An Investigation of Fuel Mixing and Reaction in a CH4/Syngas/Air Premixed Impinging Flame with Varied H2/CO Proportion
Abstract
:1. Introduction
2. Experiments
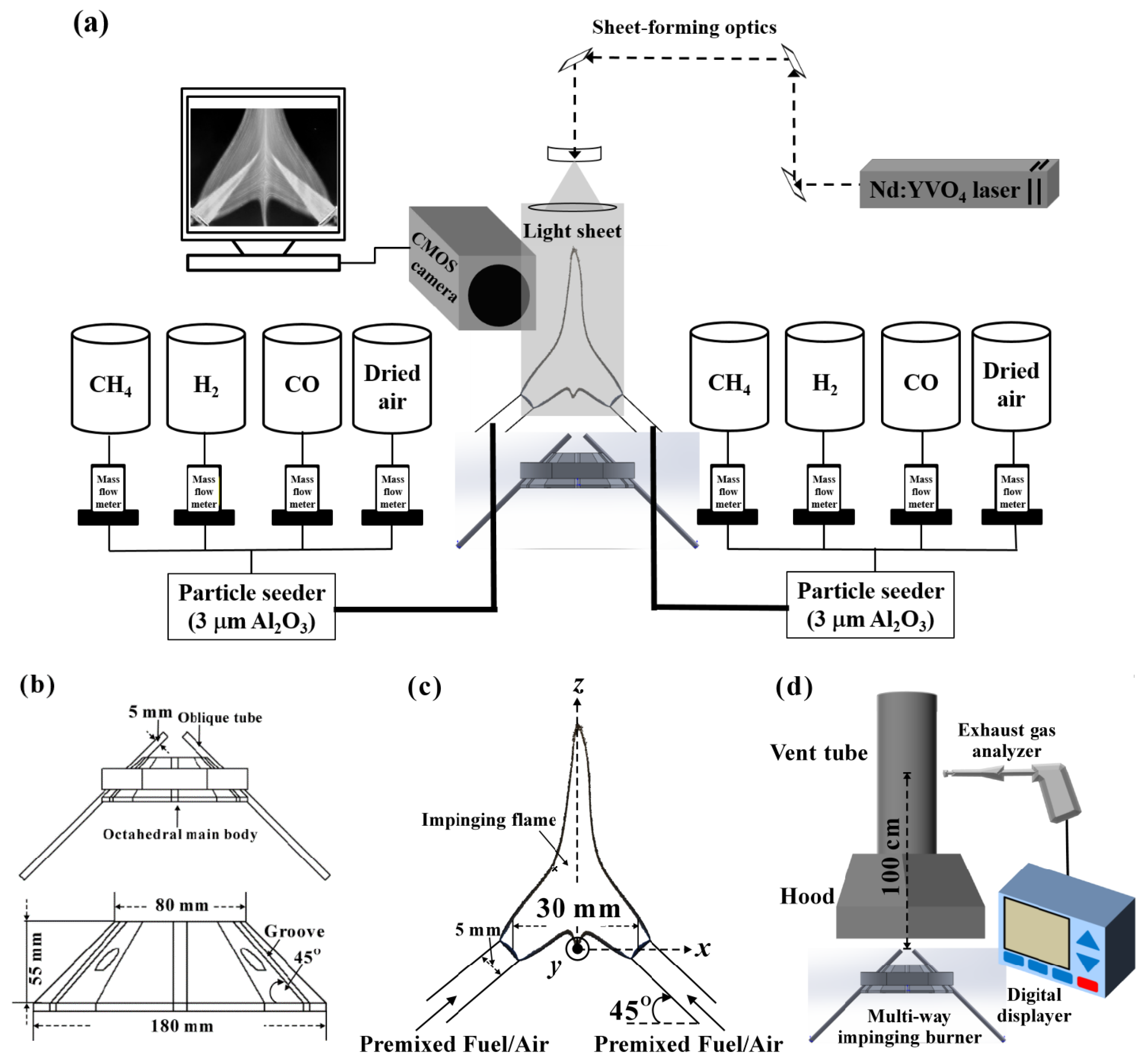
2.1. Experimental Setup and Parameters
2.2. Particle Image Velocimetry and OH* Chemiluminescence
2.3. Flame Temperature and Exhaust Gas
3. Results and Discussion
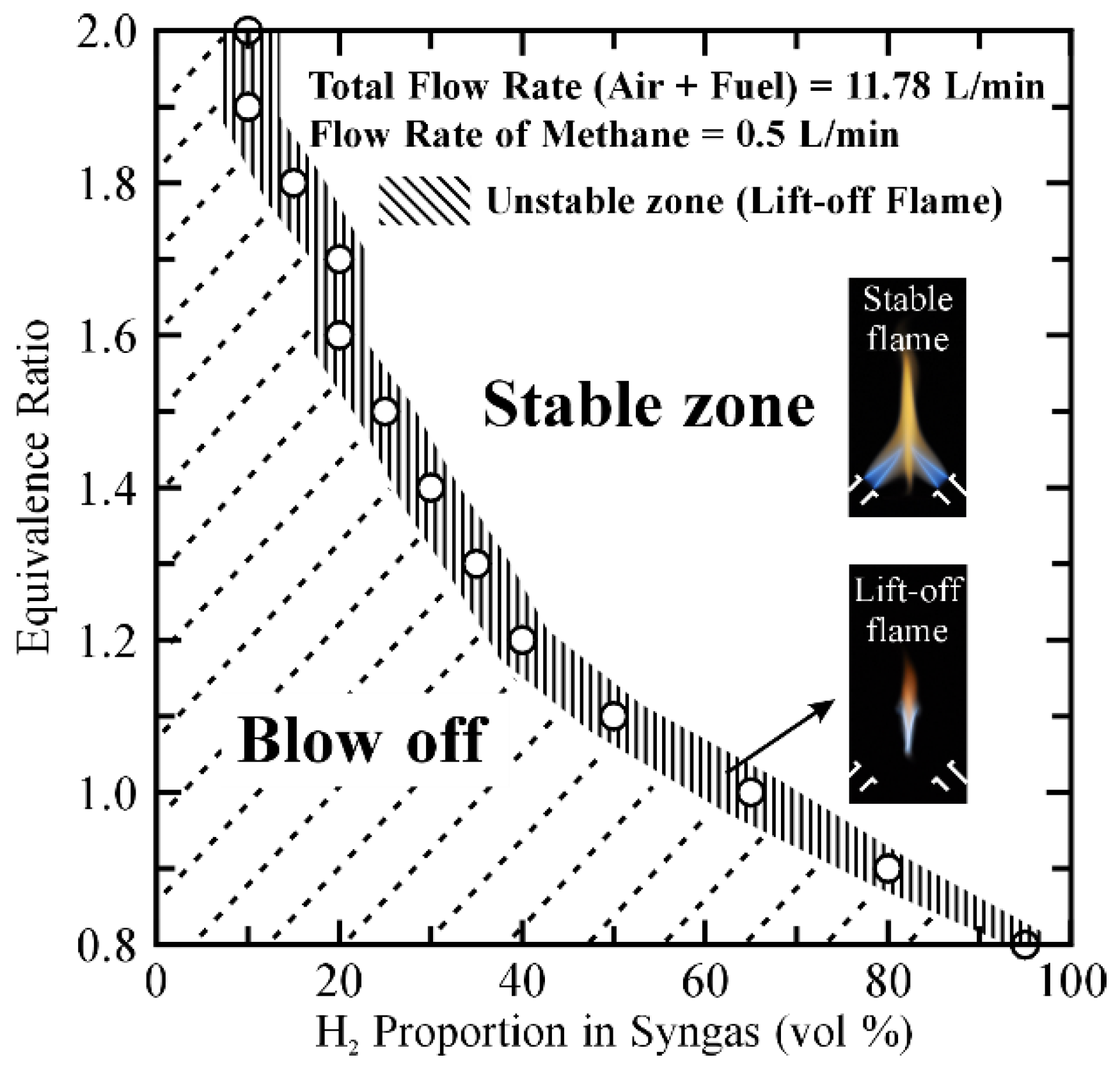
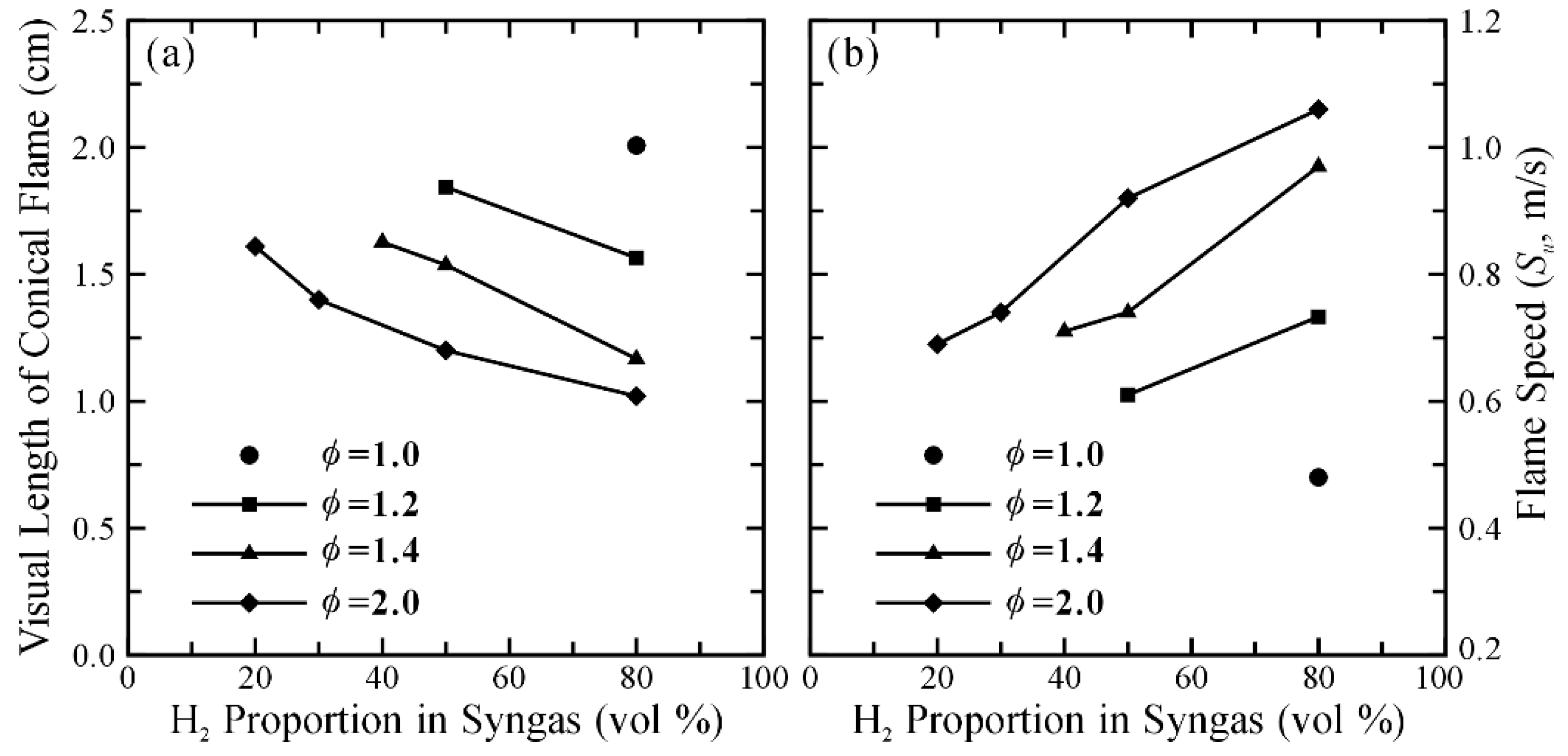
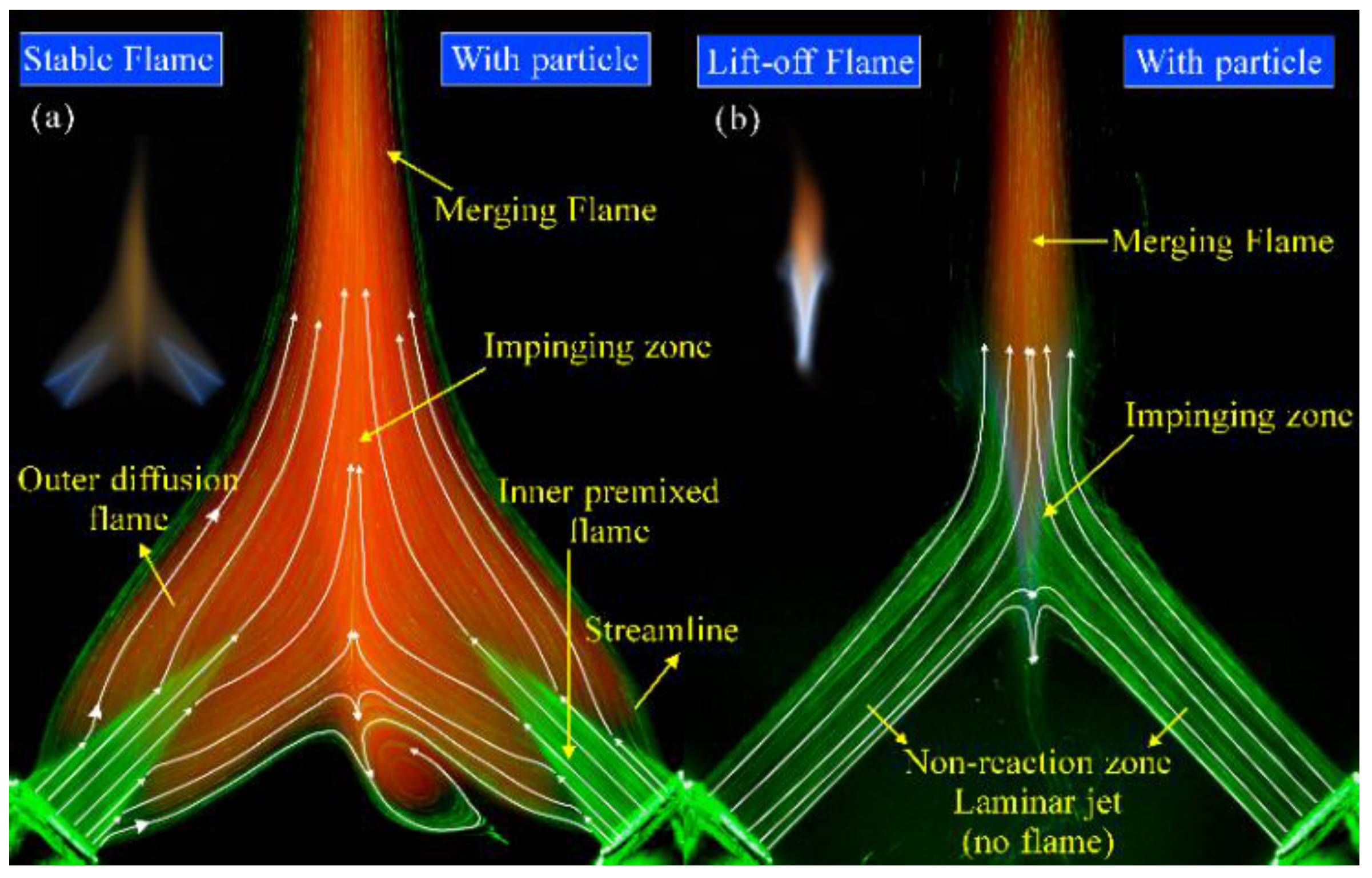
3.1. Operating Range and Flame Patterns
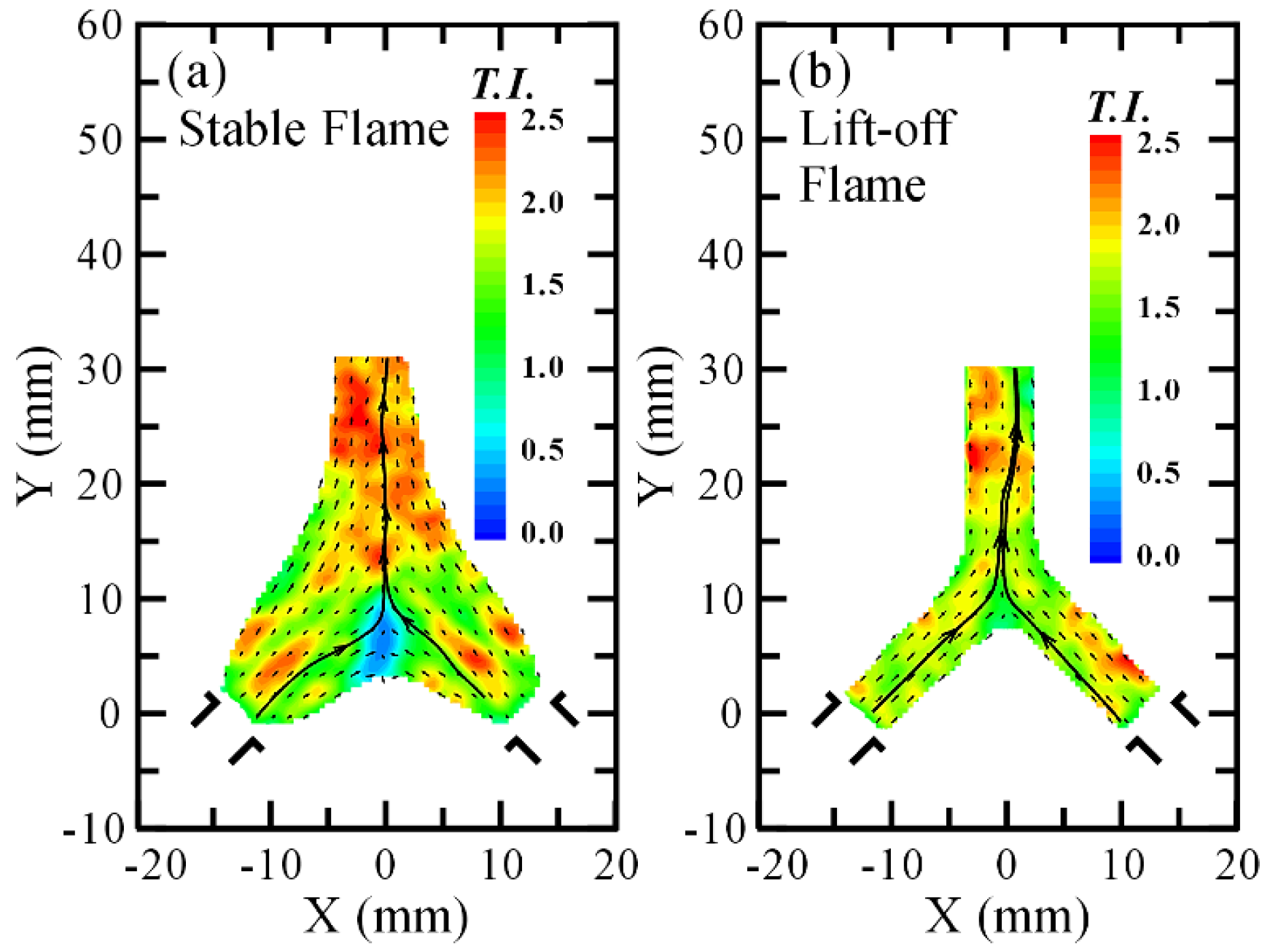
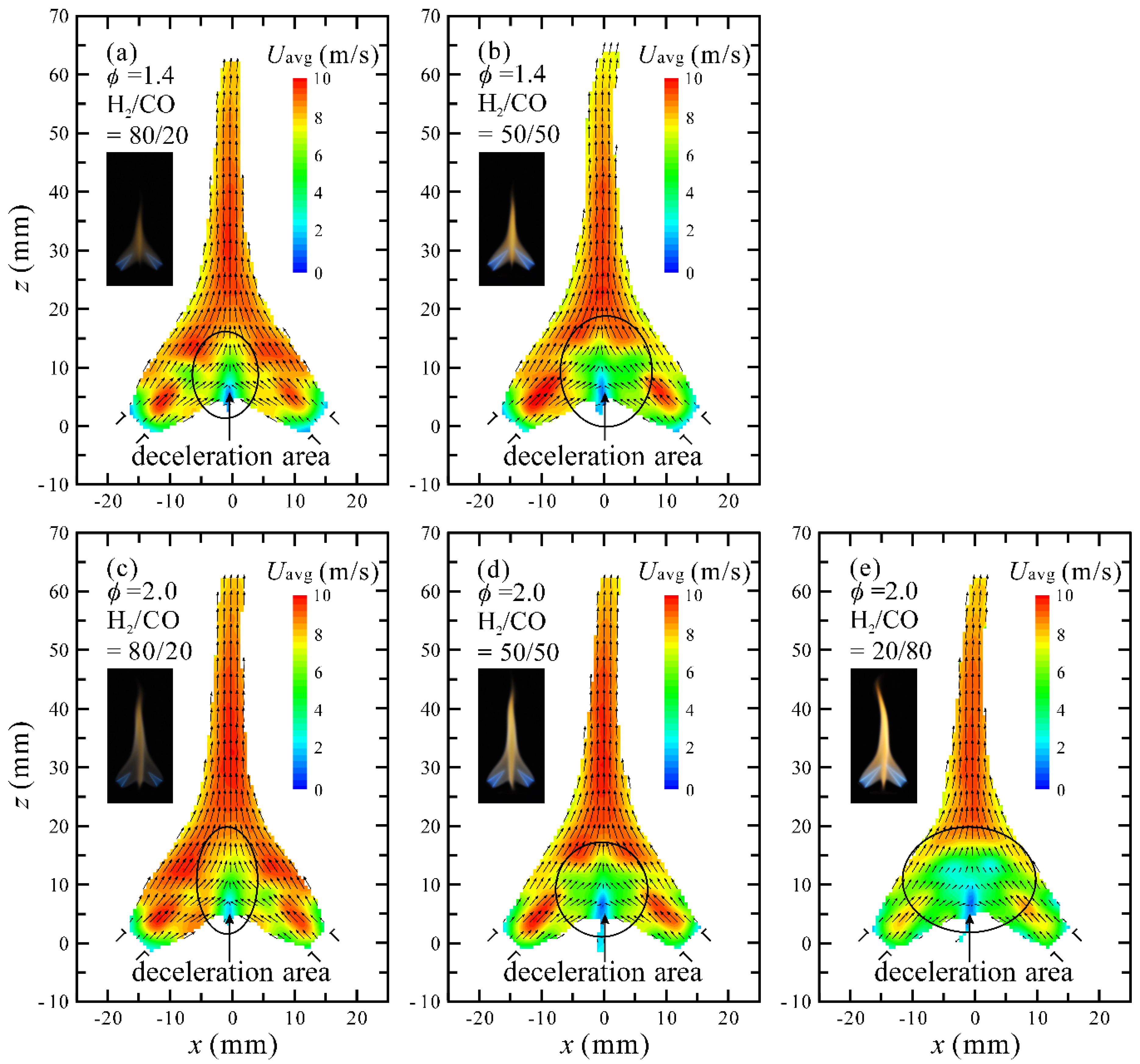
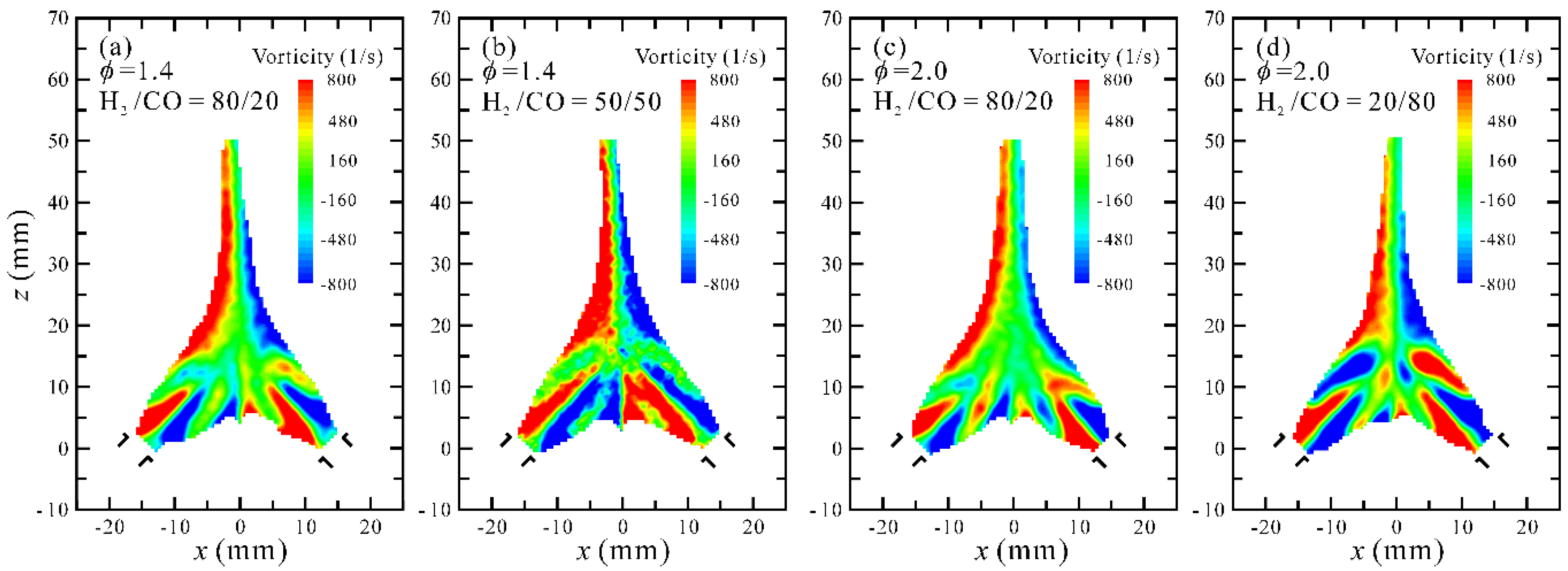
3.2. Flow Visualization, Distribution of Velocity and Vorticity in the Combustion Flow Field
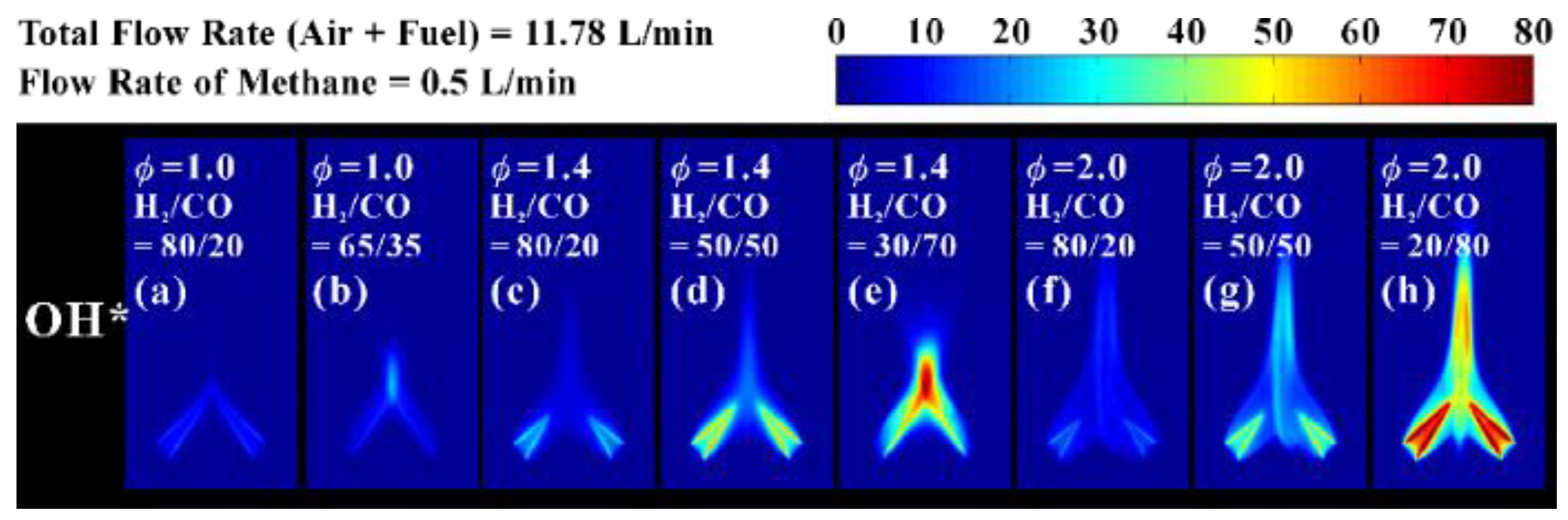
3.3. Distribution of OH* Chemiluminescence
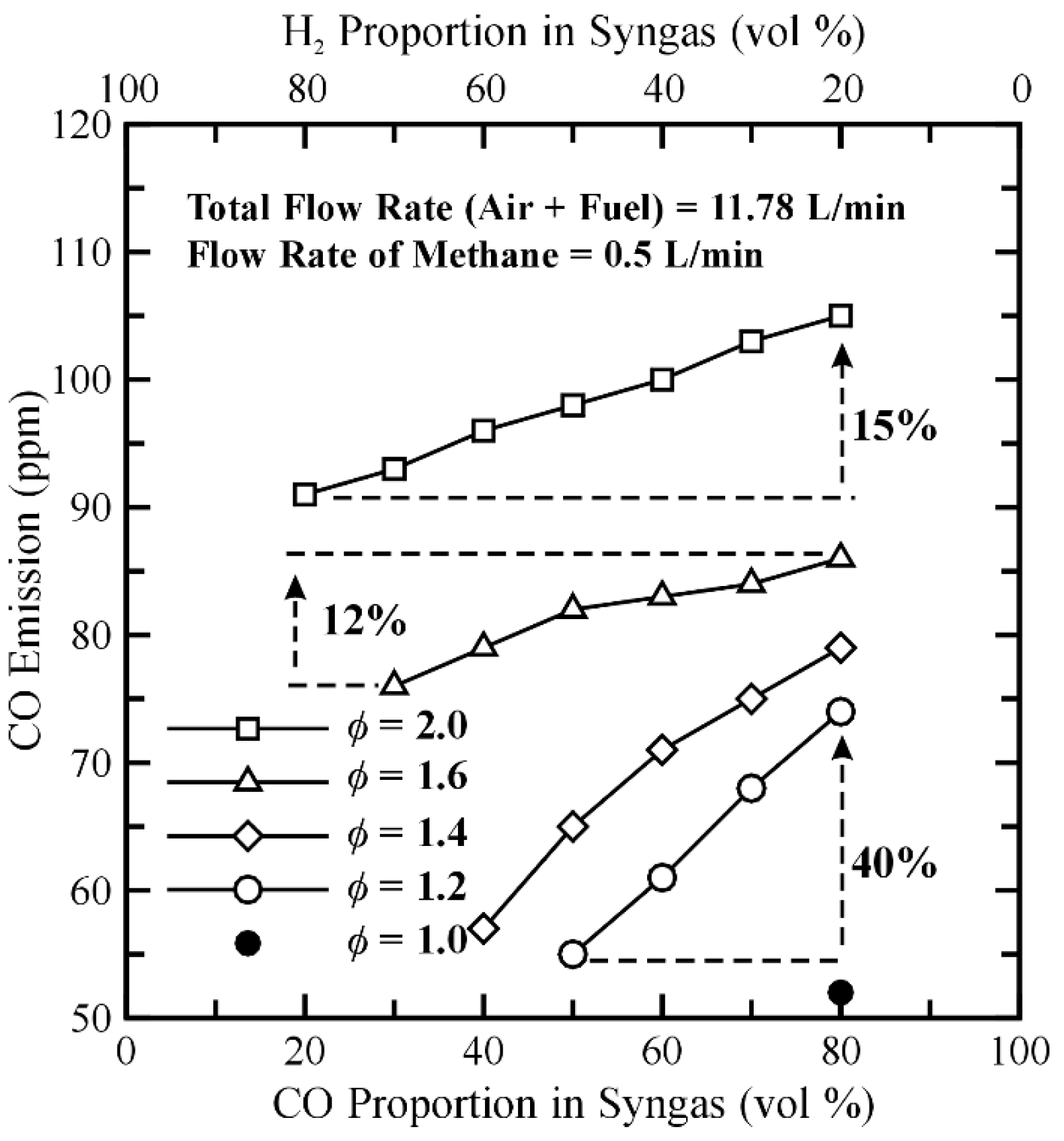
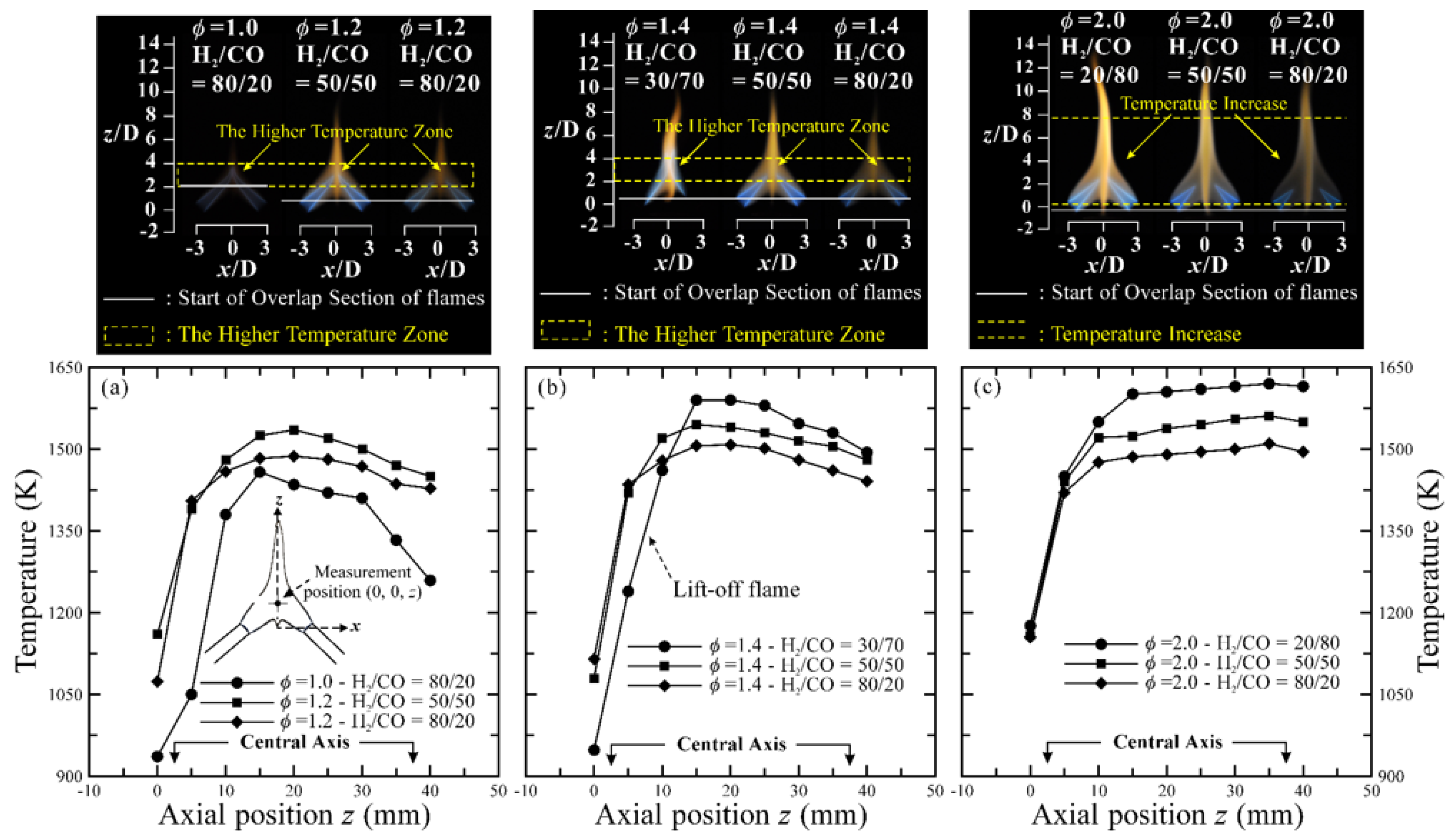
3.4. Temperature Distribution and CO Emission
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| D | inner diameter of the fuel tube, mm |
| μi | dynamic viscosity of component i, N s/m2 |
| Uexit | flow velocity at the burner exit, m/s |
| Yi | mole fraction of component i, % |
| Vtotal | volume flow rate of total gas, m3/s |
| Mi | molecular weight of component i, % |
| Re | Reynolds number of the mixture jet |
| ρi | density of component i, kg/m3 |
| ρmix | density of the mixture, kg/m3 |
| Vi | volume flow rate of component i, m3/s |
| μmix | dynamic viscosity of the mixture, N s/m2 |
| Xi | volume concentration of component i in entire fuel, % |
| T.I. | turbulence intensity, % |
| u’ | velocity fluctuation of in u direction, m/s |
