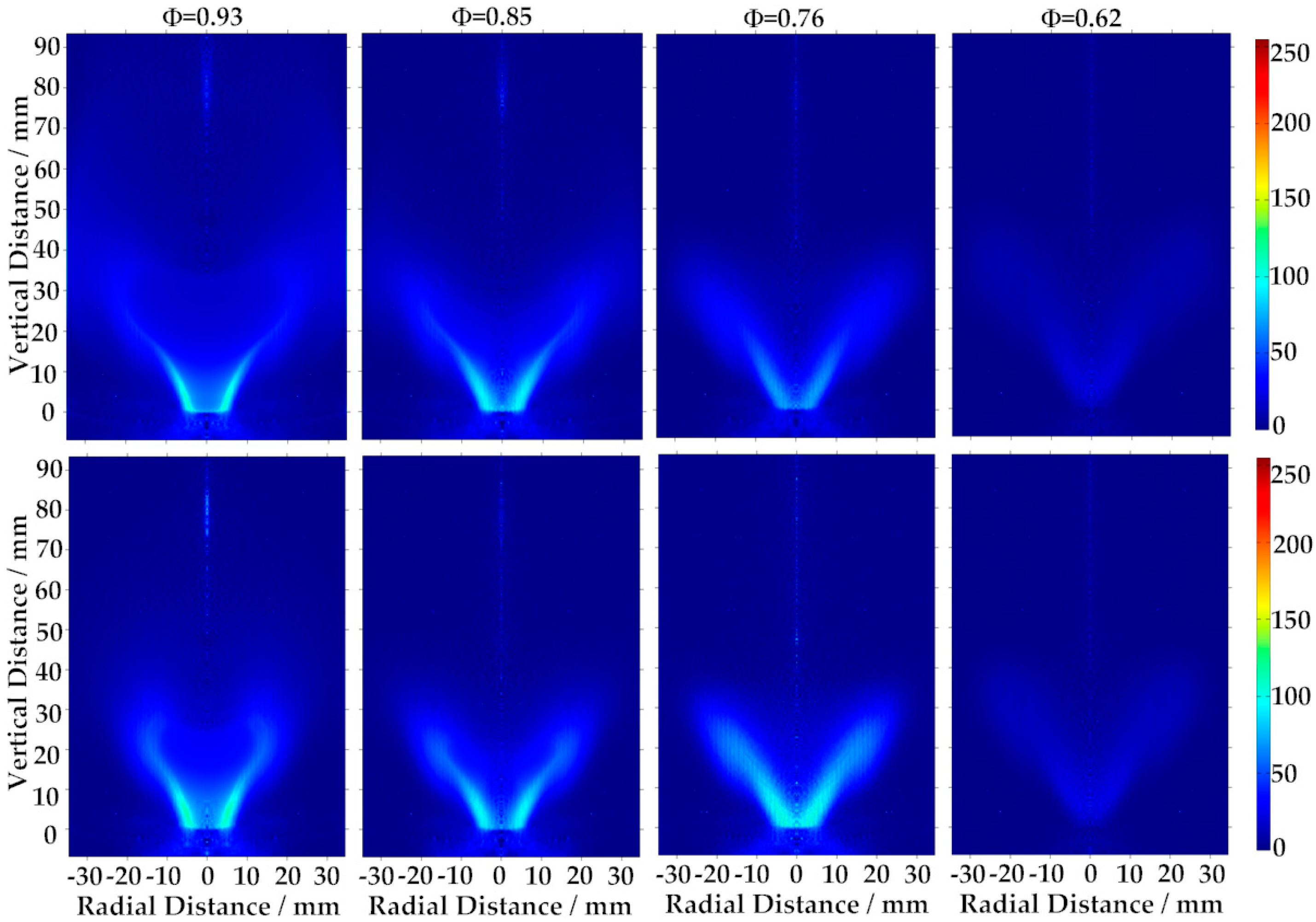
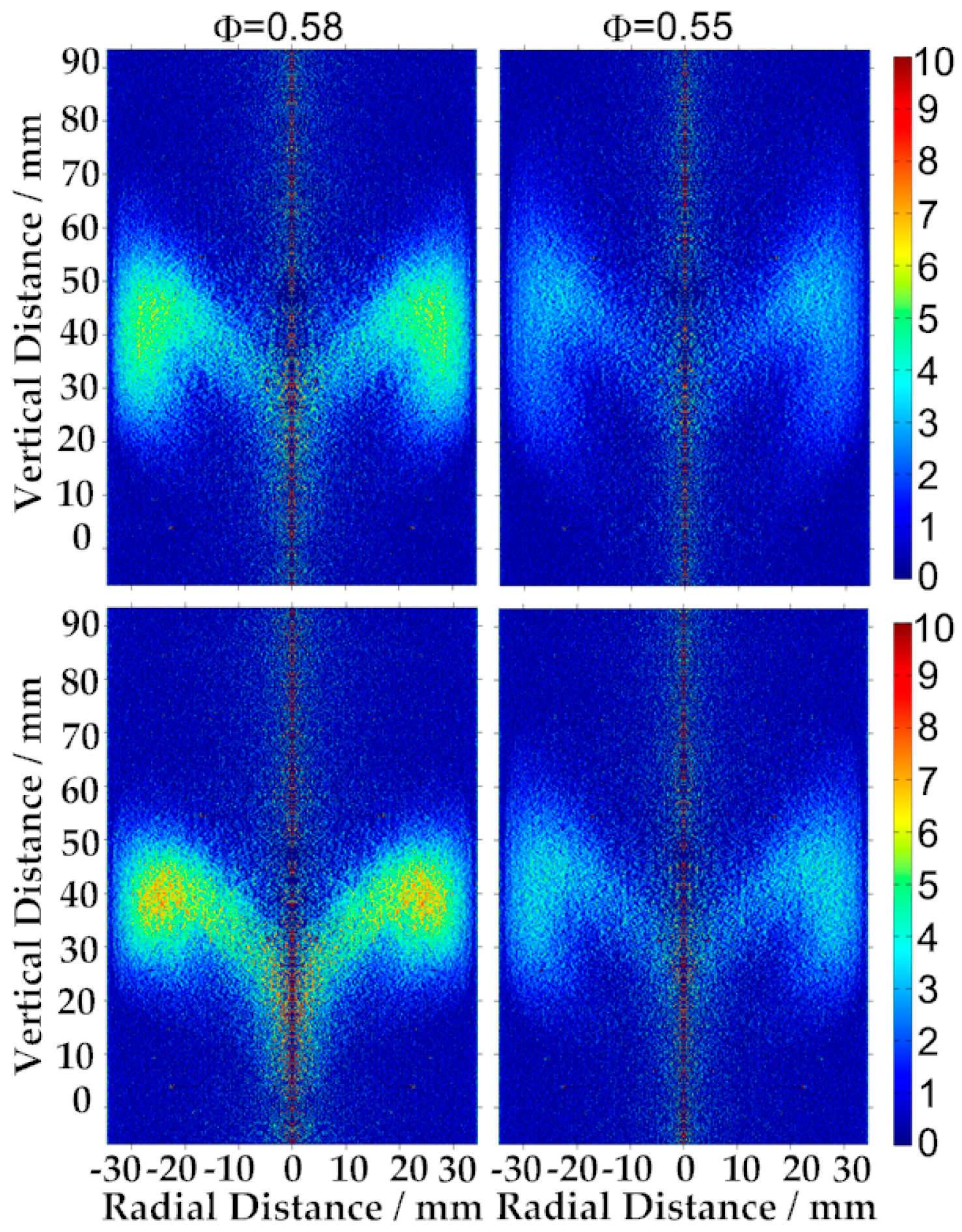
3.4. Flame Observation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Anderson, R.E.; Mac Adam, S.; Viteri, F.; Davies, D.O.; Downs, J.P.; Paliszewski, A. Adapting gas turbines to zero emission oxy-fuel power plants. In Proceedings of the ASME Turbo Expo 2008: Power for Land, Sea, and Air, Berlin, Germany, 9–13 June 2008; pp. 781–791. [Google Scholar]
- Heddle, G.; Herzog, H.; Klett, M. The Economics of CO2 Storage; Massachusetts Institute of Technology, Laboratory for Energy and the Environment: Cambridge, MA, USA, 2003. [Google Scholar]
- Hustad, C.-W. The Norwegian CO2-infrastructure initiative: A feasibility study. In Proceedings of the 5th International Conference on Greenhouse Gas Control Technology, Cairns, Australia, 13–16 August 2000. [Google Scholar]
- Stanger, R.; Wall, T.; Spoerl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Maruta, K.; Abe, K.; Hasegawa, S.; Maruyama, S.; Sato, J. Extinction characteristics of CH4/CO2 versus O2/CO2 counterflow non-premixed flames at elevated pressures up to 0.7 MPa. Proc. Combust. Inst. 2007, 31, 1223–1230. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, X.; Xu, B.; Ju, Y.; Liu, F. Studies of radiation absorption on flame speed and flammability limit of CO2 diluted methane flames at elevated pressures. Proc. Combust. Inst. 2007, 31, 2693–2700. [Google Scholar] [CrossRef]
- Ruan, J.; Kobayashi, H.; Niioka, T.; Ju, Y. Combined effects of nongray radiation and pressure on premixed CH4/O2/CO2 flames. Combust. Flame 2001, 124, 225–230. [Google Scholar] [CrossRef]
- Guo, H.; Jub, Y.; Maruta, K.; Niioka, T.; Liu, F. Numerical investigation of CH4/CO2/air and CH4/CO2/O2 counterflow premixed flames with radiation reabsorption. Combust. Sci. Technol. 1998, 135, 49–64. [Google Scholar] [CrossRef]
