The Performance of a Direct Borohydride/Peroxide Fuel Cell Using Graphite Felts as Electrodes
Abstract
:1. Introduction
2. Experimental Details
2.1. Single-Cell Design
2.2. Experimental Procedure
3. Results and Discussion
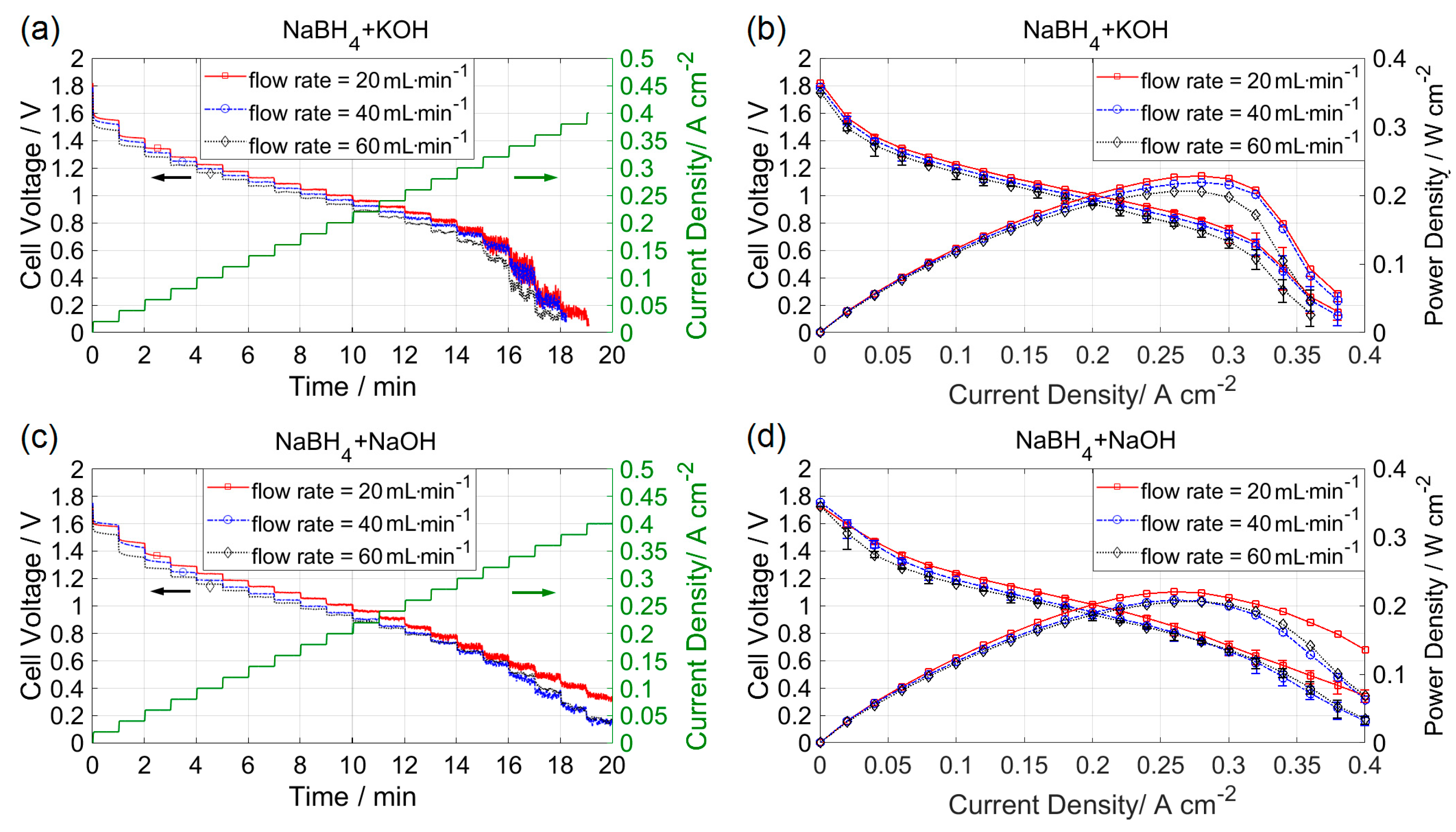
3.1. Effect of Flow Rate
3.2. Effect of Electrolyte Temperature
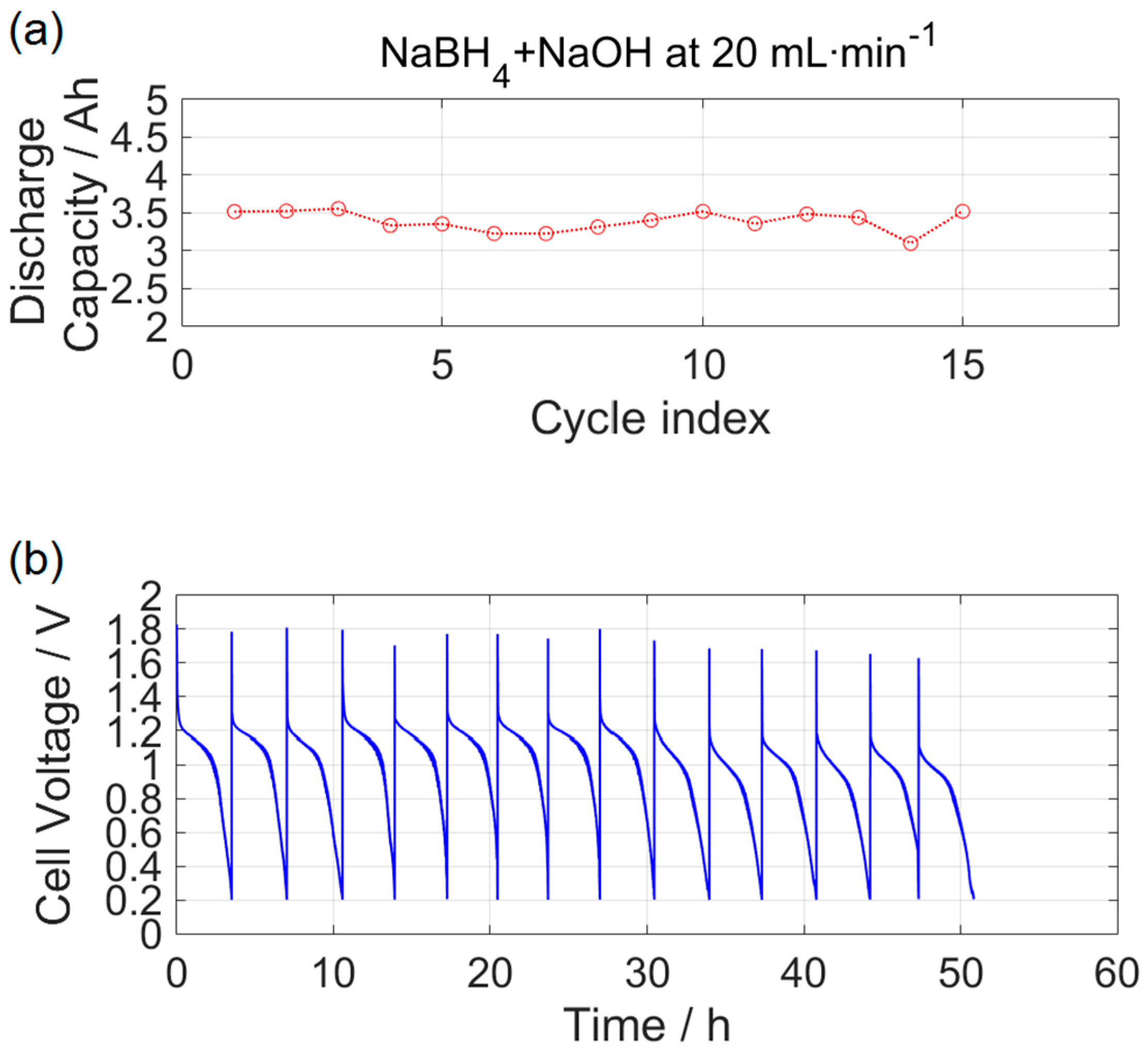
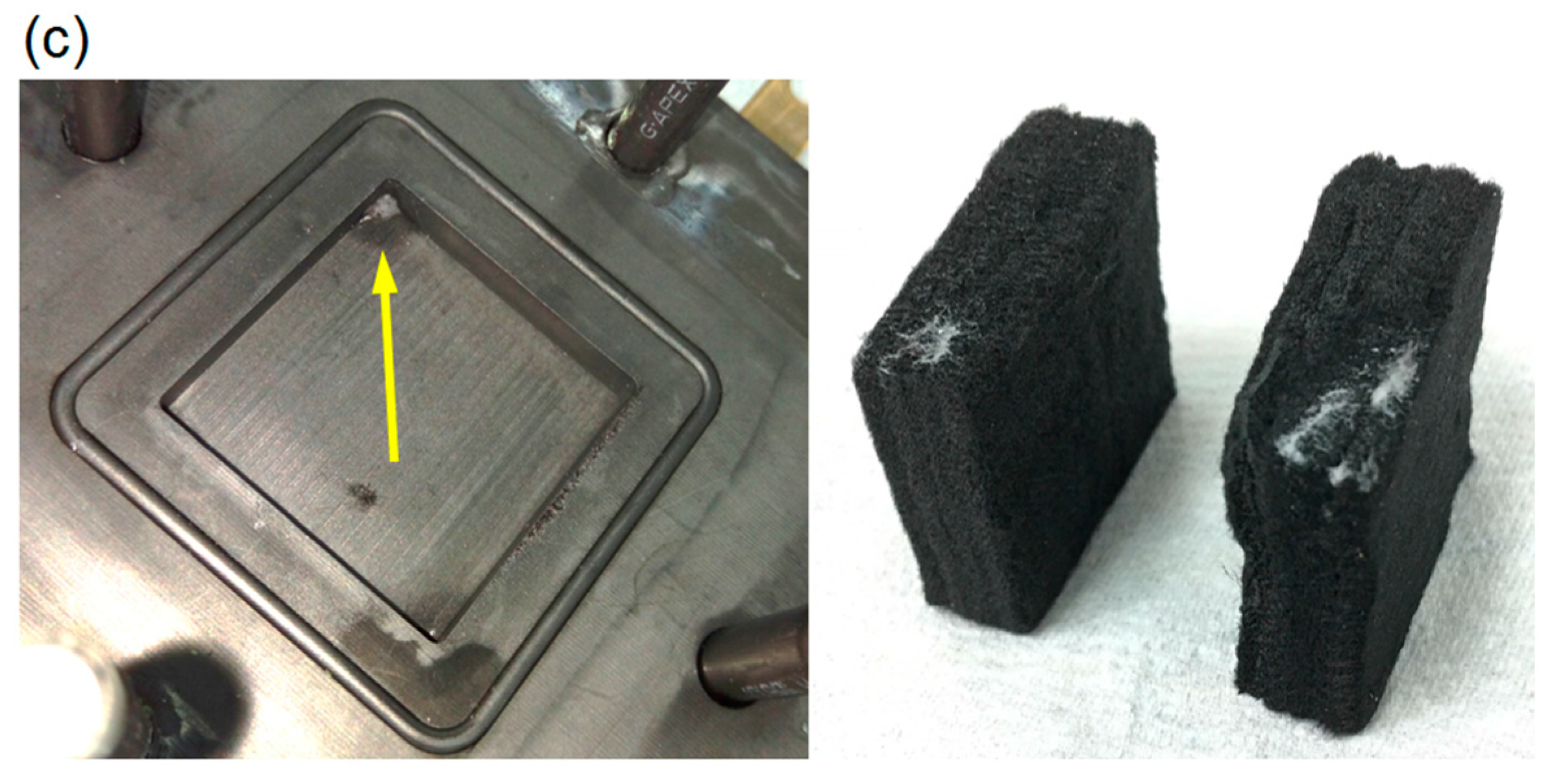
3.3. Durability Analysis
4. Conclusions
- The DBPFC with NaOH as the anolyte demonstrated more stable performance in the concentration overpotential region than the one using KOH as the anolyte.
- The anolyte/catholyte flow rate showed no significant effect on the DBPFC performance due to the large ratio of DL thickness to active area in this study.
- A maximum power density of 0.24 W∙cm−2 was obtained in the DBPFC with graphite felts as the DLs.
- After 50 h of galvanostatic operation, the DBPFC showed a slight drop in performance as a result of the accumulation of sodium borate in the graphite felts.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, X.; Yi, L.; Wang, X.; Su, J.; Song, Y.; Liu, J. Graphene supported platinum nanoparticles as anode electrocatalyst for direct borohydride fuel cell. Int. J. Hydrog. Energy 2012, 37, 17984–17991. [Google Scholar] [CrossRef]
- Briega-Martos, V.; Herrero, E.; Feliu, J.M. Borohydride electro-oxidation on Pt single crystal electrodes. Electrochem. Commun. 2015, 51, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Olu, P.-Y.; Gilles, B.; Job, N.; Chatenet, M. Influence of the surface morphology of smooth platinum electrodes for the sodium borohydride oxidation reaction. Electrochem. Commun. 2014, 43, 47–50. [Google Scholar] [CrossRef]