| Su | laminar flame speed, m/s |
| v’ | velocity fluctuation of in v direction, m/s |
References
- Lieuwen, T.; Yang, V.; Yetter, R. Synthesis Gas Combustion: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wender, I. Reactions of synthesis gas. Fuel Process. Technol. 1996, 48, 189–297. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, J.J.; Kim, T.S.; Sohn, J.L. Effects of syngas type on the operation and performance of a gas turbine in integrated gasification combined cycle. Energy Convers. Manag. 2011, 52, 2262–2271. [Google Scholar] [CrossRef]
- Bouvet, N.; Chauveau, C.; Gökalp, I.; Lee, S.Y.; Santoro, R. Characterization of syngas laminar flames using the Bunsen burner configuration. Int. J. Hydrogen Energy 2011, 36, 992–1005. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.; Fan, M.; Zhang, H.; Li, S. Laminar flame speed studies of lean premixed H2/CO/air flames. Combust. Flame 2014, 161, 2492–2495. [Google Scholar] [CrossRef]
- Yasiry, A.S.; Shahad, H.A. An experimental study of the effect of hydrogen blending on burning velocity of LPG at elevated pressure. Int. J. Hydrogen Energy 2016, 41, 19269–19277. [Google Scholar] [CrossRef]
- Lee, H.; Jiang, L.; Mohamad, A. A review on the laminar flame speed and ignition delay time of Syngas mixtures. Int. J. Hydrogen Energy 2014, 39, 1105–1121. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.J. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef]
