Numerical Investigations of the Combined Effects of Flow Rate and Methanol Concentration on DMFC Performance
Abstract
:1. Introduction
2. Methods and Models
2.1. Problem Description
2.2. Governing Equations and Models
2.3. Numerical Implementations
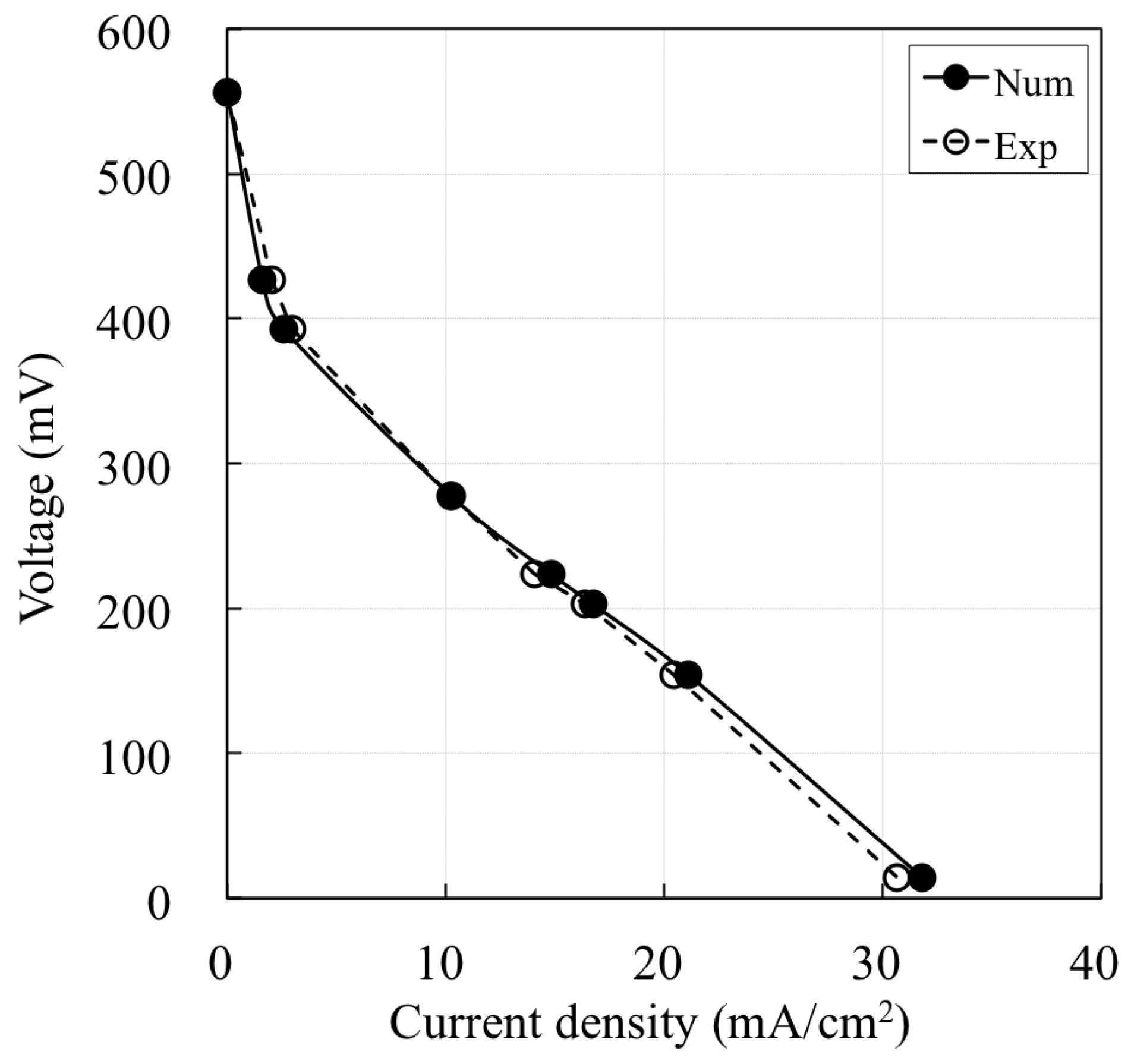
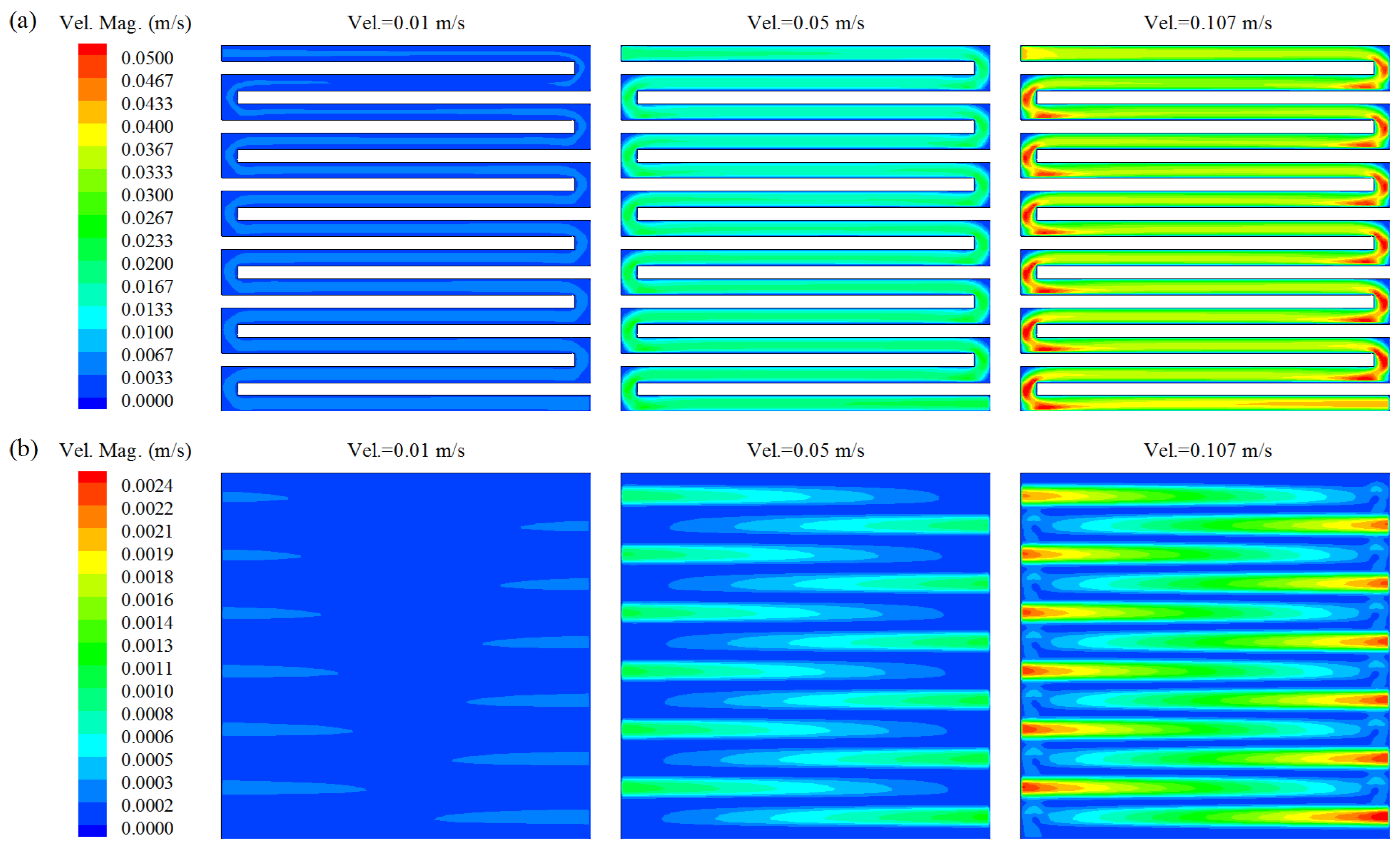
