The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
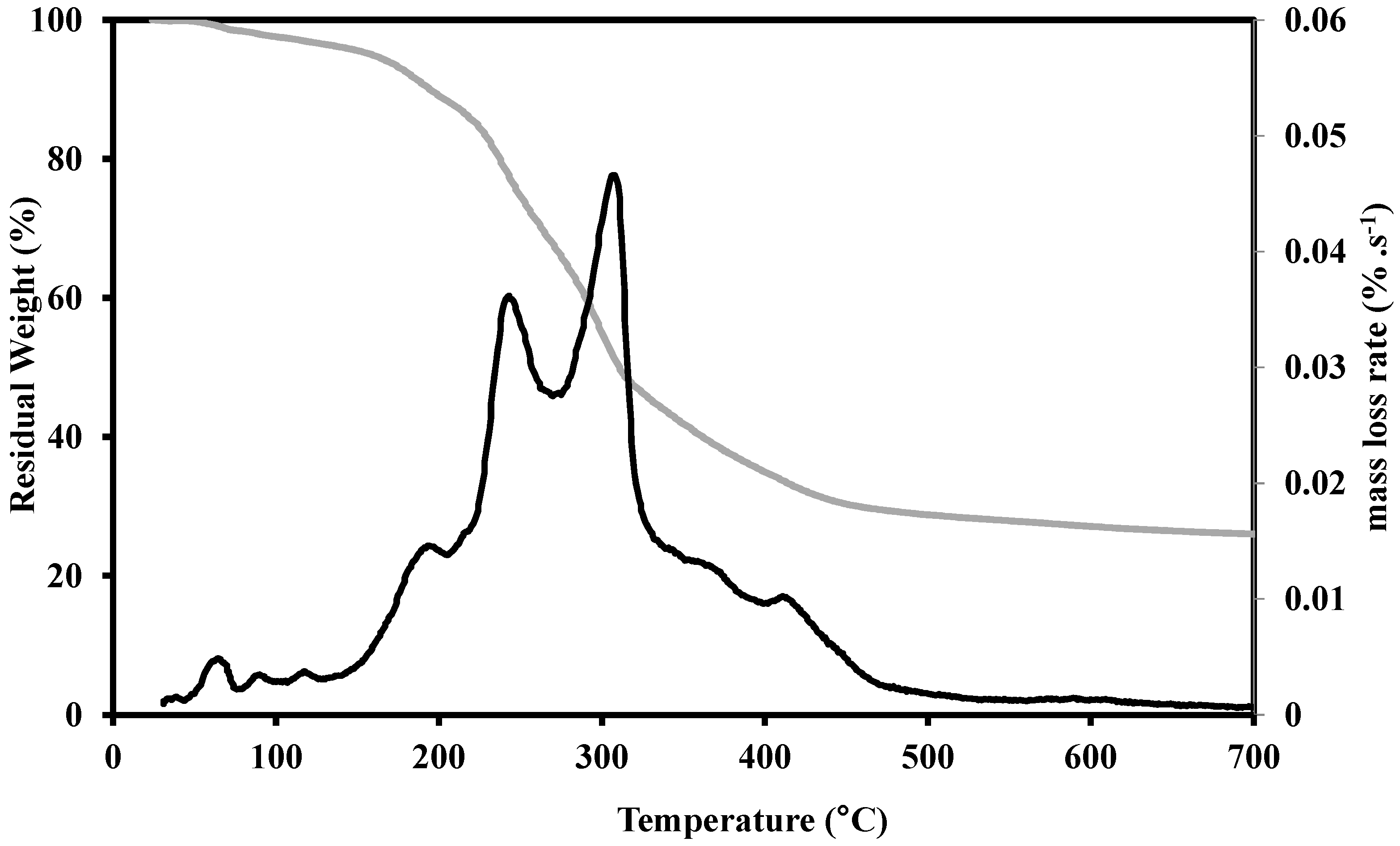
2.1.1. Thermogravimetric Study
2.1.2. Olive Charcoal Preparation
2.1.3. Olive Pomace Charcoal Characterization
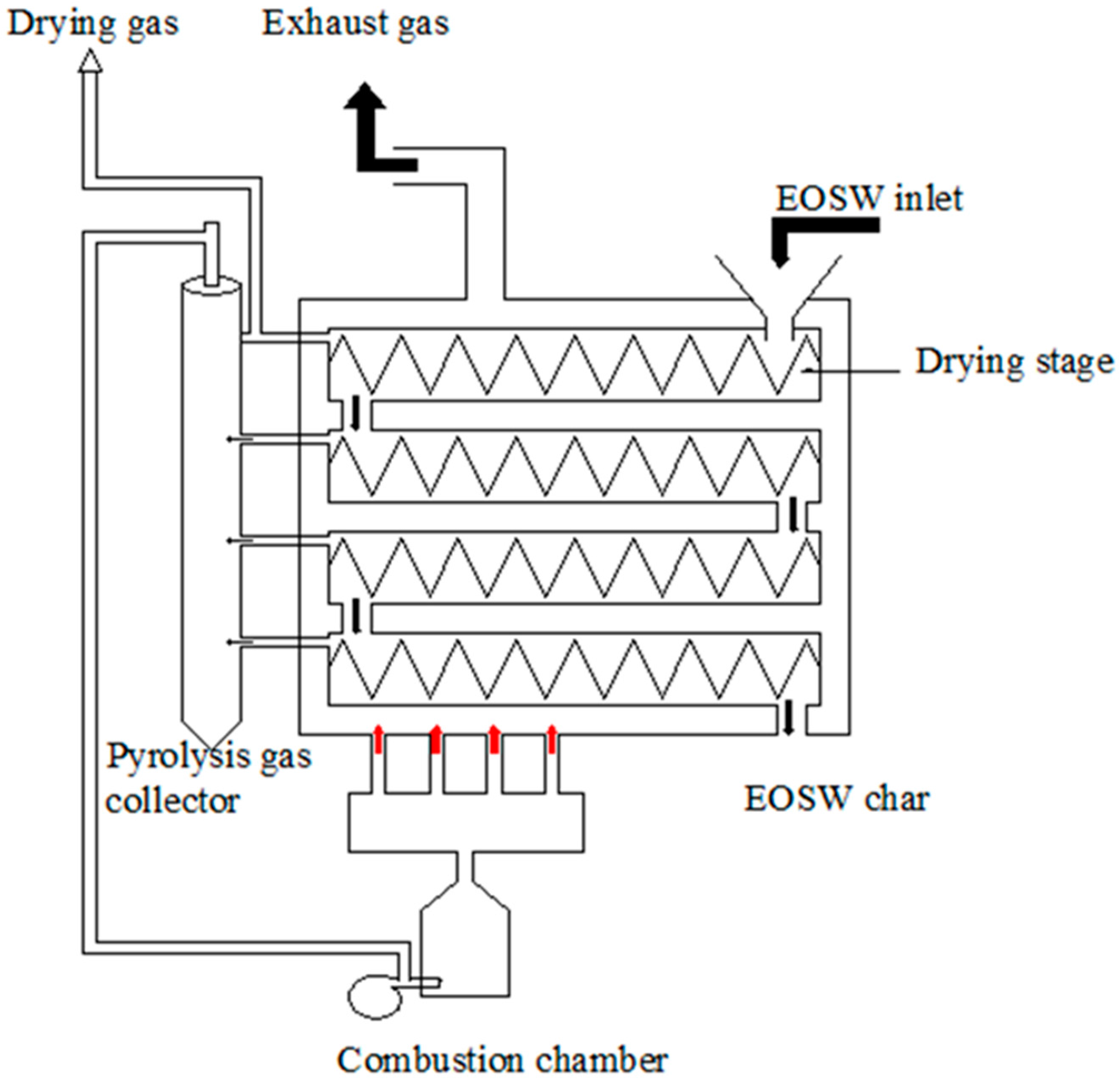
2.2. Plasma Gasification Setup