- Varga, T.; Nagy, T.; Olm, C.; Zsély, I.G.; Pálvölgyi, R.; Valkó, É. Optimization of a hydrogen combustion mechanism using both direct and indirect measurements. Proc. Combust. Inst. 2015, 35, 589–596. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Huang, Z.; Kudo, T.; Kobayashi, H. Measurement of the instantaneous flame front structure of syngas turbulent premixed flames at high pressure. Combust. Flame 2013, 160, 2434–2441. [Google Scholar] [CrossRef]
- Jin, W.; Wang, J.; Nie, Y.; Yu, S.; Huang, Z. Experimental study on flame instabilities of laminar premixed CH4/H2/air non-adiabatic flat flames. Fuel 2015, 159, 599–606. [Google Scholar] [CrossRef]
- Law, C.K.; Kwon, O. Effects of hydrocarbon substitution on atmospheric hydrogen–air flame propagation. Int. J. Hydrogen Energy 2004, 29, 867–879. [Google Scholar] [CrossRef]
- Chen, J.W.; Chiu, C.P.; Mo, S.H.; Yang, J.T. Combustion characteristics of premixed propane flame with added H2 and CO on a V-shaped impinging burner. Int. J. Hydrogen Energy 2015, 40, 1244–1255. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Z.; Yu, S.; Jin, W.; Xie, Y.; Zhang, M. Effects of stretch and preferential diffusion on tip opening of laminar premixed Bunsen flames of syngas/air mixtures. Fuel 2015, 148, 1–8. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: New York, USA, 2010. [Google Scholar]
- Mueller, M.; Yetter, R.; Dryer, F. Flow reactor studies and kinetic modeling of the H2/O2/NOx and CO/H2O/O2/NOx reactions. Int. J. Chem. Kinet. 1999, 31, 705–724. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chao, Y.C.; Cheng, T.; Chen, C.P.; Ho, C.T. Effects of CO addition on the characteristics of laminar premixed CH4/air opposed-jet flames. Combust. Flame 2009, 156, 362–373. [Google Scholar] [CrossRef]