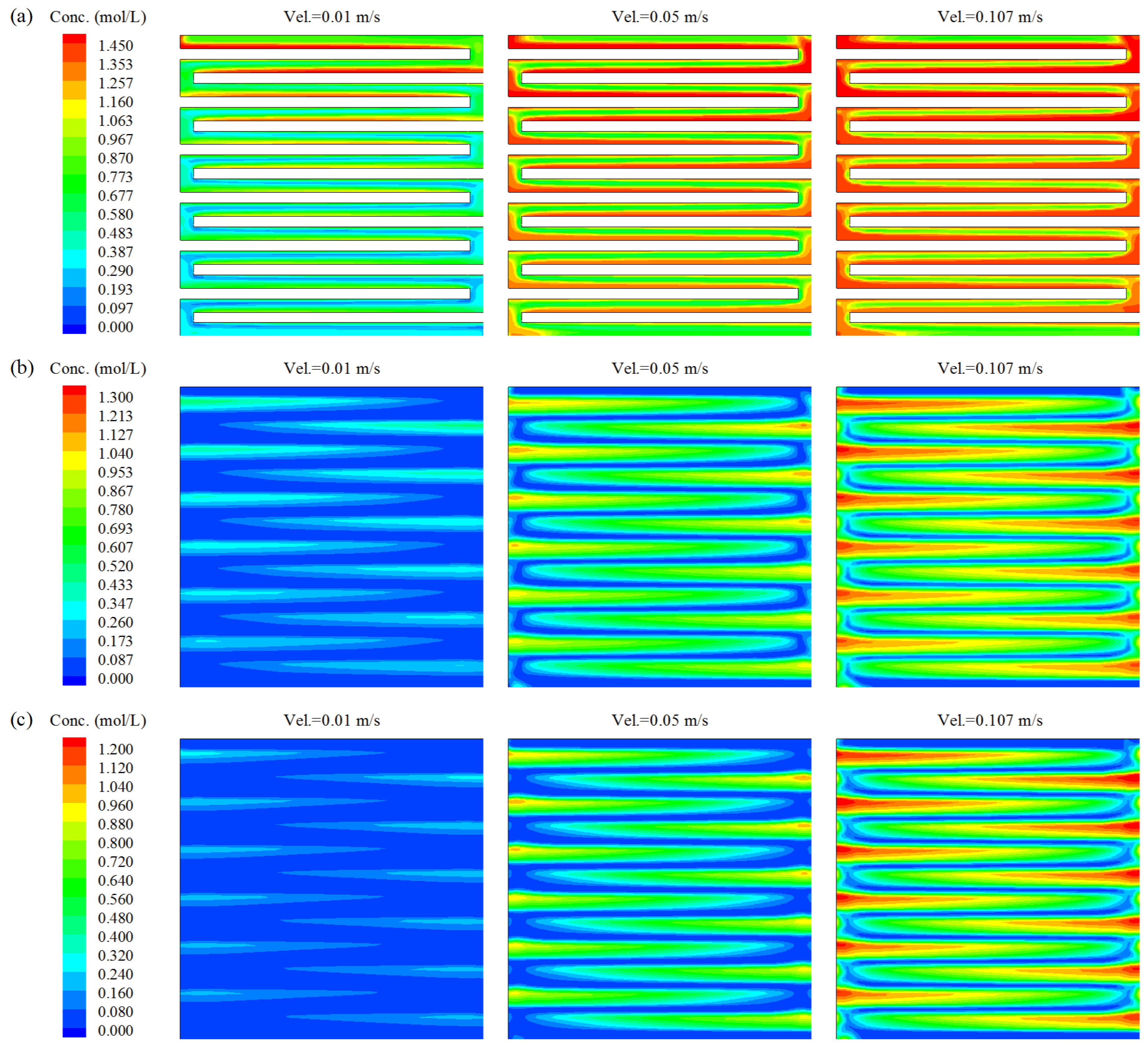
3. Results and Discussion
4. Conclusions
- (1)
- A parameter was introduced to quantify the effect of oxygen availability in model construction. It can be one of the earliest studies, to the best of our knowledge, for considering the experimental facts that DMFC’s performance depends largely on the air flow rate.