- Olu, P.-Y.; Deschamps, F.; Caldarella, G.; Chatenet, M.; Job, N. Investigation of platinum and palladium as potential anodic catalysts for direct borohydride and ammonia borane fuel cells. J. Power Sources 2015, 297, 492–503. [Google Scholar] [CrossRef]
- De Ponce León, C.; Walsh, F.C.; Patrissi, C.J.; Medeiros, M.G.; Bessette, R.R.; Reeve, R.W.; Lakeman, J.B.; Rose, A.; Browning, D. A direct borohydride-peroxide fuel cell using a Pd/Ir alloy coated microfibrous carbon cathode. Electrochem. Commun. 2008, 10, 1610–1613. [Google Scholar] [CrossRef]
- Yi, L.; Song, Y.; Wang, X.; Yi, L.; Hu, J.; Su, G.; Yi, W.; Yan, H. Carbon supported palladium hollow nanospheres as anode catalysts for direct borohydride-hydrogen peroxide fuel cells. J. Power Sources 2012, 205, 63–70. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Pasqualeti, A.M.; Molina Concha, M.B.; Chatenet, M.; Ticianelli, E.A. Borohydride electrooxidation on Au and Pt electrodes. Electrochim. Acta 2012, 84, 202–212. [Google Scholar] [CrossRef]
- Pei, F.; Wang, Y.; Wang, X.; He, P.Y.; Liu, L.; Xu, Y.; Wang, H. Preparation and performance of highly efficient Au nanoparticles electrocatalyst for the direct borohydride fuel cell. Fuel Cells 2011, 11, 595–602. [Google Scholar] [CrossRef]