- Nosseir, N.; Peled, U.; Hildebrand, G. Pressure field generated by jet-on-jet impingement. AIAA J. 1987, 25, 1312–1317. [Google Scholar] [CrossRef]
- Disimile, P.; Savory, E.; Toy, N. Mixing characteristics of twin impinging circular jets. J. Propul. Power 1995, 11, 1118–1124. [Google Scholar] [CrossRef]
- Rho, B.J.; Kim, J.K.; Dwyer, H.A. Experimental study of a turbulent cross jet. AIAA J. 1990, 28, 784–789. [Google Scholar] [CrossRef]
- Elangovan, S.; Solaiappan, A.; Rathakrishnan, E. Studies on twin non-parallel unventilated axisymmetric jets. Proc. Inst. Mech. Eng. G J. Aerosp. Eng. 1996, 210, 309–321. [Google Scholar] [CrossRef]
- Landers, B.D.; Disimile, P.J. The Growth Characteristics of Transient Impinging Axisymmetric Turbulent Jets. J. Flow Visual. Image Process. 2015, 22, 117–129. [Google Scholar] [CrossRef]
- Landers, B.D. Mixing Characteristics of Turbulent Twin Impinging Axisymmetric Jets at Various Impingement Angles. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2016. [Google Scholar]
- Su, A.; Liu, Y.C. Investigation of impinging diffusion flames with inert gas. Int. J. Heat Mass Transf. 2002, 45, 3251–3257. [Google Scholar] [CrossRef]
- Boushaki, T.; Sautet, J.C. Characteristics of flow from an oxy-fuel burner with separated jets: Influence of jet injection angle. Exp. Fluids 2010, 48, 1095–1108. [Google Scholar] [CrossRef]
- Boushaki, T.; Guessasma, S.; Sautet, J.C. Predictive analysis of combined burner parameter effects on oxy-fuel flames. Appl. Therm. Eng. 2011, 31, 202–212. [Google Scholar] [CrossRef]
- Li, C.C.; Chen, J.W.; Yang, J.T. Stabilization of double flames interacting with the intersecting flow on a V-shaped burner. Combust. Sci. Technol. 2012, 184, 2117–2135. [Google Scholar] [CrossRef]