- (2)
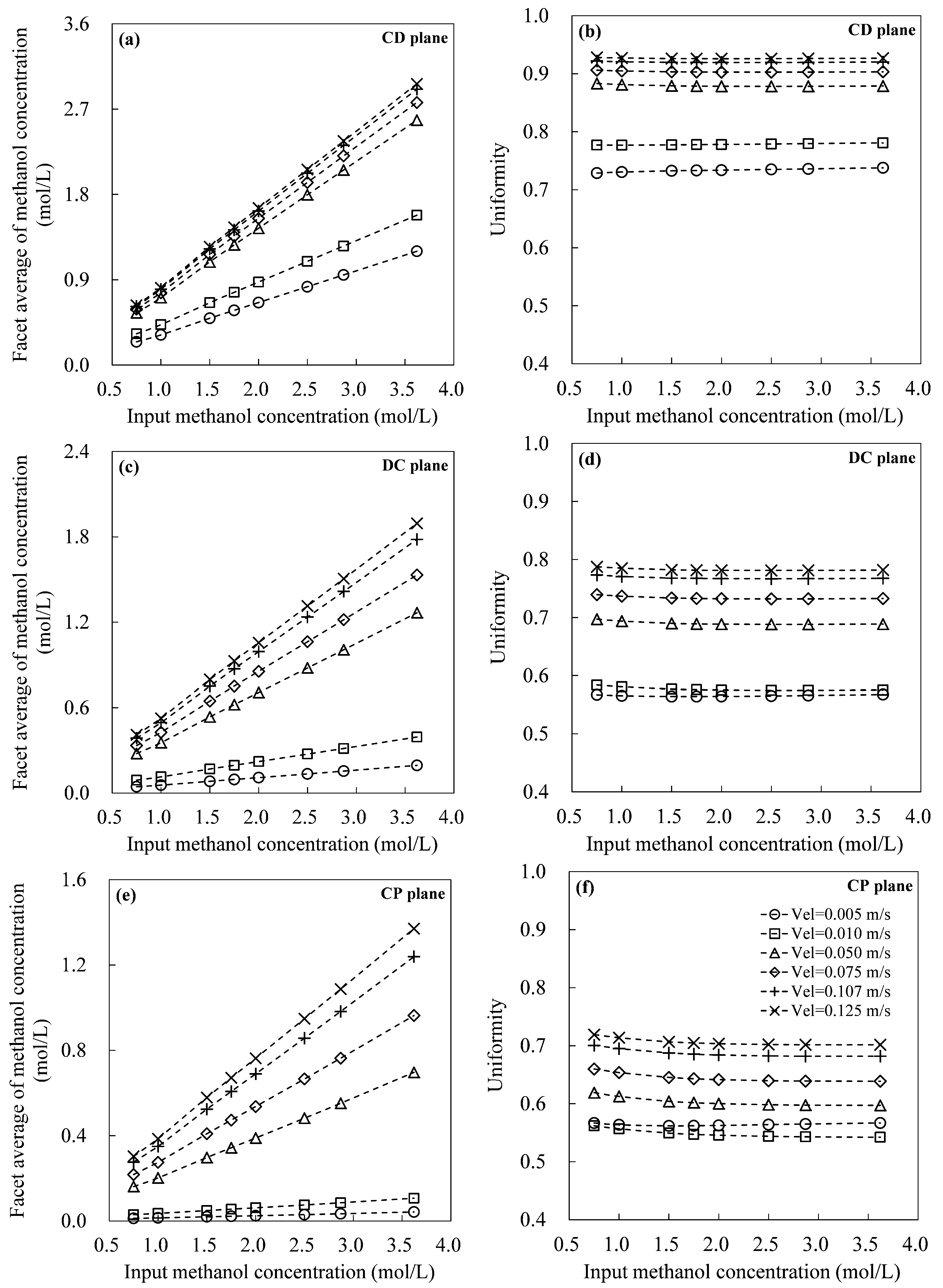
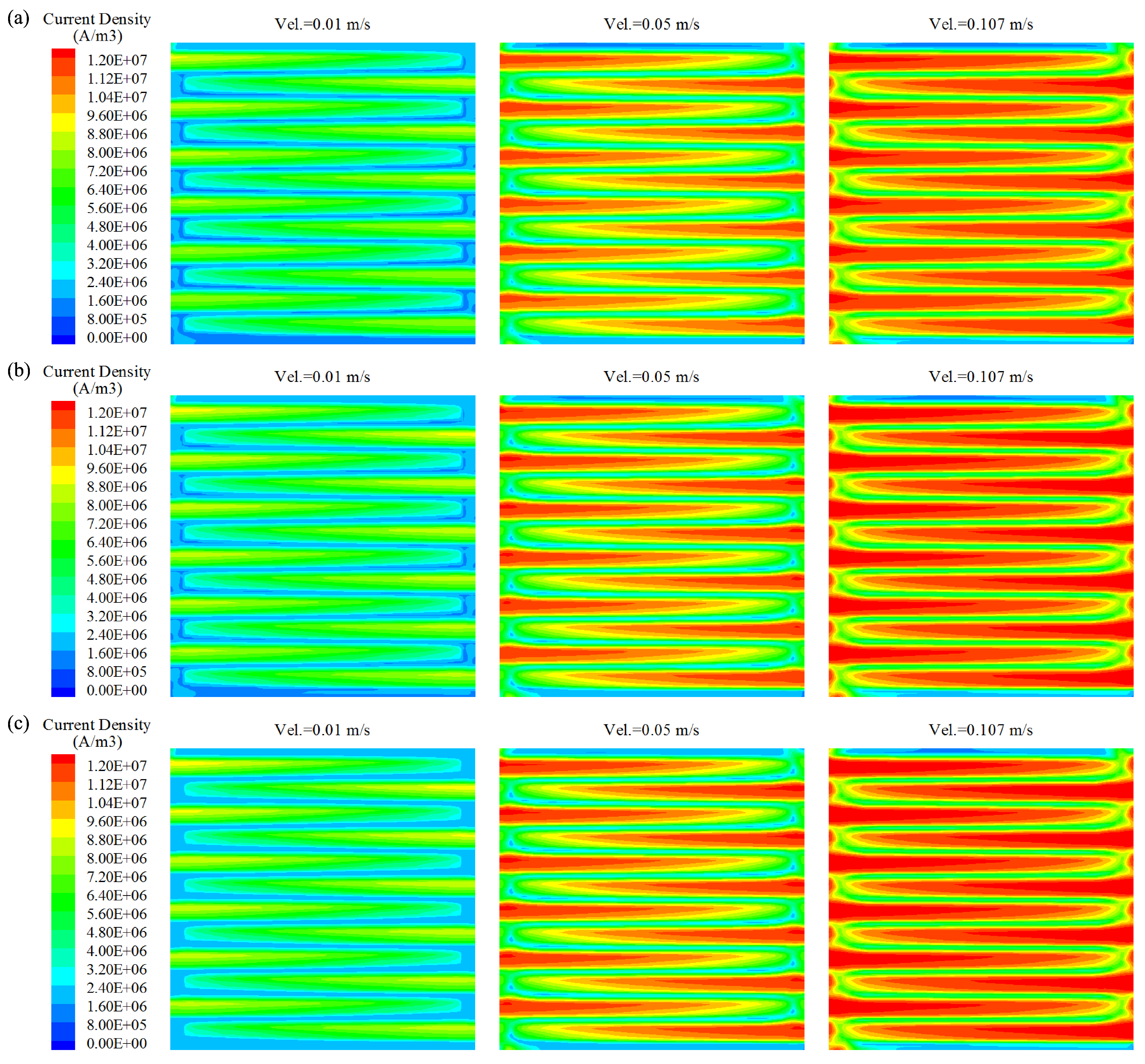
- Flow characteristics inside the DMFC system were systematically examined by numerical simulations. The increasing of inlet velocity was observed to be an effective strategy to accelerate the internal flow fields in DMFC. Careful analysis was also carried out on the the distributions of methanol concentration on three typical planes. From a hydrodynamic point of view, the diffusion layer is a critical component in the design of high-performance DMFC, as it was observed to be highly associated with the distribution uniformities of both flow velocity and methanol concentration.