- He, P.; Wang, X.; Fu, P.; Wang, H.; Yi, L. The studies of performance of the Au electrode modified by Zn as the anode electrocatalyst of direct borohydride fuel cell. Int. J. Hydrog. Energy 2011, 36, 8857–8863. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Abdolmaleki, M. Synthesis and characterization of porous nanostructured Ni/PdNi electrode towards electrooxidation of borohydride. Int. J. Hydrog. Energy 2013, 38, 5449–5456. [Google Scholar] [CrossRef]
- Hasan, M.; Newcomb, S.B.; Rohan, J.F.; Razeeb, K.M. Ni nanowire supported 3D flower-like Pd nanostructures as an efficient electrocatalyst for electrooxidation of ethanol in alkaline media. J. Power Sources 2012, 218, 148–156. [Google Scholar] [CrossRef]
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar] [CrossRef] [PubMed]
- Seymour, I.D.; Tarancón, A.; Chroneos, A.; Par, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ion. 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Park, K.T.; Jung, U.H.; Jeong, S.U.; Kim, S.H. Influence of anode diffusion layer properties on performance of direct borohydride fuel cell. J. Power Sources 2006, 162, 192–197. [Google Scholar] [CrossRef]
- Wang, G.; Gao, Y.; Wang, Z.; Du, C.; Yin, G. A membrane electrode assembly with high fuel coulombic efficiency for passive direct borohydride fuel cells. Electrochem. Commun. 2010, 12, 1070–1073. [Google Scholar] [CrossRef]