- Dinesh, K.R.; Jiang, X.; van Oijen, J.A. Hydrogen-enriched non-premixed jet flames: Analysis of the flame surface, flame normal, flame index and Wobbe index. Int. J. Hydrogen Energy 2014, 39, 6753–6763. [Google Scholar] [CrossRef]
- Dinesh, K.R.; van Oijen, J.A.; Luo, K.H.; Jiang, X. Nitric oxide pollutant formation in high hydrogen content (HHC) syngas flames. Int. J. Hydrogen Energy 2015, 40, 13621–13634. [Google Scholar] [CrossRef]
- Dinesh, K.R.; Jiang, X.; van Oijen, J.A. Hydrogen-enriched non-premixed jet flames: Compositional structures with near-wall effects. Int. J. Hydrogen Energy 2013, 38, 5150–5164. [Google Scholar] [CrossRef]
- Dinesh, K.R.; Jiang, X.; van Oijen, J.A.; Bastiaans, R.J.M.; de Goey, L.P.H. Influence of fuel variability on the characteristics of impinging non-premixed syngas burning. Proc. Combust. Inst. 2013, 34, 3219–3229. [Google Scholar] [CrossRef]
- Zhen, H.S.; Leung, C.W.; Cheung, C.S.; Huang, Z.H. Characterization of biogas-hydrogen premixed flames using Bunsen burner. Int. J. Hydrogen Energy 2014, 39, 13292–13299. [Google Scholar] [CrossRef]
- Pan, K.L.; Li, C.C.; Juan, W.C.; Yang, J.T. Low-frequency oscillation of a non-premixed flame on a bluff-body burner. Combust. Sci. Technol. 2009, 181, 1217–1230. [Google Scholar] [CrossRef]
- Raffel, M.; Willert, C.E.; Wereley, S.; Kompenhans, J. Particle Image Velocimetry: A Practical Guide; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ballester, J.; García-Armingol, T. Diagnostic techniques for the monitoring and control of practical flames. Prog. Energy Combust. Sci. 2010, 36, 375–411. [Google Scholar] [CrossRef]