2.3. Main Chemical Reactions
3. Results and Discussion
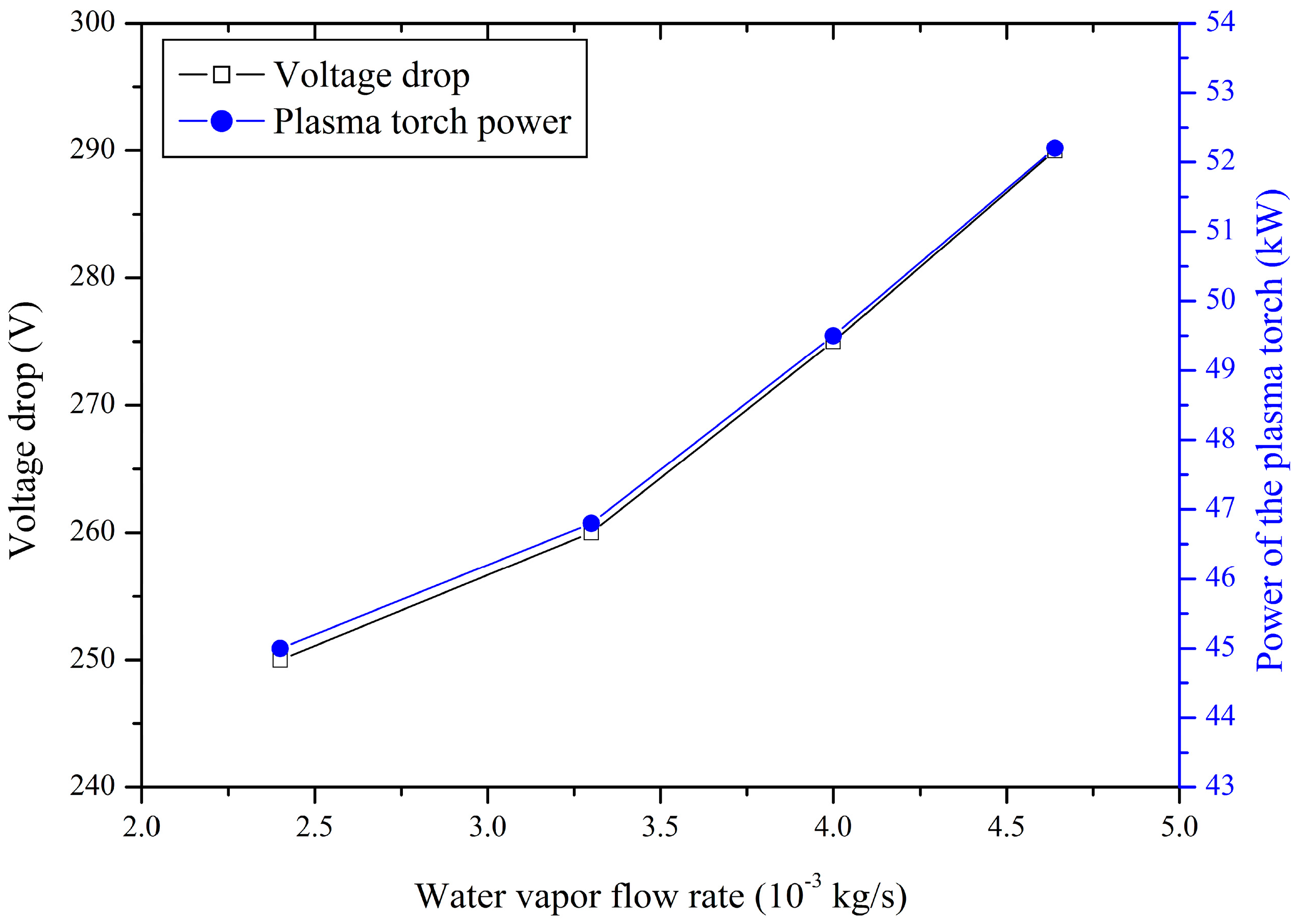
3.1. Relation between the Water Vapour Flow Rate and the Power of the Plasma Torch
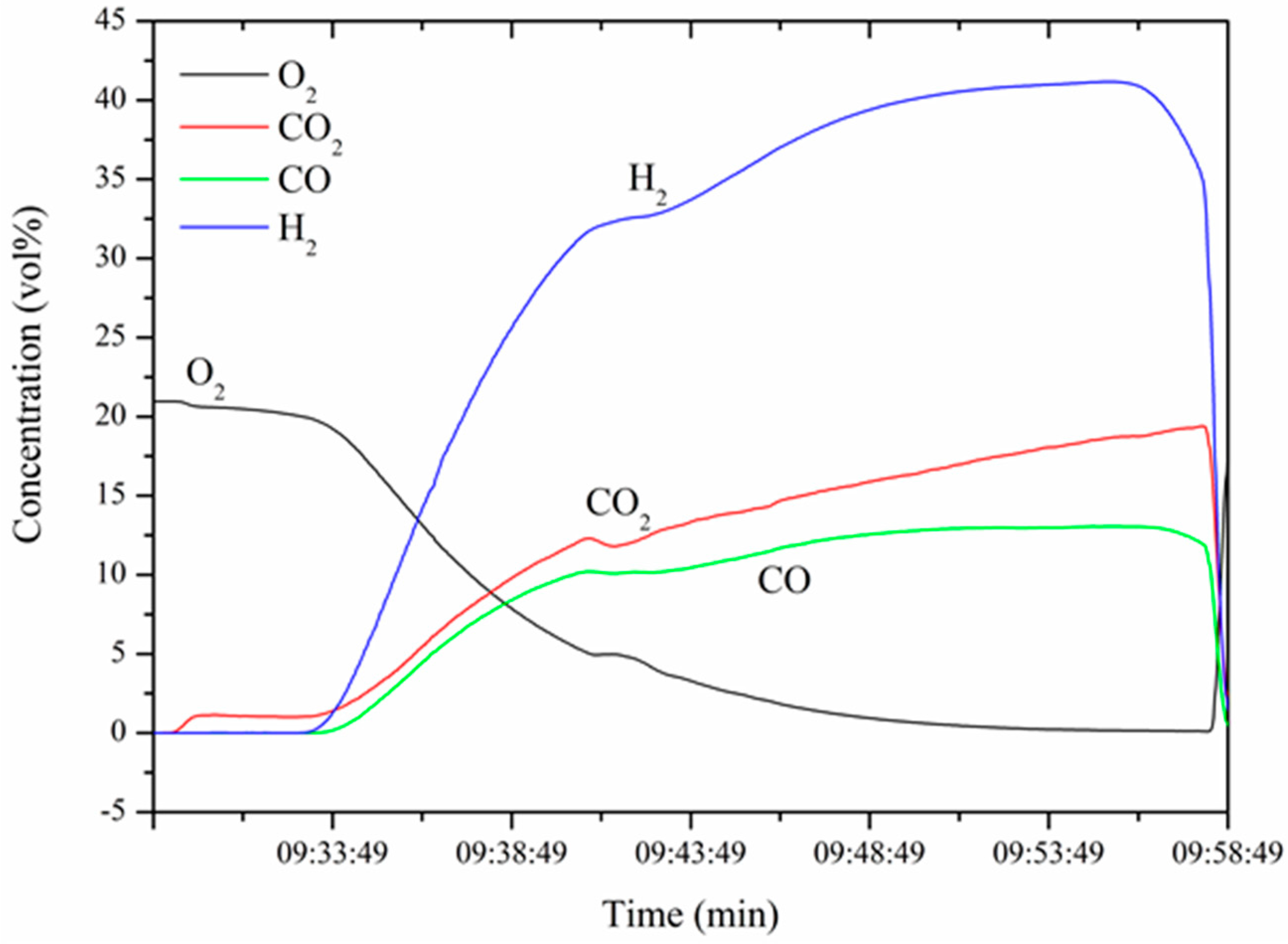
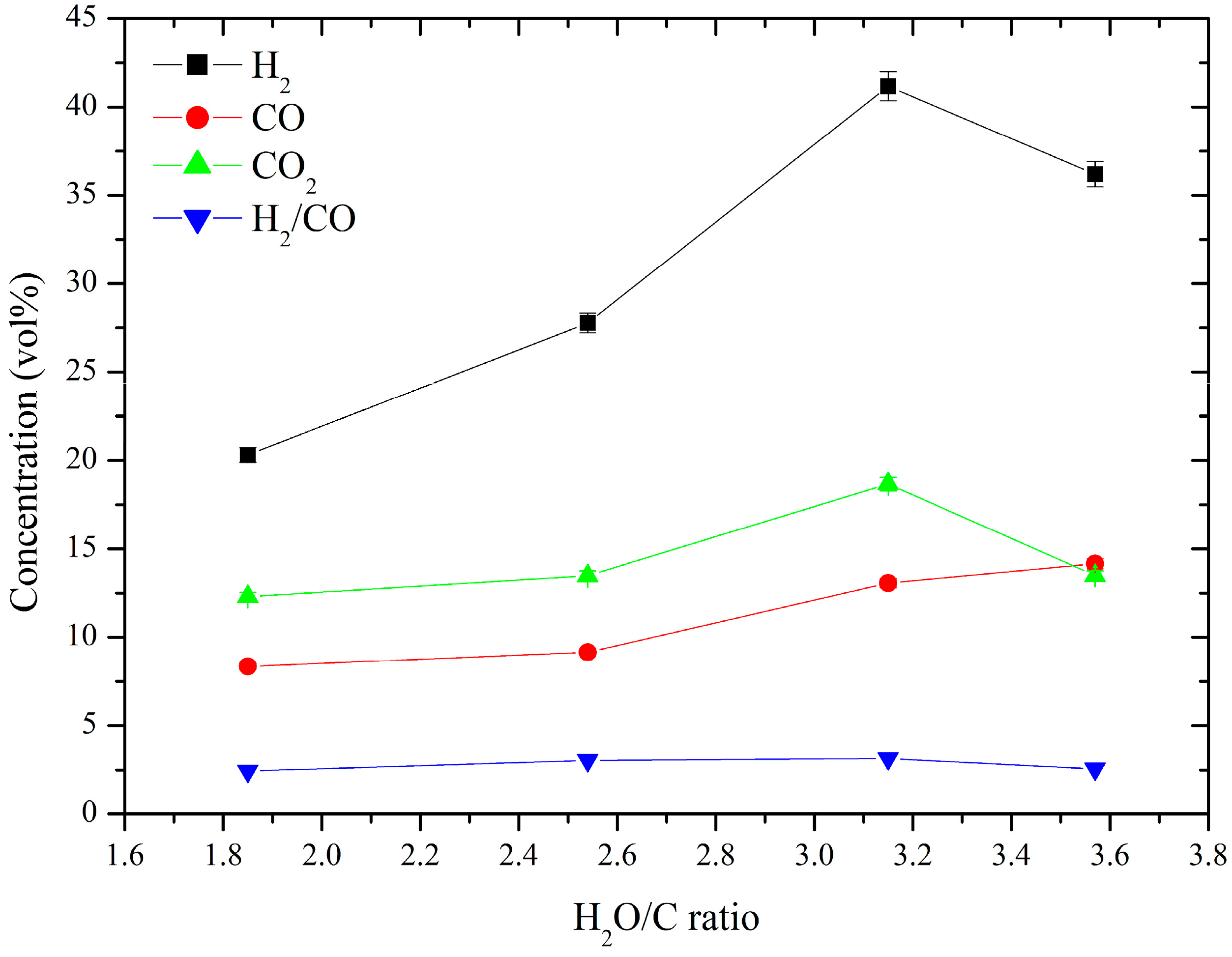