- (3)
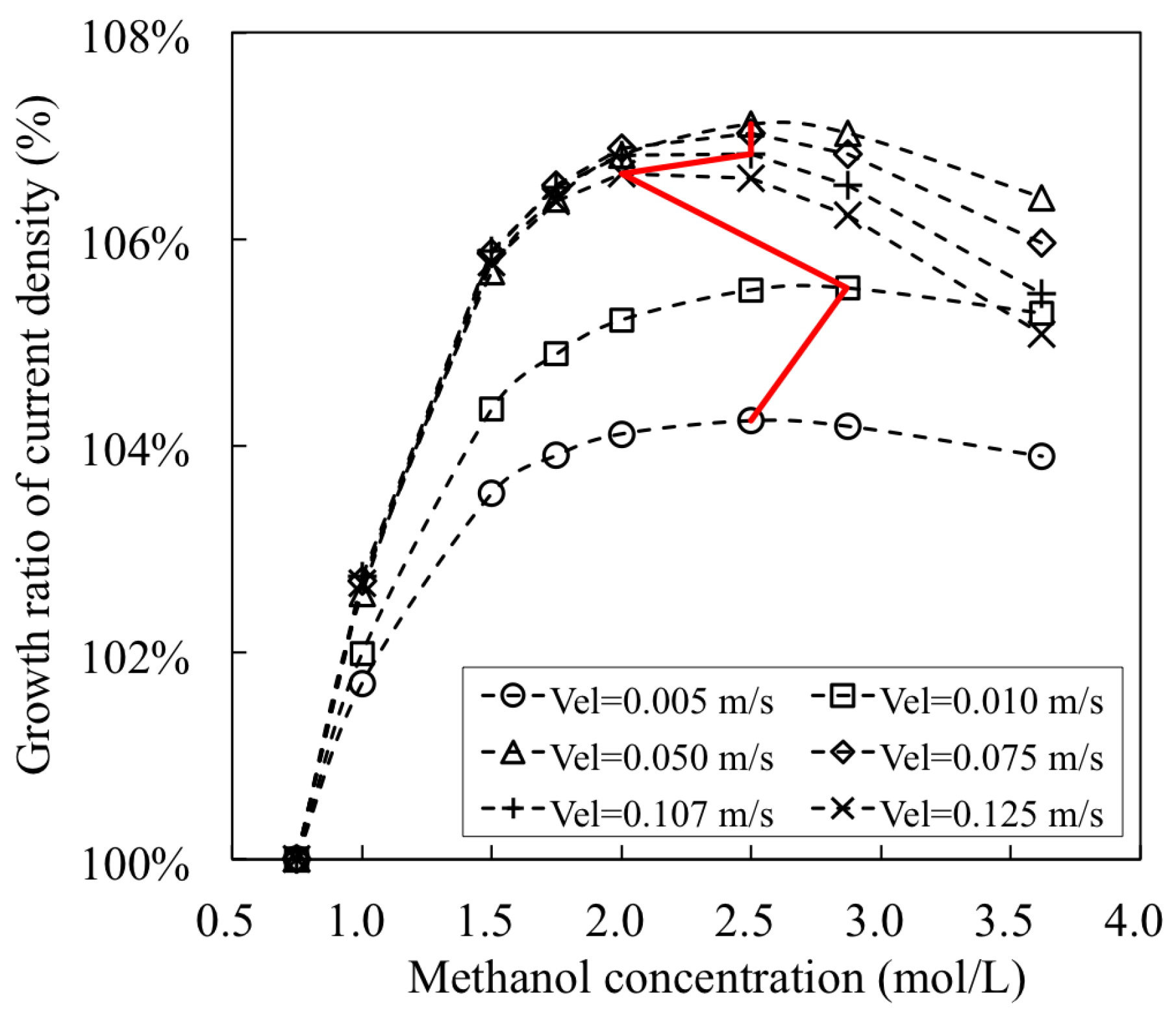
- The combined effects of inlet flow rate and input methanol concentration on the facet average (also the facet uniformity) of methanol concentration were summarized. The simultaneous increases in both flow velocity and methanol concentration were proposed to be applied, if a quick increase in facet average of methanol concentration is required in practice. The proposed quantitative results can be used as a numerical database to analyze experimental results in future. The frontier for optimal conditions of DMFC’s output were determined, which is helpful to improve the energy conversion performance of DMFC in practical applications.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMFC | Direct Methanol Fuel Cell |
| CFD | Computational Fluid Dynamics |
| PEM | Polymer Electrolyte Membrane |
| WUI | Weltens’ Uniformity Index |
| CD plane | Interface plane between Channel and Diffusion layers |
| DC plane | Interface plane between Diffusion and Catalyst layers |
| CP plane | Interface plane between Catalyst and PEM layers |
Appendix A
| Definition | Notation | Value | Unit | Ref. |
|---|---|---|---|---|
| Water density | 1000 | kg· m | [20] | |
| Water viscosity | kg· ms | [26] | ||
| Air density | 1.29 | kg· m | [20] | |
| Air viscosity | kg· ms | [26] | ||
| Diffusion coefficient | ms | [26] | ||
| of in water | ||||
| Diffusion coefficient | ms | [26] | ||
| of in water | ||||
| Permeability of | m | [20] | ||
| diffusion layer | ||||
| Permeability of | m | [20] | ||
| catalyst layer | ||||
| Porosity of | 0.6 | − | [20] | |
| diffusion layer | ||||
| Porosity of | 0.4 | − | [20] | |
| catalyst layer | ||||
| Faraday constant | F | C· k mol | [17] | |
| Gas constant | R | J molK | [17] | |
| Molecular weight | 0.032 | kg· mol | [17] | |
| of | ||||
| Molecular weight | 0.018 | kg· mol | [17] | |
| of | ||||
| Molecular weight | 0.044 | kg· mol | [27] | |
| of | ||||
| Molecular weight | 0.032 | kg· mol | [27] | |
| of | ||||
| Electro-osmotic drag | − | [28] | ||
| coefficient of | ||||
| Electro-osmotic drag | − | [14] | ||
| coefficient of | ||||
| Oxygen availability | 1.75 | − | Determined from Exp. |
| Unit | Value | |||||