- Kaskan, W. The dependence of flame temperature on mass burning velocity. Symp. Int. Combust. 1957, 6, 134–143. [Google Scholar] [CrossRef]
- Hindasageri, V.; Vedula, R.; Prabhu, S. Thermocouple error correction for measuring the flame temperature with determination of emissivity and heat transfer coefficient. Rev. Sci. Instrum. 2013, 84, 024902. [Google Scholar] [CrossRef] [PubMed]
- Samiran, N.A.; Ng, J.H.; Jaafar, M.N.M.; Valera-Medina, A.; Chong, C.T. H2-rich syngas strategy to reduce NOx and CO emissions and improve stability limits under premixed swirl combustion mode. Int. J. Hydrogen Energy 2016, 41, 19243–19255. [Google Scholar] [CrossRef]
- Lee, I.B.; Woo, I.S.; Lee, M.C. Effects of nitrogen dilution on the NOx and CO emission of H2/CO/CH4 syngases in a partially-premixed gas turbine model combustor. Int. J. Hydrogen Energy 2016, 41, 15841–15851. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C. Emission of impinging swirling and non-swirling inverse diffusion flames. Appl. Energy 2011, 88, 1629–1634. [Google Scholar] [CrossRef]
- Moffat, R.J. Contributions to the theory of single-sample uncertainty analysis. J. Fluids Eng. 1982, 104, 250–260. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Q.; Zhao, Q.; Zhang, Y.; Xu, T.; Hui, S. Experimental study on the laminar flame speed of hydrogen/carbon monoxide/air mixtures. Fuel 2009, 88, 1858–1863. [Google Scholar] [CrossRef]
- Zhen, H.; Leung, C.; Cheung, C. A comparison of the heat transfer behaviors of biogas–H2 diffusion and premixed flames. Int. J. Hydrogen Energy 2014, 39, 1137–1144. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion, 2nd ed.; McGraw-Hill: New York, NY, USA, 2006; pp. 256–257. [Google Scholar]