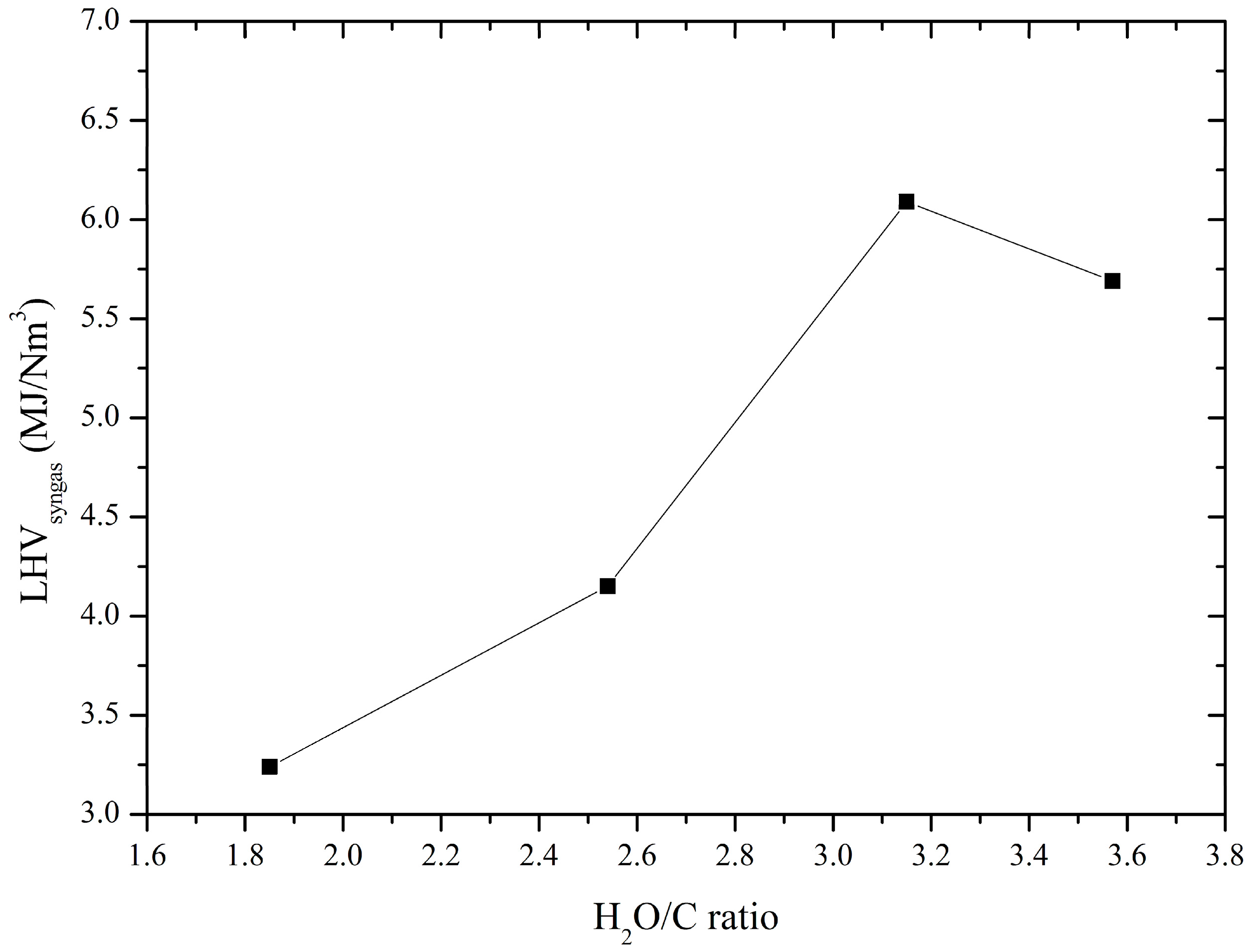
3.2. Produced Syngas Composition
3.3. Comparison with Similar Gasification Methods
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Eurostat. Statistics Explained. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Waste_statistics (accessed on 8 February 2017).
- Directive of the European Parliament and of the Council amending Directive 2008/98/EC on Waste. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52015PC0595 (accessed on 12 February 2015).
- Food and Agriculture Organization of the United Nations Statistical Databases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; Available online: www.faostat.fao.org/ (accessed on 8 February 2017).
- European Commission. Agriculture and Rural Development. Available online: http://ec.europa.eu/agriculture/olive-oil_en (accessed on 8 February 2017).