- Ju, Y.; Masuya, G.; Ronney, P.D. Effects of radiative emission and absorption on the propagation and extinction of premixed gas flames. Symp. (Int.) Combust. 1998, 27, 2619–2626. [Google Scholar] [CrossRef]
- Yossefi, D.; Belmont, M.; Maskell, S.; Ben-Dor, G. Stimulation and implementation of laminar flow reactors for the study of combustion systems of ethane, methane and deborane. Fuel 1998, 77, 173–181. [Google Scholar] [CrossRef]
- Yossefi, D.; Ashcroft, S.; Hacohen, J.; Belmont, M.; Thorpe, I. Combustion of methane and ethane with CO2 replacing N2 as a diluent. Modelling of combined effects of detailed chemical kinetics and thermal properties on the early stages of combustion. Fuel 1995, 74, 1061–1071. [Google Scholar] [CrossRef]
- Natarajan, J.; Lieuwen, T.; Seitzman, J. Laminar flame speeds of H2/CO mixtures: Effect of CO2 dilution, preheat temperature, and pressure. Combust. Flame 2007, 151, 104–119. [Google Scholar] [CrossRef]
- Walton, S.; He, X.; Zigler, B.; Wooldridge, M. An experimental investigation of the ignition properties of hydrogen and carbon monoxide mixtures for syngas turbine applications. Proc. Combust. Inst. 2007, 31, 3147–3154. [Google Scholar] [CrossRef]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Inst. 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Liu, J.; Sun, N. Investigation of laminar flame speeds of CH4/O2/CO2 mixtures at ordinary pressure and kinetic simulation. Energy 2014, 70, 626–634. [Google Scholar] [CrossRef]
- Ditaranto, M.; Hals, J. Combustion instabilities in sudden expansion oxy-fuel flames. Combust. Flame 2006, 146, 493–512. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Q.; Sun, N.; Qin, Q. Experimental study of flammability limits of oxy-methane mixture and calculation based on thermal theory. Int. J. Hydrogen Energy 2014, 39, 9527–9533. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liaw, H.