- Tsuji, H.; Yamaoka, I. The counterflow diffusion flame in the forward stagnation region of a porous cylinder. Symp. Int. Combust. 1967, 11, 979–984. [Google Scholar] [CrossRef]
- Rolon, J.C.; Veynante, D.; Martin, J.P.; Durst, F. Counter jet stagnation flows. Exp. Fluids 1991, 11, 313–324. [Google Scholar] [CrossRef]
- Waitz, I.A.; Qiu, Y.J.; Manning, T.A.; Fung, A.K.S.; Elliot, J.K.; Kerwin, J.M.; Marble, F.E. Enhanced mixing with streamwise vorticity. Prog. Aerospace Sci. 1997, 33, 323–351. [Google Scholar] [CrossRef]

| Constant Parameters | Given Parameters | Variation Parameters |
|---|---|---|
| Total volume flow rate, Vtotal = 11.78 L/min | equivalence ratio, φ from 0.8 to 2.0 | Volume concentration of component i in entire fuel, Xi, % |
| CH4 volume flow rate, Vmethane = 0.5 L/min | H2/CO proportion 1 from 100/0 to 0/100 | H2 volume flow rate, VHydrogen |
| Flow velocity of burner exit, Uexit = 5.0 m/s | - | CO volume flow rate, VCarbon monoxide |
| Vair (L/min) | Vmethane (L/min) | VHydrogen (L/min) | VCarbon monoxide (L/min) | H2/CO (Syngas) | φ | Re |
|---|---|---|---|---|---|---|
| 9.35 | 0.50 | 1.25 | 0.68 | 65/35 | 1.0 | 1622 |
| 9.35 | 0.50 | 1.54 | 0.39 | 80/20 | 1.0 | 1602 |
| 8.83 | 0.50 | 0.98 | 1.47 | 40/60 | 1.2 | 1636 |
| 8.83 | 0.50 | 1.23 | 1.23 | 50/50 | 1.2 | 1620 |
| 8.83 | 0.50 | 1.96 | 0.49 | 80/20 | 1.2 | 1571 |
| 8.36 | 0.50 | 0.88 | 2.04 | 30/70 | 1.4 | 1640 |
| 8.36 | 0.50 | 1.17 | 1.75 | 40/60 | 1.4 | 1621 |
| 8.36 | 0.50 | 1.46 | 1.46 | 50/50 | 1.4 | 1602 |
| 8.36 | 0.50 | 2.33 | 0.58 | 80/20 | 1.4 | 1542 |
