Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
2.4. Surface Breakdown Test Apparatus
3. Results and Discussion
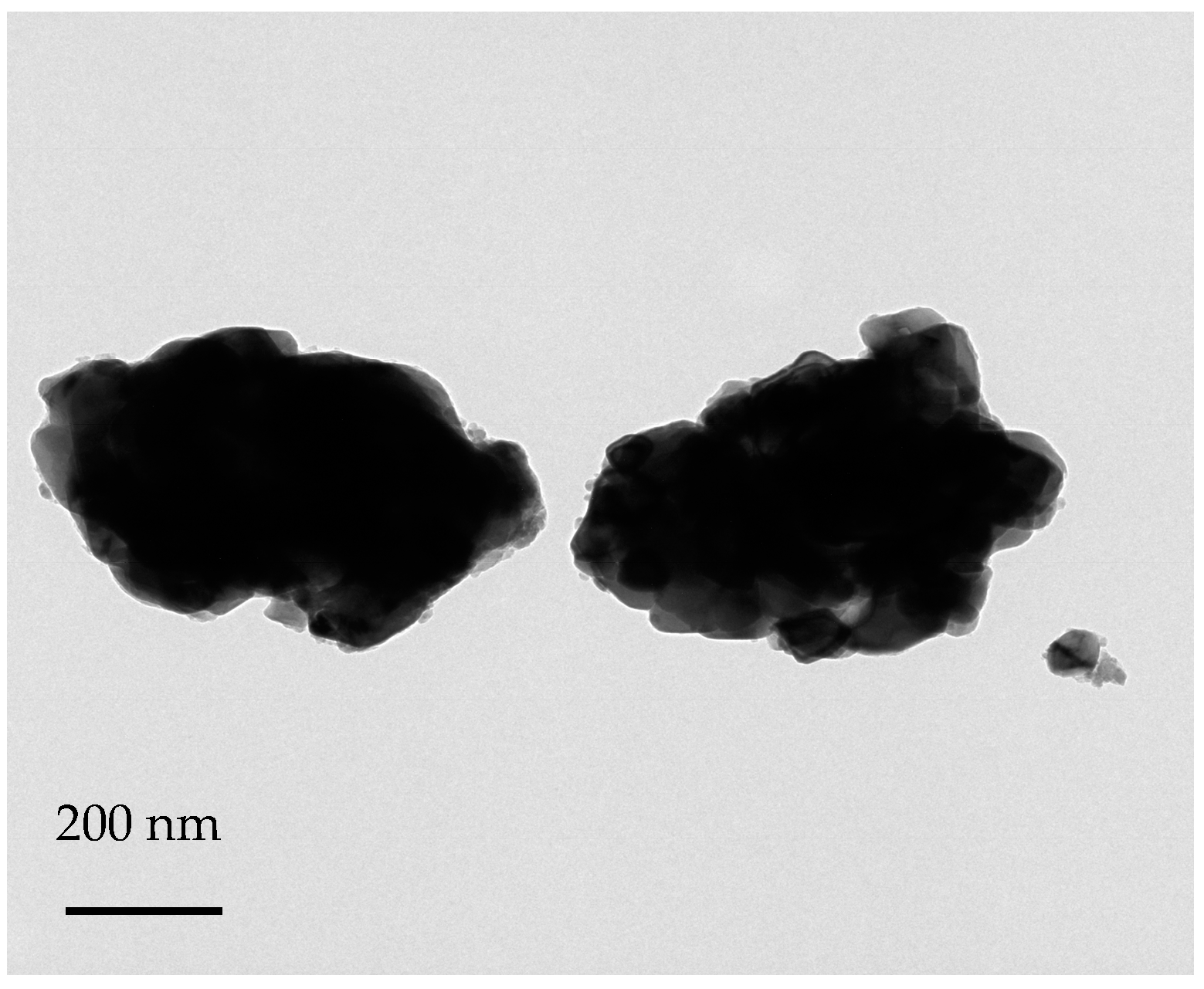
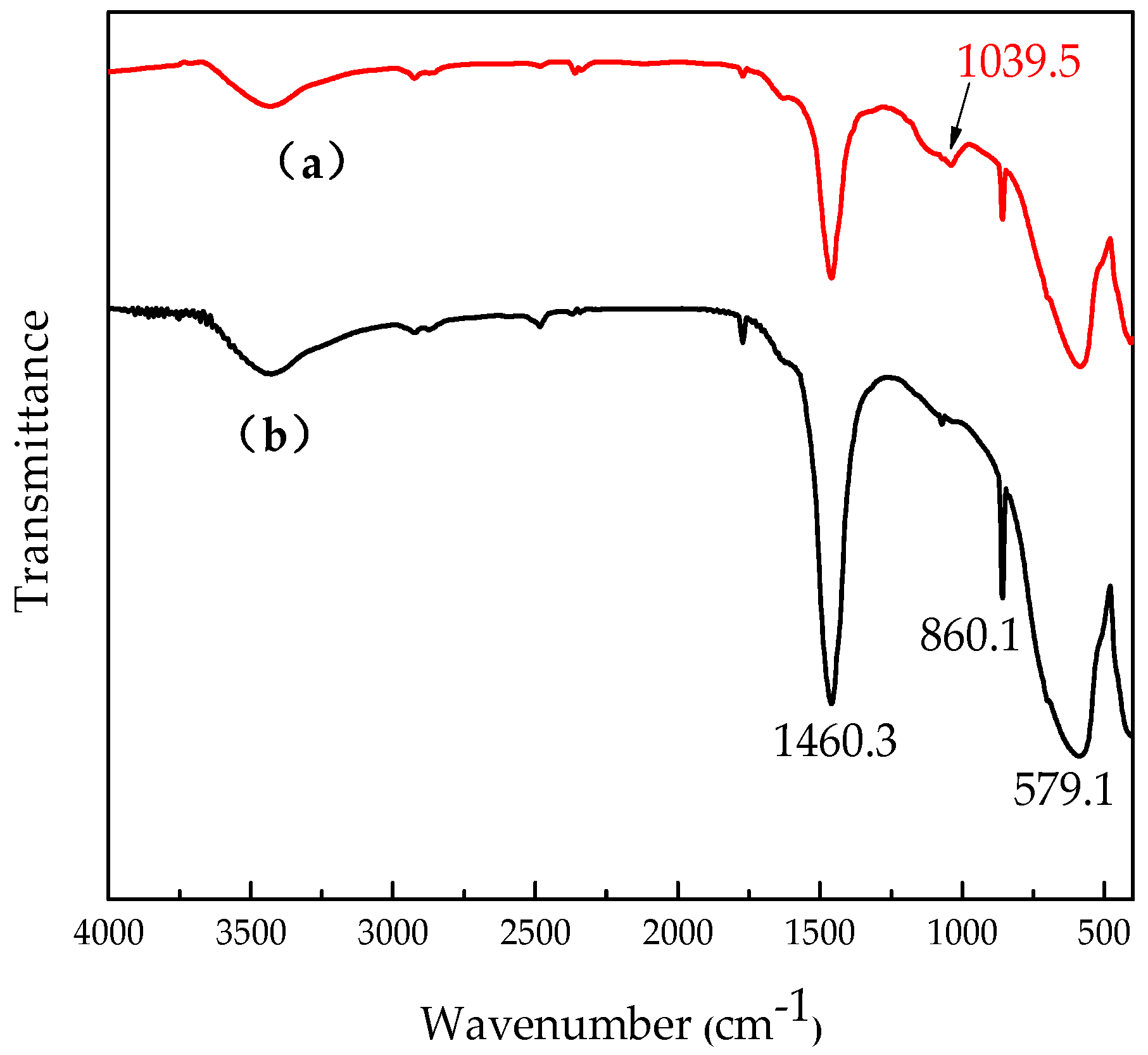
3.1. Surface Treatment of SrTiO3
3.2. Morphology of the Cured Nanocomposites
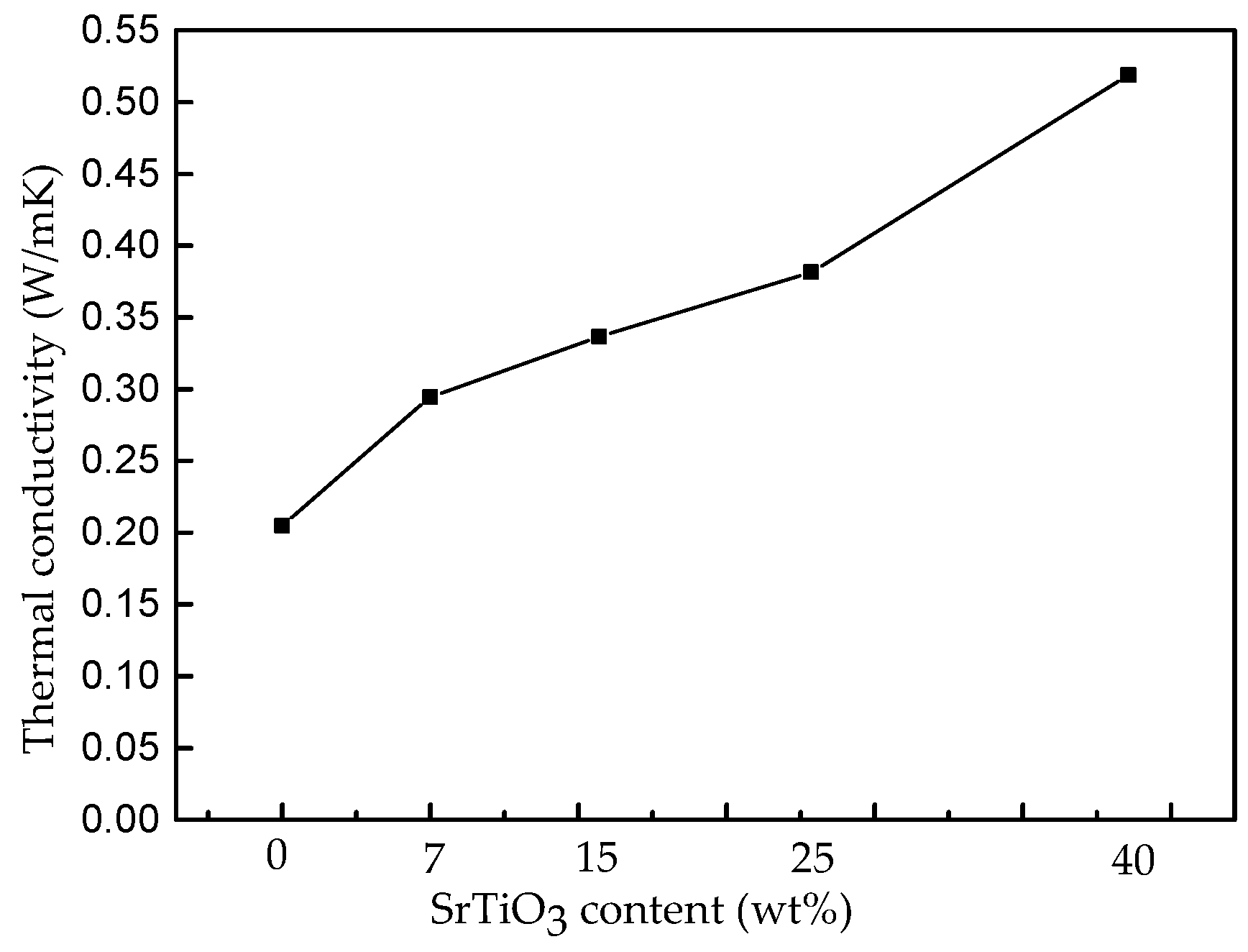
3.3. Thermal Properties of the Nanocomposites
3.4. Electrical Properties of the Nanocomposites
3.5. Surface Breakdown Tests of the Nanocomposites
4. Conclusions
- The uniformly-distributed nanocomposites exhibit an excellent thermal conductivity. The 40 wt % nanocomposites obtain a 0.52 W/m·K thermal conductivity, which is more than two times larger than that of the pure epoxy resin. The new nanocomposites provide an efficient heat transmission pathway to reduce the chances for a thermal breakdown to occur. The unique positive temperature coefficient characteristic of the particles, as well as the intimate connection between the particles and the epoxy host, may provide important explanations for the results.
- The dielectric constant of the nanocomposites increased along with their weight fractions and fit well with the calculated value from the Lichtenecher’s logarithmic mixture formula. The dielectric constant of the 40 wt % composites increased to 6.15, which was 44.7% higher than that of the pure epoxy resin. The relative dielectric loss tangent also increased along with frequency from 1 kHz to 1 MHz yet remained at a low level (approximately 0.02).
- The Weibull distribution shows that the breakdown strength at 63.2% probability of the 25 wt % nanocomposites increased by 17%. All nanocomposites showed a slightly higher breakdown strength, thereby preventing a potential decrease in breakdown strength after their amount of particles increases.
- The surface breakdown tests reveal that the surface breakdown voltage of the 40 wt % nanocomposites shows an increasing tendency. These composites also have a faster temperature decline rate (6.77 °C/s) compared with the pure epoxy resin. The 40 wt % nanocomposites and the pure epoxy resin had surface breakdown voltages of 11.2 kV and 7.6 kV, respectively.