|---|---|---|---|---|---|---|
| m/s | 0.005 | 0.010 | 0.050 | 0.075 | 0.107 | 0.125 |
| ccm | 0.2055 | 0.4110 | 2.0550 | 3.0825 | 4.4000 | 5.1375 |
References
- Dillon, R.; Srinivasan, S.; Aricò, A.S.; Antonucci, V. International activities in DMFC R&D: Status of technologies and potential applications. J. Power Sources 2004, 127, 112–126. [Google Scholar]
- Guo, Z.; Faghri, A. Development of planar air breathing direct methanol fuel cell stacks. J. Power Sources 2006, 160, 1183–1194. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Zhang, Y.; He, H.; Li, J.; Wang, S.; Yuan, Z.; Deng, H. Development and characterization of a novel air-breathing micro direct methanol fuel cell stack for portable applications. J. Micromech. Microeng. 2010, 20, 104008. [Google Scholar] [CrossRef]
- Feng, L.; Cai, W.; Li, C.; Zhang, J.; Liu, C.; Xing, W. Fabrication and performance evaluation for a novel small planar passive direct methanol fuel cell stack. Fuel 2012, 94, 401–408. [Google Scholar] [CrossRef]
- San Martín, I.; Ursúa, A.; Sanchis, P. Modelling of PEM fuel cell performance: Steady-state and dynamic experimental validation. Energies 2014, 7, 670–700. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Hu, Y.; Zhang, Y.; Liu, X.; Wang, G. A “4-cell” modular passive DMFC (direct methanol fuel cell) stack for portable applications. Energy 2015, 82, 229–235. [Google Scholar] [CrossRef]
- Barbera, O.; Stassi, A.; Sebastian, D.; Bonde, J.L.; Giacoppo, G.; D’Urso, C.; Baglio, V.; Aricò, A.S. Simple and functional direct methanol fuel cell stack designs for application in portable and auxiliary power units. Int. J. Hydrogen Energy 2016, 41, 12320–12329. [Google Scholar] [CrossRef]
- Na, Y.; Zenith, F.; Krewer, U. Increasing fuel efficiency of direct methanol fuel cell systems with feedforward control of the operating concentration. Energies 2015, 8, 10409–10429. [Google Scholar] [CrossRef]
- Vinh, N.D.; Kim, H.M. Comparison of numerical and experimental studies for flow-field optimization based on under-rib convection in polymer electrolyte membrane fuel cells. Energies 2016, 9, 844. [Google Scholar] [CrossRef]