| 7.21 | 0.50 | 0.81 | 3.25 | 20/80 | 2.0 | 1637 |
| 7.21 | 0.50 | 1.22 | 2.84 | 30/70 | 2.0 | 1611 |
| 7.21 | 0.50 | 2.03 | 2.03 | 50/50 | 2.0 | 1556 |
| 7.21 | 0.50 | 3.25 | 0.81 | 80/20 | 2.0 | 1464 |
| φ | H2/CO (Syngas) | Xmethane (vol %) (in Total Fuel) | XHydrogen (vol %) (in Total Fuel) | XCarbon monoxide (vol %) (in Total Fuel) |
|---|---|---|---|---|
| 1.0 | 65/35 | 20.60 | 51.44 | 27.96 |
| 1.0 | 80/20 | 20.60 | 63.37 | 16.03 |
| 1.2 | 40/50 | 16.95 | 33.22 | 49.83 |
| 1.2 | 50/50 | 16.95 | 41.55 | 41.50 |
| 1.2 | 80/20 | 16.95 | 66.44 | 16.61 |
| 1.4 | 30/70 | 14.63 | 25.73 | 59.64 |
| 1.4 | 40/60 | 14.63 | 34.21 | 51.16 |
| 1.4 | 50/50 | 14.63 | 42.69 | 42.68 |
| 1.4 | 80/20 | 14.63 | 68.33 | 17.04 |
| 2.0 | 20/80 | 10.96 | 17.76 | 71.28 |
| 2.0 | 30/70 | 10.96 | 26.75 | 62.29 |
| 2.0 | 50/50 | 10.96 | 44.52 | 44.52 |
| 2.0 | 80/20 | 10.96 | 71.27 | 17.77 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-P.; Yeh, S.-I.; Tsai, Y.-C.; Yang, J.-T. An Investigation of Fuel Mixing and Reaction in a CH4/Syngas/Air Premixed Impinging Flame with Varied H2/CO Proportion. Energies 2017, 10, 900. https://doi.org/10.3390/en10070900
Chiu C-P, Yeh S-I, Tsai Y-C, Yang J-T. An Investigation of Fuel Mixing and Reaction in a CH4/Syngas/Air Premixed Impinging Flame with Varied H2/CO Proportion. Energies. 2017; 10(7):900. https://doi.org/10.3390/en10070900
Chicago/Turabian StyleChiu, Chih-Pin, Szu-I Yeh, Yu-Ching Tsai, and Jing-Tang Yang. 2017. "An Investigation of Fuel Mixing and Reaction in a CH4/Syngas/Air Premixed Impinging Flame with Varied H2/CO Proportion" Energies 10, no. 7: 900. https://doi.org/10.3390/en10070900