- Raman, R.K.; Prashant, S.K.; Shukla, A.K. A 28-W portable direct borohydride-hydrogen peroxide fuel-cell stack. J. Power Sources 2006, 162, 1073–1076. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.-J.; Ha, M.Y. Investigation of the characteristics of a stacked direct borohydride fuel cell for portable applications. J. Power Sources 2008, 180, 114–121. [Google Scholar] [CrossRef]
- Khadke, P.S.; Sethuraman, P.; Kandasamy, P.; Parthasarathi, S.; Shukla, A.K. A self-supported direct borohydride-hydrogen peroxide fuel cell system. Energies 2009, 2, 190–201. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Condeço, J.A.D.; Franco, M.W.; Sequeira, C.A.C. An Improved Borohydride-H2O2 Laboratory Fuel Cell. ECS Trans. 2007, 3, 19–30. [Google Scholar] [CrossRef]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Jamard, R.; Salomon, J.; Martinent-Beaumont, A.; Coutanceau, C. Life time test in direct borohydride fuel cell system. J. Power Sources 2009, 193, 779–787. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Hsu, Y.-H.; Tsai, P.-H.; Lee, J.-Y.; Chen, Y.-S. The Performance of a Direct Borohydride/Peroxide Fuel Cell Using Graphite Felts as Electrodes. Energies 2017, 10, 1124. https://doi.org/10.3390/en10081124
Lee H-Y, Hsu Y-H, Tsai P-H, Lee J-Y, Chen Y-S. The Performance of a Direct Borohydride/Peroxide Fuel Cell Using Graphite Felts as Electrodes. Energies. 2017; 10(8):1124. https://doi.org/10.3390/en10081124
Chicago/Turabian StyleLee, Heng-Yi, Yi-Hsuan Hsu, Po-Hong Tsai, Jiunn-Yih Lee, and Yong-Song Chen. 2017. "The Performance of a Direct Borohydride/Peroxide Fuel Cell Using Graphite Felts as Electrodes" Energies 10, no. 8: 1124. https://doi.org/10.3390/en10081124