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Jeguirim, M.; Elmay, Y.; Limousy, L.; Lajili, M.; Said, R. Devolatilization behaviour and pyrolysis kinetics of potential Tunisian biomass fuels. Environ. Prog. Sustain. Energy 2014, 33, 1452–1458. [Google Scholar]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy. Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Bosmans, A.; Vanderreydt, I.; Geysen, D.; Helsen, L. The crucial role of waste-to-energy technologies in enhanced landfill mining: A technology review. J. Clean. Prod. 2013, 55, 10–23. [Google Scholar] [CrossRef]
- Siciliano, A.; Stillitano, M.A.; De Rosa, S. Biogas production from wet olive mill wastes pretreated with hydrogen peroxide in alkaline conditions. Renew. Energy 2016, 85, 903–916. [Google Scholar] [CrossRef]
- De Rosa, S.; Siciliano, A. A catalytic oxidation process of olive oil mill wastewaters using hydrogen peroxide and copper. Desalination Water Treat. 2010, 23, 187–193. [Google Scholar] [CrossRef]
- Giordano, G.; Perathoner, S.; Centi, G.; De Rosa, S.; Granato, T.; Katovic, A.; Siciliano, A.; Tagarelli, A.; Tripicchio, F. Wet hydrogen peroxide catalytic oxidation of olive oil mill wastewaters using Cu-zeolite and Cu-pillared clay catalysts. Catal. Today 2007, 124, 240–246. [Google Scholar] [CrossRef]
- Al-Mallhi, J.; Furuichi, T.; Ishii, K. Appropriate conditions for applying NaOH-pretreated two-phase olive milling waste for codigestion with food waste to enhance biogas production. Waste Manag. 2016, 48, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, E.; Fokaides, P.A. A review of olive solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.; Jurado, F.; Margaritis, N.K.; Grammelis, P. Experimental and economic study of a gasification plant fueled with olive industry wastes. Energy Sustain. Dev. 2014, 23, 247–257. [Google Scholar] [CrossRef]
- Vera, D.; de Mena, B.; Jurado, F.; Schories, G. Study of a downdraft gasifier and gas engine fueled with olive oil industry wastes. Appl. Therm. Eng. 2013, 51, 119–129. [Google Scholar] [CrossRef]
- Kipcak, E.; Akgun, M. Oxidative gasification of olive mill wastewater as a biomass source in supercritical water: Effects on gasification yield and biofuel composition. J. Supercrit. Fluids 2012, 69, 57–63. [Google Scholar] [CrossRef]
- Margaritis, N.; Grammelis, P.; Vera, D.; Jurado, F. Assessment of Operational Results of a Downdraft Biomass Gasifier Coupled with a Gas Engine. Procedia Soc. Behav. Sci. 2012, 48, 857–867. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L.; Jones, D.D.; Hanna, M.A. Contemporary issues in thermal gasification of biomass and its application to electricity and fuel production. Biomass Bioenergy 2008, 32, 573–581. [Google Scholar] [CrossRef]
- Vera, D.; Jurado, F.; Panopoulos, K.D.; Grammelis, P. Modelling of biomass gasifier and microturbine for the olive oil industry. Int. J. Energy Res. 2012, 36, 355–367. [Google Scholar] [CrossRef]
- Evangelisti, S.; Tagliaferri, C.; Clift, R.; Lettieri, P.; Taylor, R.; Chapman, C. Life cycle assessment of conventional and two-stage advanced energy-from-waste technologies for municipal solid waste treatment. J. Clean. Prod. 2015, 100, 212–223. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; van der Mullen, J. Gas discharge plasmas and their applications. Spectrochim. Acta B 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Huang, H.; Tang, L. Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers. Manag. 2007, 48, 1331–1337. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Gimžauskaitė, D.; Jeguirim, M.; Mėčius, V.; Aikas, M. Energy recovery from waste glycerol by utilizing thermal water vapor plasma. Environ. Sci. Pollut. Res. 2016, in press. [Google Scholar] [CrossRef]
- Fabry, F.; Rehmet, C.; Rohani, V.; Fulcheri, L. Waste gasification by thermal plasma: A review. Waste Biomass Valoriz. 2013, 4, 421–439. [Google Scholar] [CrossRef]
- Rutberg, G.Ph.; Bratsev, A.N.; Kuznersov, V.A.; Popov, V.E.; Ufimtsev, A.A.; Shtengel, S.V. On efficiency of plasma gasification of wood residues. Biomass Bioenergy 2011, 35, 495–504. [Google Scholar] [CrossRef]
- Arabi, K.; Aubry, O.; Khacef, A.; Cormier, J.M. Syngas production by plasma treatments of alcohols, bio-oils and wood. J. Phys. Conf. Ser. 2012, 406, 1–8. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Valinčius, V.; Levinskas, R. Biomass conversion to hydrogen-rich synthesis fuels using water steam plasma. CR Chim. 2016, 19, 433–440. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Grigaitienė, V.; Valinčius, V.; Striūgas, N. A cleaner production of synthesis gas from glycerol using thermal water steam plasma. J. Clean. Prod. 2016, 130, 187–194. [Google Scholar] [CrossRef]