-J.; Wang, T.-C.; Lin, C.-Y. Carbon dioxide dilution effect on flammability limits for hydrocarbons. J. Hazard. Mater. 2009, 163, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Takizawa, K.; Takahashi, A.; Tokuhashi, K. Extended Le Chatelier’s formula for carbon dioxide dilution effect on flammability limits. J. Hazard. Mater. 2006, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kutne, P.; Kapadia, B.K.; Meier, W.; Aigner, M. Experimental analysis of the combustion behaviour of oxyfuel flames in a gas turbine model combustor. Proc. Combust. Inst. 2011, 33, 3383–3390. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Habib, M.A. Experimental and numerical investigations of an atmospheric diffusion oxy-combustion flame in a gas turbine model combustor. Appl. Energy 2013, 111, 401–415. [Google Scholar] [CrossRef]
- Lee, M.C.; Seo, S.B.; Yoon, J.; Kim, M.; Yoon, Y. Experimental study on the effect of N2, CO2, and steam dilution on the combustion performance of H2 and CO synthetic gas in an industrial gas turbine. Fuel 2012, 102, 431–438. [Google Scholar] [CrossRef]
- Saanum, I.; Ditaranto, M. Experimental study of oxy-fuel combustion under gas turbine conditions. Energy Fuels 2017, 31, 4445–4451. [Google Scholar] [CrossRef]
- Nori, V.; Seitzman, J. Evaluation of chemiluminescence as a combustion diagnostic under varying operating conditions. In Proceedings of the 46th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 7–10 January 2008; p. 953. [Google Scholar]
- Amato, A.; Hudak, B.; D’Carlo, P.; Noble, D.; Scarborough, D.; Seitzman, J.; Lieuwen, T. Methane oxycombustion for low CO2 cycles: Blowoff measurements and analysis. J. Eng. Gas Turbines Power 2011, 133, 061503. [Google Scholar] [CrossRef]
- Procedure for the Analysis and Evaluation of Gaseous Emissions from Aircraft Engines; ARP1533A; SAE International: Warrendale, PA, USA, 2004.