- The novel dielectric material exhibits improvements in its thermal and dielectric properties. The corona resistance of the new nanocomposites warrants further investigation to explain the relationship between their thermal and electrical properties and to enhance their practical application in the manufacture of key electrical equipment, such as gas-insulated switchgear and efficient transformers.
Author Contributions
Conflicts of Interest
References
- Venkatesulu, B.; Thomas, M.J. Corona aging studies on silicone rubber nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 615–624. [Google Scholar] [CrossRef]
- Du, B.X.; Zhang, J.W.; Liu, Y. Effects of concentration on tracking failure of epoxy/TiO2 nanocomposites under DC voltage. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1750–1759. [Google Scholar] [CrossRef]
- Nysten, B.; Gonry, P.; Issi, J.P. Intra-and inter-chain thermal conduction in polymers. Synth. Met. 1995, 69, 67–68. [Google Scholar] [CrossRef]
- Choy, C.L.; Young, K. Thermal conductivity of semicrystalline polymers-A model. Polymer 1977, 18, 769–776. [Google Scholar] [CrossRef]
- Kurabayashi, K. Anisotropic thermal properties of solid polymers. Int. J. Thermophys. 2001, 22, 277–288. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Qi, S.H.; Zhao, H.Z.; Wang, C.; Kou, J. Effect of particle size of Al2O3 on the properties of filled thermal conductive silicone rubber. J. Appl. Polym. Sci. 2007, 104, 1312–1318. [Google Scholar] [CrossRef]
- Zhang, X.L.; Shen, L.Y.; Wu, H.; Guo, S. Enhanced thermally conductivity and mechanical properties of polyethylene(PE)/boron nitride(BN) composites through multistage stretching extrusion. Compos. Sci. Technol. 2013, 89, 24–28. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Mcnamara, A.; Liu, Y.; Moon, K.S.; Wong, C.P. Exfoliated hexagonal boron nitride-based polymer nanocomposte with enhanced thermal conductivity for electronic encapsulation. Compos. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, C.F.; Ai, T.; Wu, K.; Zhao, F.; Gu, H. A novel fiber-reinforced polyethylene composite with added silicon nitride particles for enhanced thermal conductivity. Compos. Part A 2009, 40, 830–836. [Google Scholar] [CrossRef]
- Gu, J.W.; Zhang, Q.Y.; Dang, J.; Zhang, J.; Yang, Z. Thermal conductivity and mechanical properties of aluminum nitride filled linear low-density polyethylene composites. Polym. Eng. Sci. 2009, 49, 1030–1034. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Fieder, B.; Kinloch, I.A.; Bauhofer, W.; Windle, A.H.; Schulte, K. Evaluation and identification of electrical and thermal conduction mechanism in carbon nanotube/epoxy composites. Polymer 2006, 47, 2036–2045. [Google Scholar] [CrossRef]
- Yu, J.H.; Huo, R.M.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Influence of interface structure on dielectric properties of epoxy/alumina nanocomposites. Macromol. Res. 2012, 20, 816–826. [Google Scholar] [CrossRef]
- Wang, T.T.; Li, W.P.; Luo, L.H.; Zhu, Y. Ultrahigh dielectric constant composites based on the oleic acid modified ferroferric oxide nanoparticles and polyvinylidene fluoride. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Tang, H.X.; Sodano, H.A. High energy density nanocomposite capacitors using non-ferroelectric nanowires. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Dang, Z.M.; Wang, H.Y.; Xu, H.P. Influence of silane coupling agent on morphology and dielectric property in BaTiO3/polyvinylidene fluoride composites. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Kraemer, K.L.; Reding, N.A.; Skomski, R.; Ducharme, S.; Sellmyer, D.J. Synthesis of monodisperse TiO2-paraffin core-shell nanoparticles for improved dielectric properties. ACS Nano 2010, 4, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Kraemer, K.L.; Valloppilly, S.R.; Ducharme, S.; Sellmyer, D.J. Cluster synthesis of monodisperse rutile-TiO2 nanoparticles and dielectric TiO2-vinylidene fluoride oligomer nanocomposites. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fujinami, K.; Zhang, R.; Wang, N.; Ba, Y.; Koumoto, K. Interfacial thermal resistance and thermal conductivity in nanaograined SrTiO3. Appl. Phys. Express 2010, 3. [Google Scholar] [CrossRef]