- Ko, J.; Chippar, P.; Ju, H. A one-dimensional, two-phase model for direct methanol fuel cells-Part I: Model development and parametric study. Energy 2010, 35, 2149–2159. [Google Scholar] [CrossRef]
- Birgersson, E.; Nordlund, J.; Ekström, H.; Vynnycky, M.; Lindbergh, G. Reduced two-dimensional one-phase model for analysis of the anode of a DMFC. J. Electrochem. Soc. 2003, 150, A1368–A1376. [Google Scholar] [CrossRef]
- Yan, T.Z.; Jen, T.C. Two-phase flow modeling of liquid-feed direct methanol fuel cell. Int. J. Heat Mass Transf. 2008, 51, 1192–1204. [Google Scholar] [CrossRef]
- Ge, J.; Liu, H. A three-dimensional mathematical model for liquid-fed direct methanol fuel cells. J. Power Sources 2006, 160, 413–421. [Google Scholar] [CrossRef]
- Danilov, V.A.; Lim, J.; Moon, I.; Changb, H. Three-dimensional, two-phase, CFD model for the design of a direct methanol fuel cell. J. Power Sources 2006, 162, 992–1002. [Google Scholar] [CrossRef]
- Yang, W.W.; Zhao, T.S.; Xu, C. Three-dimensional two-phase mass transport model for direct methanol fuel cells. Electrochim. Acta 2007, 53, 853–862. [Google Scholar] [CrossRef]
- Vera, M. A single-phase model for liquid-feed DMFCs with non-Tafel kinetics. J. Power Sources 2007, 171, 763–777. [Google Scholar] [CrossRef]
- Yang, Q.; Kianimanesh, A.; Freiheit, T.; Park, S.S.; Xue, D. A semi-empirical model considering the influence of operating parameters on performance for a direct methanol fuel cell. J. Power Sources 2011, 196, 10640–10651. [Google Scholar] [CrossRef]
- Kianimanesh, A.; Yu, B.; Yang, Q.; Freiheit, T.; Xue, D.; Park, S.S. Investigation of bipolar plate geometry on direct methanol fuel cell performance. Int. J. Hydrogen Energy 2012, 37, 18403–18411. [Google Scholar] [CrossRef]
- Yu, B.; Yang, Q.; Kianimanesh, A.; Freiheit, T.; Park, S.S.; Zhao, H.; Xue, D. A CFD model with semi-empirical electrochemical relationships to study the influence of geometric and operating parameters on DMFC performance. Int. J. Hydrogen Energy 2013, 38, 9873–9885. [Google Scholar] [CrossRef]
- Jeng, K.T.; Chen, C.W. Modeling and simulation of a direct methanol fuel cell anode. J. Power Sources 2002, 112, 367–375. [Google Scholar] [CrossRef]