- Kee, R.; Rupley, F.; Miller, J. CHEMKIN-PRO 15112; Reaction Design: San Diego, CA, USA, 2011. [Google Scholar]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr.; et al. GRI-Mech 3.0. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 28 April 2017).
- Glarborg, P.; Bentzen, L.L. Chemical effects of a high CO2 concentration in oxy-fuel combustion of methane. Energy Fuels 2007, 22, 291–296. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.S.; Chung, J.O.; Yun, J.H.; Keel, S.I. Chemical effects of added CO2 on the extinction characteristics of H2/CO/CO2 syngas diffusion flames. Int. J. Hydrogen Energy 2009, 34, 8756–8762. [Google Scholar] [CrossRef]
- Design, R. CHEMKIN/CHEMKIN-PRO Theory Manual CHEMKIN® Software. No August, 2010, 1–360.
- Kobayashi, H.; Kitano, M. Extinction characteristics of a stretched cylindrical premixed flame. Combust. Flame 1989, 76, 285–295. [Google Scholar] [CrossRef]
- Wang, P.; Hu, S.; Wehrmeyer, J.; Pitz, R. Stretch and curvature effects on flames. In Proceedings of the 42nd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 5–8 January 2004. [Google Scholar]
- Plee, S.; Mellor, A. Characteristic time correlation for lean blowoff of bluff-body-stabilized flames. Combust. Flame 1979, 35, 61–80. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Heywood, J.B.; Tabaczynski, R.J. Premixed turbulent flame blowoff velocity correlation based on coherent structures in turbulent flows. Combust. Flame 1981, 42, 19–33. [Google Scholar] [CrossRef]
- Weber, R.; Breussin, F. Scaling properties of swirling pulverized coal flames: From 180 kW to 50 MW thermal input. Symp. (Int.) Combust. 1998, 27, 2957–2964. [Google Scholar] [CrossRef]


| Operation Parameter | CH4/Air | OF30 |
|---|---|---|
| Fuel | CH4 (99.98%) | CH4 (99.98%) |
| Fuel flow rate (g/s) | 0.04–0.09 | 0.045–0.12 |
| Equivalence ratio | 0.48–1 | 0.53–1 |
| Preheated temperature (K) | 396.15–466.15 K | 396.15–466.15 K |
| Reynolds number (-) | 5300–4800 | 4950–5600 |
| Air flow rate (g/s) | 1.438 | - |
| O2/CO2 flow rate (g/s) | - | 1.405–2.012 |
| Swirl number (-) | 0.58 | 0.58 |
| Power (kW) | 2.02–4.16 | 2.24–5.99 |
| Preheated Temperature | CH4/Air | OF30 |
|---|---|---|
| 396.15 K | 0.913 mm | 1.014 mm |
| 431.15 K | 1.019 mm | 1.168 mm |
| 466.15 K | 1.234 mm | 1.362 mm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tong, Y.; Thern, M.; Klingmann, J. Investigation of Methane Oxy-Fuel Combustion in a Swirl-Stabilised Gas Turbine Model Combustor. Energies 2017, 10, 648. https://doi.org/10.3390/en10050648
Li M, Tong Y, Thern M, Klingmann J. Investigation of Methane Oxy-Fuel Combustion in a Swirl-Stabilised Gas Turbine Model Combustor. Energies. 2017; 10(5):648. https://doi.org/10.3390/en10050648
Chicago/Turabian StyleLi, Mao, Yiheng Tong, Marcus Thern, and Jens Klingmann. 2017. "Investigation of Methane Oxy-Fuel Combustion in a Swirl-Stabilised Gas Turbine Model Combustor" Energies 10, no. 5: 648. https://doi.org/10.3390/en10050648
APA StyleLi, M., Tong, Y., Thern, M., & Klingmann, J. (2017). Investigation of Methane Oxy-Fuel Combustion in a Swirl-Stabilised Gas Turbine Model Combustor. Energies, 10(5), 648. https://doi.org/10.3390/en10050648