- Seetawan, T.; Wong-ud-dee, G.; Thanachayanont, C.; Amornkitbumrung, V. Molecular dynamics simulation of strontium titanate. Chin. Phys. Lett. 2010, 27, 217–219. [Google Scholar] [CrossRef]
- Kim, P.; Jones, S.C.; Hotchkiss, P.J.; Haddock, J.N.; Kippelen, B.; Marder, S.R.; Perry, J.W. Phosphonic acid-modified barium titanate polymer nanocomposites with high permittivity and dielectric strength. Adv. Mater. 2010, 19, 1001–1005. [Google Scholar] [CrossRef]
- Chon, J.; Ye, S.; Cha, K.J.; Lee, S.C.; Koo, Y.S.; Jung, J.H.; Kwon, Y.K. High-k dielectric sol-gel hybrid materials containing barium titanate nanocomposites. Chem. Mater. 2010, 22, 5445–5452. [Google Scholar] [CrossRef]
- Li, J.; Claude, J.; Norena-Franco, L.E.; Seok, S.I.; Wang, Q. Electrical energy storage in ferroelectric polymer nanocomposites containing surface-functionalized BaTiO3 nanoparticles. Chem. Mater. 2008, 20, 6304–6306. [Google Scholar] [CrossRef]
- Tang, H.X.; Lin, Y.R.; Sodano, H.A. Synthesis of high aspect ratio BaTiO3 nanowires for high energy density nanocomposite capacitors. Adv. Energy Mater. 2013, 3, 451–456. [Google Scholar] [CrossRef]
- Beier, C.W.; Cuevas, M.A.; Brutchey, R.L. Effect of surface modification on the dielectric properties of BaTiO3 nanocrystals. Langmuir 2010, 26, 5067–5071. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, H.; Zhou, Y.C.; Bai, Y.; Niu, Y. Enhanced dielectric properties of BaTiO3/poly(vinylidene fluoride) nanocomposites for energy storage applications. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Xie, L.Y.; Huang, X.Y.; Yang, K.; Li, S.; Jiang, P. “Grafting to” route to PVDF-HFP-GMA/BaTiO3 nanocomposites with high dielectric constant and high thermal conductivity for energy storage and thermal management application. J. Mater. Chem. A 2014, 2, 5244–5251. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wen, H.; Chen, X.X.; Wu, Y. Improving electrical properties of SrTiO3/epoxy nanocomposites with high thermal conductivity. In Proceedings of the 2016 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Chengdu, China, 19–22 September 2016. [Google Scholar]
- Zhou, Q.Z.; Vilarinho, P.M.; Baptista, J.L. Dielectric properties of bismuth doped Ba1−xSrxTiO3 ceramics. J. Eur. Ceram. Soc. 2001, 21, 531–534. [Google Scholar] [CrossRef]
- Chen, W.P.; Zhu, Q.A. Synthesis of barium strontium titanate nanorods in reverse microemulsion. Mater. Lett. 2007, 61, 3378–3380. [Google Scholar] [CrossRef]
- Uvarov, N.F. Estimation of composites conductivity using a general mixing rule. Solid State Ion. 2000, 136–137, 1267–1272. [Google Scholar] [CrossRef]










| Samples | Measured Value at 1 kHz | Calculated Value at 1 kHz | Measured Value at 100 kHz | Calculated Value at 100 kHz |
|---|---|---|---|---|
| 7 wt %→1.72 vol % | 4.65 | 4.55 | 4.46 | 4.37 |
| 15 wt %→3.95 vol % | 4.75 | 4.98 | 4.56 | 4.79 |
| 25 wt %→7.20 vol % | 5.51 | 5.69 | 5.25 | 5.48 |
| 40 wt %→13.44 vol % | 6.15 | 7.33 | 5.81 | 7.08 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wen, H.; Chen, X.; Wu, Y.; Xiao, S. Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites. Energies 2017, 10, 692. https://doi.org/10.3390/en10050692
Zhang X, Wen H, Chen X, Wu Y, Xiao S. Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites. Energies. 2017; 10(5):692. https://doi.org/10.3390/en10050692
Chicago/Turabian StyleZhang, Xiaoxing, Hao Wen, Xiaoyu Chen, Yunjian Wu, and Song Xiao. 2017. "Study on the Thermal and Dielectric Properties of SrTiO3/Epoxy Nanocomposites" Energies 10, no. 5: 692. https://doi.org/10.3390/en10050692