- Qiu, J.; He, X.; Sun, T.; Zhao, Z.; Zhou, Y.; Guo, Sh.; Zhang, J.; Ma, T. Coal gasification in steam and air medium under plasma conditions: A preliminary study. Fuel Process. Technol. 2004, 85, 969–982. [Google Scholar] [CrossRef]
- Agon, N.; Hrabovsky, M.; Chumak, O.; Hlina, M.; Kopecky, V.; Mašlani, A.; Bosmans, A.; Helsen, L.; Skoblja, S.; Van Oost, G.; et al. Plasma gasification or refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag. 2016, 47, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wu, J.; Ma, D.; Liu, Y.; Qiu, P.; Qiu, R.; Liao, S.; Gao, D. Gasification of corn cob using non-thermal arc plasma. Int. J. Hydrogen Energy 2015, 40, 12634–12649. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the behaviour of ash from agricultural wastes during combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]
- Kraiem, N.; Lajili, M.; Limousy, L.; Said, R.; Jeguirim, M. Energy recovery from Tunisian agri-food wastes: Evaluation of combustion performance and emissions characteristics of green pellets prepared from tomato residues and grape marc. Energy 2016, 107, 409–418. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Jeguirim, M.; Guizani, C.; Limousy, L.; Dupont, C.; Gadiou, R. Thermogravimetric study on the influence of structural, textural and chemical properties of biomass chars on CO2 gasification reactivity. Energy 2015, 88, 703–710. [Google Scholar] [CrossRef]
- Jeguirim, M.; Bikai, J.; Elmay, Y.; Limousy, L.; Njeugna, E. Thermal characterization and pyrolysis kinetics of tropical biomass feedstocks for energy recovery. Energy Sustain. Dev. 2014, 23, 188–193. [Google Scholar] [CrossRef]
- Blanco-López, M.C.; Blanco, C.G.; Martimez-Alonso, A.; Tascou, J.M.D. Composition of gases released during olive stones pyrolysis. J. Anal. Appl. Pyrolysis 2002, 65, 313–322. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Favre-Reguillon, A.; Trouvé, G.; Le Buzit, G.; Khiari, B.; Zagrouba, F. Energetic valorisation of olive mill wastewater impregnated on low cost absorbent: Sawdust versus Olive Solid Waste. Energy 2012, 39, 74–81. [Google Scholar] [CrossRef]
- Ozveren, U.; Ozdogan, Z.S. Investigation of the slow pyrolysis kinetics of olive oil pomace using thermo-gravimetric analysis coupled with mass spectrometry. Biomass Bioenergy 2013, 58, 168–179. [Google Scholar] [CrossRef]
- Pantoleontos, G.; Basinas, P.; Skodras, G.; Grammelis, P.; Pintér, J.D.; Topis, S.; Sakellaropoulos, G.P. A global optimization study on the devolatilisation kinetics of coal, biomass and waste fuels. Fuel Process. Technol. 2009, 90, 762–769. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguirim, M.; Khiari, B.; Trouvé, G.; Zagrouba, F. Thermal degradation behavior of olive solid waste: Influence of the particle size and oxygen atmosphere. Resour. Conserv. Recycl. 2010, 54, 271–277. [Google Scholar] [CrossRef]
- Hmid, A.; Mondelli, D.; Fiore, S.; Fanizzi, F.P.; Al Chami, Z.; Dumontet, S. Production and characterization of biochar from three-phase olive mill waste through slow pyrolysis. Biomass Bioenergy 2014, 71, 330–339. [Google Scholar] [CrossRef]
- Abenavoli, L.M.; Longo, L.; Roto, A.R.; Gallucci, F.; Ghigoli, A.; Zimbalatti, G.; Russo, D.; Colantoni, A. Characterization of biochar obtained from olive and hazelnut prunings and comparison with the standards of European Biochar Certificate (EBC). Procedia Soc. Behav. Sci. 2016, 223, 698–705. [Google Scholar] [CrossRef]
- Kim, Y.; Ferreri, V.W.; Rosocha, L.A.; Anderson, G.K.; Abbate, A.; Kim, K.T. Effect of plasma chemistry on activated propane/air flames. IEEE Trans. Plasma Sci. 2006, 34, 2532–2536. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Valatkevičius, P.; Grigaitienė, V.; Valinčius, V. Production of synthesis gas from propane using thermal water vapor plasma. Int. J. Hydrogen Energy 2014, 39, 2078–2086. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Tamošiūnas, A.; Valatkevičius, P.; Grigaitienė, V.; Valinčius, V. Water vapor plasma torch: Design, characteristics and applications. World Acad. Sci. Eng. Technol. 2012, 6, 10–13. [Google Scholar]
- De Souza-Santos, M.L. Solid Fuels Combustion and Gasification: Modeling, Simulation, and Equipment Operations, 2nd ed.; CRC Press: Boka Raton, FL, USA, 2010; p. 508. [Google Scholar]