- He, Y.L.; Li, X.L.; Miao, Z.; Liu, Y.W. Two-phase modeling of mass transfer characteristics of a direct methanol fuel cell. Appl. Therm. Eng. 2009, 29, 1998–2008. [Google Scholar] [CrossRef]
- Le, A.D.; Zhou, B. A general model of proton exchange membrane fuel cell. J. Power Sources 2008, 182, 197–222. [Google Scholar] [CrossRef]
- Sivertsen, B.R.; Djilali, N. CFD-based modelling of proton exchange membrane fuel cells. J. Power Sources 2005, 141, 65–78. [Google Scholar] [CrossRef]
- Weltens, H.; Bressler, H.; Terres, F.; Neumaier, H.; Rammoser, D. Optimisation of Catalytic Converter Gas Flow Distribution by CFD Prediction. SAE Pap. 1993, 930780. [Google Scholar]
- Wang, Z.H.; Wang, C.Y. Mathematical modeling of liquid-feed direct methanol fuel cells. J. Electrochem. Soc. 2003, 150, A508–A519. [Google Scholar] [CrossRef]
- Divisek, J.; Fuhrmann, J.; Gärtner, K.; Jung, R. Performance modeling of a direct methanol fuel cell. J. Electrochem. Soc. 2003, 150, A811–A825. [Google Scholar] [CrossRef]
- Guo, H.; Ma, C.F. 2D analytical model of a direct methanol fuel cell. Electrochem. Commun. 2004, 6, 306–312. [Google Scholar] [CrossRef]
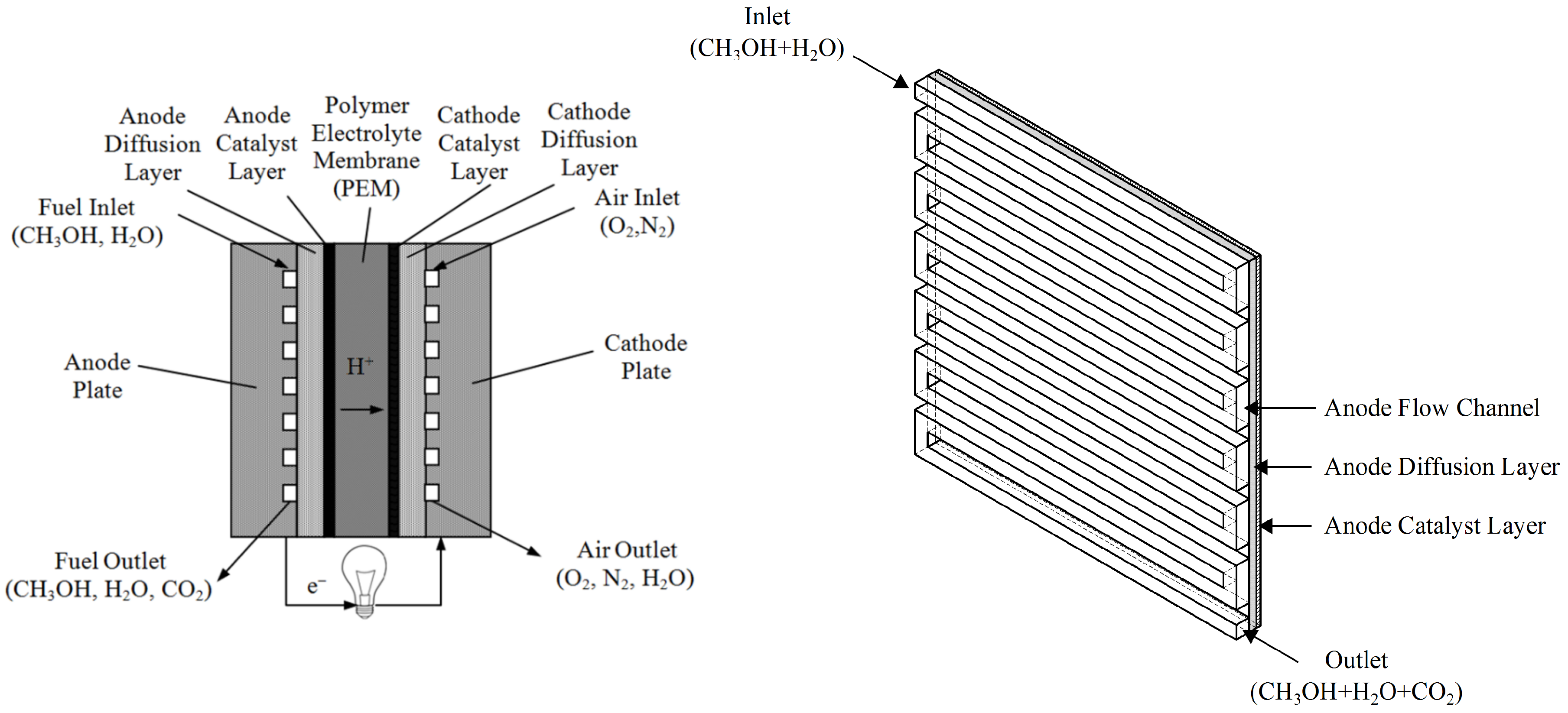
| Location | Length | Width | Height | Info |
|---|---|---|---|---|
| Anode plate | 30.95 | 30.65 | − | − |
| Channel layer | 30.95 | 30.65 | 0.50 | Serpentine channel with 12 U-turns |
| Channel cross-section | − | 1.37 | 0.50 | Square shape |
| Diffusion layer | 30.95 | 30.65 | 0.20 | − |
| Catalyst layer | 30.95 | 30.65 | 0.02 | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Wang, X.; Chen, J.; Yang, Q.; Jin, D.; Qiu, X. Numerical Investigations of the Combined Effects of Flow Rate and Methanol Concentration on DMFC Performance. Energies 2017, 10, 1094. https://doi.org/10.3390/en10081094
Hu X, Wang X, Chen J, Yang Q, Jin D, Qiu X. Numerical Investigations of the Combined Effects of Flow Rate and Methanol Concentration on DMFC Performance. Energies. 2017; 10(8):1094. https://doi.org/10.3390/en10081094
Chicago/Turabian StyleHu, Xuqu, Xingyi Wang, Juanzhong Chen, Qinwen Yang, Dapeng Jin, and Xiang Qiu. 2017. "Numerical Investigations of the Combined Effects of Flow Rate and Methanol Concentration on DMFC Performance" Energies 10, no. 8: 1094. https://doi.org/10.3390/en10081094