- Breault, R.W. Gasification processes old and new: A basic review of the major technologies. Energies 2010, 3, 216–240. [Google Scholar] [CrossRef]
- Hong, Y.C.; Lee, S.J.; Shin, D.H.; Kim, Y.J.; Lee, B.J.; Cho, S.Y.; Chang, H.S. Syngas production from gasification of brown coal in a microwave torch plasma. Energy 2012, 47, 36–40. [Google Scholar] [CrossRef]
- Yoon, S.J.; Lee, J.G. Hydrogen-rich syngas production through coal and charcoal gasification using microwave steam and air plasma torch. Int. J. Hydrogen Energy 2012, 37, 17093–17100. [Google Scholar] [CrossRef]
- Messerle, V.E.; Ustimenko, A.B.; Lavrichshev, O.A. Comparative study of coal gasification: Simulation and experiment. Fuel 2016, 164, 172–179. [Google Scholar] [CrossRef]
- Chen, Z.; Dun, Q.; Shi, Y.; Lai, D.; Zhou, Y.; Gao, S.; Xu, G. High quality syngas production from catalytic coal gasification using disposable Ca(OH)2 catalyst. Chem. Eng. J. 2017, 316, 842–849. [Google Scholar] [CrossRef]

| Biomass Type | C a (wt %) | H a (wt %) | N a (wt %) | S a (wt %) |
|---|---|---|---|---|
| Olive pomace (this study) | 47.04 | 5.73 | 0.87 | <0.06 |
| Olive tree wood [34] | 48.20 | 5.30 | 0.70 | 0.03 |
| Pine Sawdust [35] | 51.30 | 6.40 | 0.20 | 0.01 |
| Miscanthus [36] | 47.60 | 6.00 | 0.30 | 0.02 |
| Corn cob [37] | 46.40 | 5.40 | 1.0 | 0.02 |
| Biomass Type | Moisture (% Dry Basis) | Volatile Matter (% Dry Basis) | Fixed Carbon (% Dry Basis) | Ash (% Dry Basis) |
|---|---|---|---|---|
| Olive pomace char coal | 22 | 17.4 | 77 | 5.6 |
| Parameters | This Study Olive Pomace Char | Olive Mill Waste Char [43] | Olive Wood Biochar [44] | Hazelnut Wood Biochar [44] |
|---|---|---|---|---|
| Pyrolysis temperature | 450 °C | 480 ± 10 °C | 400–800 °C | 400–800 °C |
| C a (wt % ) | 80.4 | 75.3 | 90.1 | 78.1 |
| H a (wt % ) | 2.87 | 3.64 | 1.58 | 1.21 |
| N (wt %) | 0.42 | 0.94 | 0.42 | 0.64 |
| S a (mg/kg) | 271 | ND | ND | ND |
| HHV a (MJ/kg) | 30.89 | 29.21 | 31.71 | 26.62 |
| LHV a (MJ/kg) | 30.30 | 28.35 | 30.48 | 25.66 |
| Oxidation Reactions | |||
| 1. | Carbon partial oxidation | –111 MJ/kmol | |
| 2. | Carbon monoxide oxidation | –283 MJ/kmol | |
| 3. | Carbon oxidation | –394 MJ/kmol | |
| 4. | Hydrogen oxidation | –242 MJ/kmol | |
| Gasification Reactions Involving Steam | |||
| 5. | Water–gas reaction | +131 MJ/kmol | |
| 6. | Water–gas shift reaction | –41 MJ/kmol | |
| 7. | Steam methane reforming | +206 MJ/kmol | |
| Gasification Reactions Involving Hydrogen | |||
| 8. | Hydrogasification | –75 MJ/kmol | |
| 9. | Methanation | –227 MJ/kmol | |
| Gasification Reactions Involving Carbon Monoxide | |||
| 10. | Bouduard reaction | +172 MJ/kmol | |
| Method | Gasifying Agent | Material | H2 (vol %) | CO (vol %) | H2/CO | LHVsyngas (MJ/nm3) | Ref. |
|---|---|---|---|---|---|---|---|
| Conventional gasification (Lurgi, HTW, Siemens, Shell, etc.) | |||||||
| Moving Bed | Oxygen | Coal * | 28.1–42.3 | 15.1–61.2 | 0.5–2.7 | 4–11 | [47,50] |
| Steam/air | 16.2–23.3 | 17.1–27.8 | 0.69–1.35 | ||||
| Fluidized Bed | Oxygen | Coal * | 32.8–40.0 | 31.0–53.0 | 0.71–0.85 | ||
| Steam/air | 12.6–28.56 | 12.54–30.7 | 0.56–1.0 | ||||
| Entrained flow | Oxygen | Coal * | 28.1–42.3 | 15.1–61.2 | 0.51–2.72 | ||
| Steam/air | 15.7–25.5 | 16.1–31.0 | 0.82–2.00 | ||||
| Transport flow | Oxygen | Coal * | 36.2–41.9 | 25.5–39.1 | 0.92–1.64 | ||
| Steam/air | 11.8–15.7 | 13.3–23.7 | 0.5–1.18 | ||||
| Plasma gasification (MW, DC) | |||||||
| MW | Steam/air | Coal | 48 | 23 | 2.08 | - | [51] |
| O2/air | Coal | 21.3 | 51.7 | 0.41 | - | ||
| MW | Steam | Coal | 62 | 20 | 3.1 | 10.9 | [52] |
| Charcoal | 58 | 17 | 3.41 | 10.4 | |||
| DC thermal arc plasma | Steam/air | Coal | 58.7 | 35.5 | 1.65 | - | [53] |
| DC thermal arc plasma | Steam/air | Coal | 39 | 34 | 1.14 | - | [31] |
| DC thermal arc plasma | Steam | Charcoal | 41.17 | 13.06 | 3.15 | 6.09 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. https://doi.org/10.3390/en10050710
Tamošiūnas A, Chouchène A, Valatkevičius P, Gimžauskaitė D, Aikas M, Uscila R, Ghorbel M, Jeguirim M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies. 2017; 10(5):710. https://doi.org/10.3390/en10050710
Chicago/Turabian StyleTamošiūnas, Andrius, Ajmia Chouchène, Pranas Valatkevičius, Dovilė Gimžauskaitė, Mindaugas Aikas, Rolandas Uscila, Makrem Ghorbel, and Mejdi Jeguirim. 2017. "The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal" Energies 10, no. 5: 710. https://doi.org/10.3390/en10050710







